1.Introduction
BC is one of the most common diseases in the female population, in BC, the amplification of HER2 gene and overexpression of the protein are closely related to the occurrence, development, aggressiveness and prognosis of tumors. About 20-30% of primary invasive BCs have HER2 gene amplification or overexpression. Patients with HER2-positive BC typically have rapid disease progression, short remission periods with chemotherapy, poor response to endocrine therapy, and low disease-free survival and overall survival. In the treatment of HER2 BC, innovative targeted drugs have played an important role in recent decades [1]. Antibo-drug conjugates (ADCs) have been the focus of some researchers, including trastuzumab dercuxtecan (T-DXd).
It consists of an anti-HER2 humanizedmAb (MAAL-9001) combined with a cytotoxic drug named Esatiecan derivative MAAA-1181a (DXd), linked to a topoisomerase I inhibitor payload via a tetrapeptide-based cleavable splicer. Its unique design has a high drug-antibody ratio of about 8 that remains stable, thus providing an effective cytotoxic (ADCC) payload that is internalized and selectively cut by an overexpressed lysosomal enzyme in cancer cells.
Studies have shown that T-DXd has a good therapeutic effect on HER2-positive BC patients [2]. In addition, in the DESTINY-Breast03 study, the new progression-free survival (FPS) record for HER2-positive BC has established the status of second-line standard treatment internationally [2,3]. At present, several related biosimilars have been introduced into the clinic, providing different treatment schemes for trastuzumab adjuvant therapy [4].
However, in the long-term use of T-DXd, some side effects also appear, such as fatigue, decreased appetite, etc., which is similar to the characteristics of side effects of chemotherapy drugs [2]. In clinical and drug research, not only the effectiveness of drugs needs to be paid attention to, but also the safety of drugs needs to be guaranteed. Therefore, this paper focuses on the generation and analysis of side effects of T-DXd after adjuvant therapy in adult patients with HER2-positive BC, and based on this, discusses the problems that should be paid attention to in future drug development and clinical treatment of T-DXd, and puts forward the aspects that need to be focused on.
2.HER2 positive BC
2.1.Generation mechanism
The HER2 gene encodes a protein called human epidermal growth factor receptor 2 (EGFR). In some BC cells, the HER2 gene replicates multiple times in their DNA, with an increased copy number, which means that the HER2 gene is abnormally amplified, and in turn, excessive HER2 protein is produced during transcription and translation. The HER2 protein belongs to EGFR and its normal function is to regulate the growth and division of cells.
Due to overexpression of the HER2 gene, the concentration of HER2 protein on the surface of BC cells increases significantly, making it easier for HER2 receptors to form heterodimers or homologous dimers with other members of the HER family, such as HER1, HER3, and HER4. The formation of this dimer triggers the autophosphorylation of the receptor, activating multiple downstream signaling pathways.
These signaling pathways play an important role in promoting cell cycle, cell proliferation, invasion and metastasis. At the same time, their continued activation inhibits apoptosis of BC cells and affects the tumor microenvironment (including angiogenesis, immune escape, and stromal cell reprogramming), creating favorable conditions for rapid growth, deterioration, further development, and metastasis of tumor cells.
In general, after a multi-step process, involving gene variation, protein expression dysregulation, signaling abnormalities and other factors work together to promote the emergence and development of HER2-positive BC.
2.2.Treatment means
The treatment of HER2-positive BC includes anti-HER2-targeted therapy, chemotherapy, endocrine therapy (for HR+/ HER2-positive recurrent metastatic BC), surgical therapy, neoadjuvant (pre-operative) and adjuvant (post-operative) therapy, radiation therapy, etc.
Among them, anti-HER2 targeted therapy is an important treatment for HER2-positive BC, which includes large molecular clonal antibodies, small molecular tyrosine kinase inhibitors, and antibody conjugants (such as T-DM1 and T-DXd). With the development of HER2-targeting drugs, the cure rate of early stage patients and the survival rate of late stage patients have been significantly improved [5]. In addition, because T-DXd as ADCs has a high drug-antibody ratio structure and its unique mechanism of action against cancer cells, its outstanding and stable performance in clinical trials has attracted the attention of many researchers, and some people have compared the therapeutic effect of T-DXd with T-DM1, which was put into clinical trials earlier.
Therefore, the development of novel drugs and the implementation of new technologies that can specifically target cancer cells with minimal side effects will be the focus of future research [5].
3.T-DXd: a HER2-targeting drug
3.1.Structure and mechanism of action
T-DXd is a monoclonal ADC (ATC code :L01XC41). It consists of an anti-HER2 humanizedmAb (MAAL-9001) combined with a cytotoxic drug called the Esatikon derivative MAAA-1181a (DXd), linked to a topoisomerase I inhibitor payload via a tetraceptide-based cleavable adapter. Its unique design has a high drug-antibody ratio of about 8 that remains stable, thus providing an effective cytotoxic (ADCC) payload that is internalized and selectively cut by an overexpressed lysosomal enzyme in cancer cells.
T-DXd specifically binds to HER2 receptors on tumor cells. As shown in Figure 1, it is internalized by lysosomal enzymes and cleaved to intracellular junctions. After that, T-DXd enters tumor cells and plays a role in inducing DNA damage and apoptosis [6].
