1. Introduction
ALS belongs to the neurodegenerative disease (NDD). ALS is relatively strange to the public because of the low disease incidence, and it is reported this disease happens to a maximum of 4 to 6 people per 100,000. ALS patients suffer from muscle atrophy, weakness due to degeneration and death of upper and lower motor neurons, and ultimately die of respiratory and other function failure.
So far, the pathogenesis of ALS is not clear enough. It is reported a few cases are due to genetic factors. Other factors include age, gender (men under 65 have a higher incidence than women), smoking, toxic exposure (organic solvents, heavy metal elements) and abnormal immune function may lead to the occurrence of the disease. So far, researchers have found mutations in multiple genes are closely related to the occurrence of ALS, such as TDP-43, SOD1, FUS. Their mutations may lead to the aggregation of abnormal proteins, inducing ALS. Other mechanisms including Oxidative stress, mitochondrial dysfunction, neuroinflammation may also lead to the occurrence of ALS. Currently, only four drugs, riluzole, edaravone, Relyvrio and Tofersen, have been approved by the U.S. Food and Drug Administration (FDA) for the treatment of ALS, and some prescription drugs are also used to control ALS related symptoms. However, these drugs can only delay the disease or reduce the pain, but cannot completely cure ALS. Therefore, patients are now in urgent need of drugs that can slow down and possibly treat this disease.
Currently, a variety of drugs are undergoing clinical trials based on the possible pathogenic mechanisms of ALS. Among them, ropinirole, as a dopamine receptor agonist, can enhance the effect of dopamine and was originally used to treat PD. In clinical trials, it was found it can effectively improve various indicators of ALS patients, including axon length, lactate dehydrogenase (LDH) leakage protein aggregation, etc. [1]. Thus, ropinirole may become an effective drug for the treatment of ALS. This paper investigated ropinirole, including initiation of autophagy, scavenging of reactive oxygen species (ROS), and regulating the abnormal metabolism of lipids, in the hope of illustrating its therapeutic mechanism for ALS.
2. Partial pathogenesis of ALS and related genes
2.1. Aggregation of misfolded proteins
Abnormal protein aggregation is considered a pathological marker of ALS. Repeated accumulation of SOD1, TDP-43, FUS can cause great damage to neurons through gain of function or loss of function. Among them, SOD1 is the most common and earliest discovered gene, which exists in the cytoplasm, nucleus and mitochondrial membrane space. Although SOD1 only has 154 amino acids, more than 180 mutants have been found in it. Mutations in SOD1 cause conformational changes in the encoded protein, leading to abnormal protein aggregation and toxic effects. At the same time, autophagy, as the main way of clearing abnormal proteins in the body, also appears abnormal in ALS patients.
2.2. Oxidative stress damage
ROS is a byproduct of normal oxygen metabolism, Excessive ROS can cause oxidative stress. Compared with other organs, the brain is more sensitive and neurons are more susceptible to damage caused by excessive ROS. Too much ROS will cause a large amount of Ca+ to enter mitochondria and neurons and cause damage. At the same time, the mitochondrial permeability transition pore (mPTP) will open due to excessive Ca+ concentration. Mitochondria will sell and rupture due to the large influx of small molecule substances [2].
2.3. Mitochondrial dysfunction
Significant changes can be observed in the mitochondria of ALS patients, including swelling, abnormal cell distribution, and reduced activity of related enzymes. In terms of function, ALS patients show increased ROS levels, impaired energy synthesis, protein imbalance, etc. At the same time, mitochondrial autophagy is also abnormal. SOD1 mutations may lead to weakened mitophagy and further cause neurological damage. However, some studies have shown mitophagy may also be significantly enhanced due to SOD1 mutations [3]. Studies suggested mutant SOD1 in mitochondria binds to Bcl-2, and the toxic complex formed exposes the BH3 domain and then forms toxic proteins through structural rearrangements, which is responsible for the reduced permeability and hyperpolarization of mitochondria to ADP [4].
3. Mechanisms of treatment with ropinirole
As a dopamine agonist, ropinirole enhances the effects of dopamine, corrects central neurotransmitter imbalances, relieves muscle stiffness, tremors, bradykinesia and loss of voluntary movement, and maintains patient autonomy for the treatment of PD. Nowadays, induced pluripotent stem cell (IPSC) technology is widely used in the study of ALS, and researchers have established a large number of in vitro models of SLAS using IPSC technology and screened 1,232 FDA-approved drugs, finally identifying ropinirole as a potential drug for the treatment of ALS [1].
3.1. Beclin1-dependent induction of autophagy activation
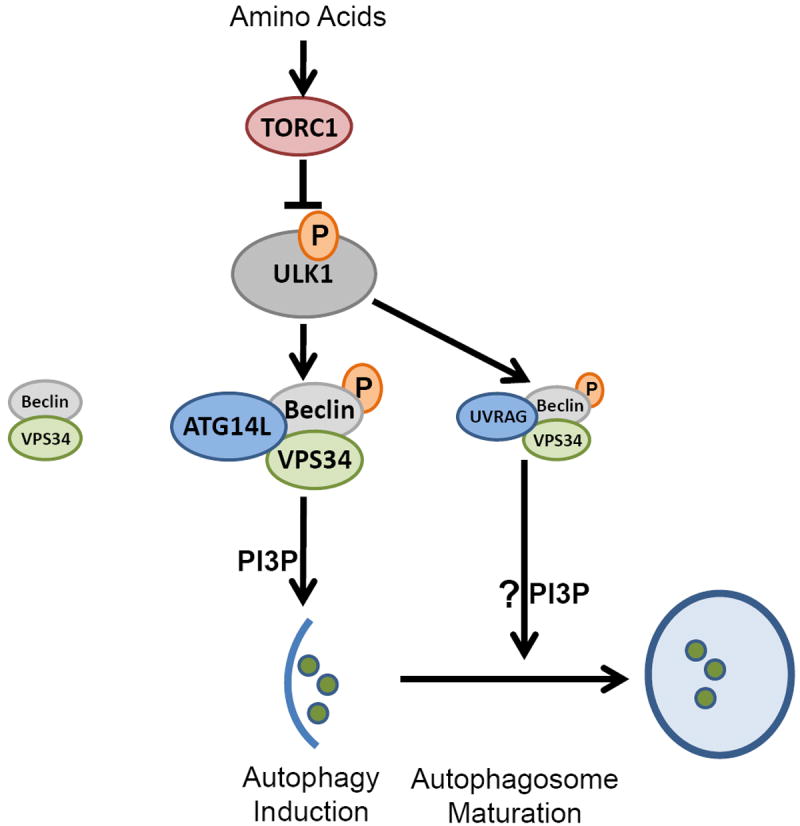
MTOR is the main regulator of autophagy, but studies suggest the mTOR pathway does not appear to be involved in agonist-induced autophagy [5]. However, receptor agonists may induce autophagy dependent on the Beclin-1 (Becn1) pathway. Becn1 is also an important factor in regulating autophagy, as shown in Figure 1, when mTOR is inhibited and in a state of amino acid starvation, a kinase ULK1 phosphorylates the Ser 14 site of Becn1 and activates it. The esterase VPS3 increases the activity of its complex to induce autophagy, and the phosphorylation of uvrag-bound Becn1 by ULK1 may promote autophagosome maturation [6].
| Figure 1. ULK1 kinase participates in Becn1-dependent autophagy [6]. |
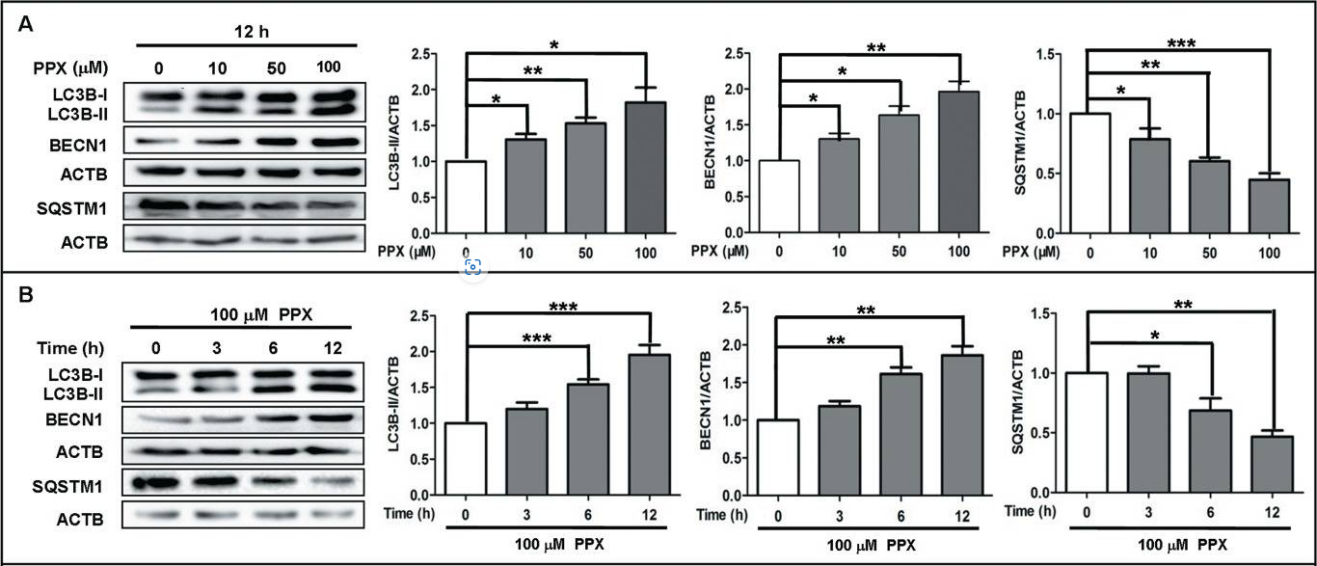
| Figure 2. Treatment of PC12 cells using PPX and quinpirole [5]. |
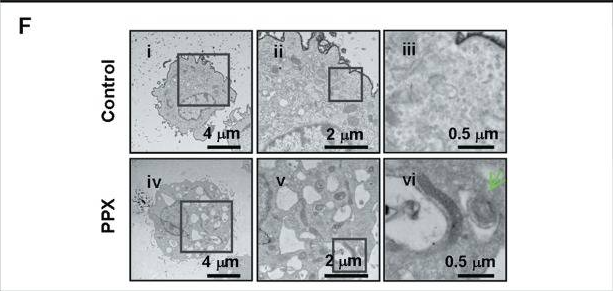
At the same time, researchers used pramipexole (PPX, a structural analog of ropinirole) and another dopamine receptor agonist quinpirole to treat PC12 cells to study the effects of D2 receptor agonists on autophagy, the results showed the levels of BECN1 and LC3B-II in PC12 cells increased after treatment with these two receptor agonists, suggesting autophagy activation in surface PC12 cells could be induced by dopamine receptor agonists, and surface PPX and LC3B-II were analyzed by co-immunoprecipitation, indicating quinpirole enhances the expression of endogenous BECN1. From figure2:When PC12 cells were treated with PPX at different concentrations (10μm,50μm,100μm), the level of BECIN1 protein increased with the increase in concentration; under the treatment of the same concentration of PPX, the level of BECN1 protein in PC12 cells gradually increased within 12 hours. From figure3: using PPX could induce the formation of autophagic vesicles. Various results show PPX and quinpirole are dependent on BECN1 to induce autophagy [5], suggesting ropinirole may share the same mechanism of inducing autophagy which relies on BECN1.
| Figure 3. After treatment with PPX, the formation of autophagic vesicles was observed by transmission electron microscopy [5]. |
These evidence show dopamine receptor agonists can induce PC12 cells to initiate autophagy, suggesting ropinirole may rely on BECN1 to initiate autophagy, clear structurally and functionally abnormal proteins, thereby protecting neurons.
In addition, a new AP-1 sequence was found in the promoter of Becn1, and the transcription factor FOS can bind to it to increase the transcription of BECN1. The researchers also demonstrated DRD2 and DRD3 receptor agonists may enhance this binding. At the same time, FOS up-regulation and induction of autophagy may be involved in Ca2+, CAMK4 signaling and CREB phosphorylation. Once DRD2 and DRD3 agonists bind to the receptor, Ca2+ from the endoplasmic reticulum is released through the PLCB1 and IP3 signaling pathways, binds to calmodulin and activates CAMK4 to phosphorylate CREB protein, resulting in enhanced FOS transcription [5].
3.2. Ropinirole inhibits mitochondrial mPTP
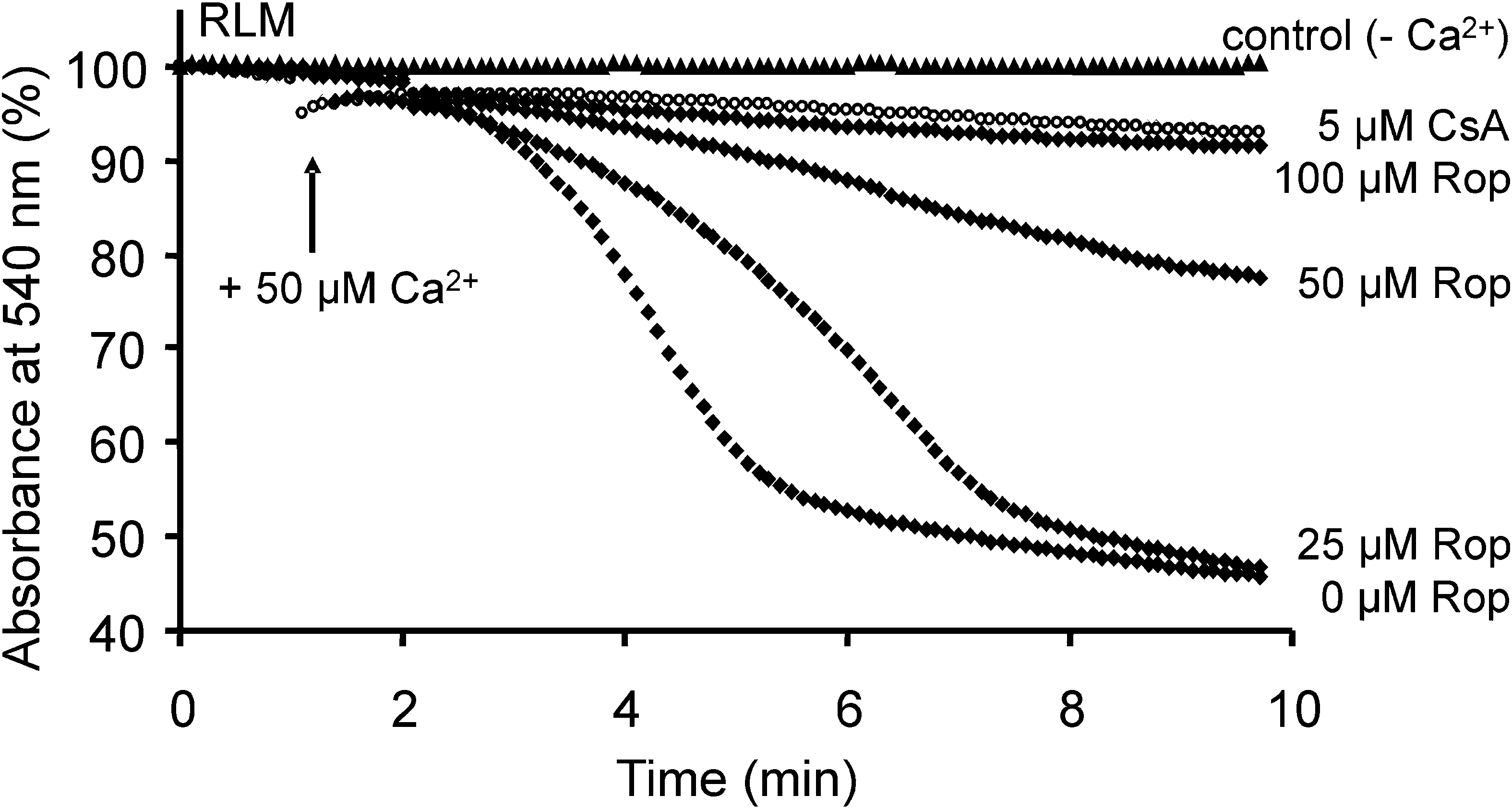
Ropinirole can inhibit the opening activity of mPTP to a certain extent, thereby protecting mitochondria and neurons. Researchers treated Wistar rat liver mitochondria (RLM) [7] with different concentrations of ropinirole, and monitored permeability transition triggered by Ca2+, mitochondrial swelling, mitochondrial membrane potential and other indicators. And results showed the relationship between the effect of ropinirole on the opening of the mPTP (mitochondrial swelling indicated the opening of the mPTP or a shift in permeability) as well as the depolarisation of mitochondria related to the concentration of ropinirole [8]. From figure4, 5 µm mPTP inhibitor CsA completely inhibited mitochondrial swelling induced by Ca2+, and as the concentration of ropinirole increased, the inhibitory effect was further enhanced. This suggests that higher concentrations of ropinirole may have a better inhibitory effect on mPTP opening and mitochondrial swelling.
| Figure 4. The relationship between mPTP opening triggered by Ca2+ in RLM treated with different concentrations of ropinirole [8]. |
These results indicated ropinirole affects the opening of mPTP in liver mitochondria, but whether ropinirole can have the same effect on mPTP opening in human brain tissue requires further study.
In addition, ropinirole exists in the form of lipophilic cations under physiological pH conditions, which makes it possible to bind to the negatively charged groups of mPTP and enter the inner mitochondrial membrane [9]. This may be why ropinirole could target, bind to mitochondria and play a protective role.
3.3. Ropinirole scavenges reactive oxygen species
Because of its unique structural and chemical characteristics, ropinirole may exert protective effects by scavenging ROS in addition to functioning as a dopamine receptor agonist. Ropinirole and its metabolite 7-hydroxyropinirole have an oxindole skeleton (the bioisostere of phenol) and N,N-di-n-propylethylamine. The former is an equivalent form of phenol and can act as an antioxidant, explaining why ropinirole can clean up ROS and protect mitochondria [10].
3.4. Current progress of ropinirole in the treatment of ALS
Ropinirole is currently approved by the FDA for the treatment of restless legs syndrome (RLS) and PD, and the treatment of ALS is still in the clinical trial phase. More recently, a phas 1/2a trial further investigated the safety, tolerability and efficacy of ropinirole in 20 patients with SALS (13 on ropinirole and 7 on placebo as the control group) [11]. Regarding its safety, subjects experienced constipation, nausea, drowsiness and other adverse events related to ropinirole itself; ropinirole was well tolerated and all patients were able to accept its maximum prescribed dose (16mg), no patient stopped taking the medication due to adverse reactions, which also proved ropinirole can be safely used for treatment; the results showed the disease course of the patients in the ropinirole group was longer, extending by about 7 months, which is meaningful for ALS patients ( considering the average time to death is 2-4 years). In addition, through patients’ IPSCs, it was found ropinirole could improve MNs neurite length. The researchers found the expression of d2r receptor under the action of ropinirole, suggesting ropinirole may indeed work through dependence on D2R receptor; in addition, considering studies have shown lipid metabolism in ALS patients appears abnormalities such as fatty acids, sterols, glycerides, etc. [12]. By analyzing cerebrospinal fluid and blood samples from ALS patients, experiments also pointed out lipid peroxidation has been identified as a marker of ALS progression and drug efficacy [13], and the treatment of ropinirole may involve the srebp2-cholesterol pathway: ropinirole inhibits the expression of SREBP2 and HMGCR to reduce toxic lipids [14], and SREBP2 and HMGCR are transcription factors and enzymes that are important for cholesterol synthesis.
4. Conclusion
Through research, this article found ropinirole may improve various indicators of ALS patients in multiple ways. As a dopamine receptor agonist, ropinirole may rely on the BECN1 to induce autophagy to clear structurally and functionally abnormal proteins, thereby protecting neurons. The new AP-1 sequence on the BECN1 promoter may also be involved in inducing autophagy. Ropinirole can inhibit the opening activity of mPTP and inhibit oxidative stress damage to protect mitochondria because of its unique chemical structure in the body; finally, ropinirole may inhibit abnormal lipid metabolism in ALS patients via the srebp-2 cholesterol pathway. Through analysis, this article summarises the possible therapeutic mechanisms of ropinirole, which provides a theoretical basis for further research on ropinirole and the development of more new drugs and reflects the importance of IPSC for ALS drug development. However, current studies face the problem of an insufficient sample size, and some of the mechanisms of ropinirole need further study. Future research can focus on the specific pathways of ropinirole in scavenging ROS and controlling abnormal lipid metabolism, thereby elucidating the mechanism of ropinirole and allowing it to be used in the treatment of ALS.
References
[1]. Fujimori K Ishikawa M Otomo A et al 2018 Modeling sporadic ALS in iPSC-derived motor neurons identifies a potential therapeutic agent Nat Med 24 10 1579-1589
[2]. Xu G 2021 Mechanism of calcium ion and reactive oxygen regulation of mitochondrial permeability transition pore opening [Dissertation Shanxi University]
[3]. Maestro I de la Ballina L R Porras G et al 2018 Discovery of Mitophagy Inhibitors with Therapeutic Potential in Different Familial Amyotrophic Lateral Sclerosis Mutations Int J Mol Sci 23 20 12676
[4]. Tan W Naniche N Bogush A Pedrini S Trotti D & Pasinelli P 2013 Small peptides against the mutant SOD1/Bcl-2 toxic mitochondrial complex restore mitochondrial function and cell viability in mutant SOD1-mediated ALS J Neurosci 33 28 11588-98
[5]. Wang J D Cao Y L Li Q et al 2015 A pivotal role of FOS-mediated BECN1/Beclin 1 upregulation in dopamine D2 and D3 receptor agonist-induced autophagy activation Autophagy 11 11 2057-2073
[6]. Russell R C Tian Y Yuan H et al 2013 ULK1 induces autophagy by phosphorylating Beclin-1 and activating VPS34 lipid kinase Nat Cell Biol 15 7 741-50
[7]. Liu Y Fiskum G & Schubert D 2002 Generation of reactive oxygen species by the mitochondrial electron transport chain J Neurochem 80 5 780-7
[8]. Parvez S Winkler-Stuck K Hertel S Schönfeld P & Siemen D 2010 The dopamine-D2-receptor agonist ropinirole dose-dependently blocks the Ca2+-triggered permeability transition of mitochondria Biochim Biophys Acta 1797 6-7 1245-50
[9]. Murphy M P 2008 Targeting lipophilic cations to mitochondria Biochim Biophys Acta 1777 7-8 1028-31
[10]. Okano H Yasuda D Fujimori K Morimoto S & Takahashi S 2020 Ropinirole a New ALS Drug Candidate Developed Using iPSCs Trends Pharmacol Sci 41 2 99-109
[11]. Morimoto S Takahashi S Ito D et al 2023 Pooled Resource Open-Access ALS Clinical Trials Consortium; Okano H Phase 1/2a clinical trial in ALS with ropinirole a drug candidate identified by iPSC drug discovery Cell Stem Cell 30 6 766-780e9
[12]. Hartmann H Ho W Y Chang J C & Ling S C 2022 Cholesterol dyshomeostasis in amyotrophic lateral sclerosis: cause consequence or epiphenomenon? FEBS J 289 24 7688-7709
[13]. Okano H Morimoto S Kato C et al 2023 Induced pluripotent stem cells-based disease modeling drug screening clinical trials and reverse translational research for amyotrophic lateral sclerosis J Neurochem 167 5 603-614
Cite this article
Man,H. (2024). Mechanism of ropinirole in the treatment of amyotrophic lateral sclerosis. Theoretical and Natural Science,49,192-197.
Data availability
The datasets used and/or analyzed during the current study will be available from the authors upon reasonable request.
Disclaimer/Publisher's Note
The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of EWA Publishing and/or the editor(s). EWA Publishing and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
About volume
Volume title: Proceedings of the 4th International Conference on Biological Engineering and Medical Science
© 2024 by the author(s). Licensee EWA Publishing, Oxford, UK. This article is an open access article distributed under the terms and
conditions of the Creative Commons Attribution (CC BY) license. Authors who
publish this series agree to the following terms:
1. Authors retain copyright and grant the series right of first publication with the work simultaneously licensed under a Creative Commons
Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this
series.
2. Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the series's published
version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial
publication in this series.
3. Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and
during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See
Open access policy for details).
References
[1]. Fujimori K Ishikawa M Otomo A et al 2018 Modeling sporadic ALS in iPSC-derived motor neurons identifies a potential therapeutic agent Nat Med 24 10 1579-1589
[2]. Xu G 2021 Mechanism of calcium ion and reactive oxygen regulation of mitochondrial permeability transition pore opening [Dissertation Shanxi University]
[3]. Maestro I de la Ballina L R Porras G et al 2018 Discovery of Mitophagy Inhibitors with Therapeutic Potential in Different Familial Amyotrophic Lateral Sclerosis Mutations Int J Mol Sci 23 20 12676
[4]. Tan W Naniche N Bogush A Pedrini S Trotti D & Pasinelli P 2013 Small peptides against the mutant SOD1/Bcl-2 toxic mitochondrial complex restore mitochondrial function and cell viability in mutant SOD1-mediated ALS J Neurosci 33 28 11588-98
[5]. Wang J D Cao Y L Li Q et al 2015 A pivotal role of FOS-mediated BECN1/Beclin 1 upregulation in dopamine D2 and D3 receptor agonist-induced autophagy activation Autophagy 11 11 2057-2073
[6]. Russell R C Tian Y Yuan H et al 2013 ULK1 induces autophagy by phosphorylating Beclin-1 and activating VPS34 lipid kinase Nat Cell Biol 15 7 741-50
[7]. Liu Y Fiskum G & Schubert D 2002 Generation of reactive oxygen species by the mitochondrial electron transport chain J Neurochem 80 5 780-7
[8]. Parvez S Winkler-Stuck K Hertel S Schönfeld P & Siemen D 2010 The dopamine-D2-receptor agonist ropinirole dose-dependently blocks the Ca2+-triggered permeability transition of mitochondria Biochim Biophys Acta 1797 6-7 1245-50
[9]. Murphy M P 2008 Targeting lipophilic cations to mitochondria Biochim Biophys Acta 1777 7-8 1028-31
[10]. Okano H Yasuda D Fujimori K Morimoto S & Takahashi S 2020 Ropinirole a New ALS Drug Candidate Developed Using iPSCs Trends Pharmacol Sci 41 2 99-109
[11]. Morimoto S Takahashi S Ito D et al 2023 Pooled Resource Open-Access ALS Clinical Trials Consortium; Okano H Phase 1/2a clinical trial in ALS with ropinirole a drug candidate identified by iPSC drug discovery Cell Stem Cell 30 6 766-780e9
[12]. Hartmann H Ho W Y Chang J C & Ling S C 2022 Cholesterol dyshomeostasis in amyotrophic lateral sclerosis: cause consequence or epiphenomenon? FEBS J 289 24 7688-7709
[13]. Okano H Morimoto S Kato C et al 2023 Induced pluripotent stem cells-based disease modeling drug screening clinical trials and reverse translational research for amyotrophic lateral sclerosis J Neurochem 167 5 603-614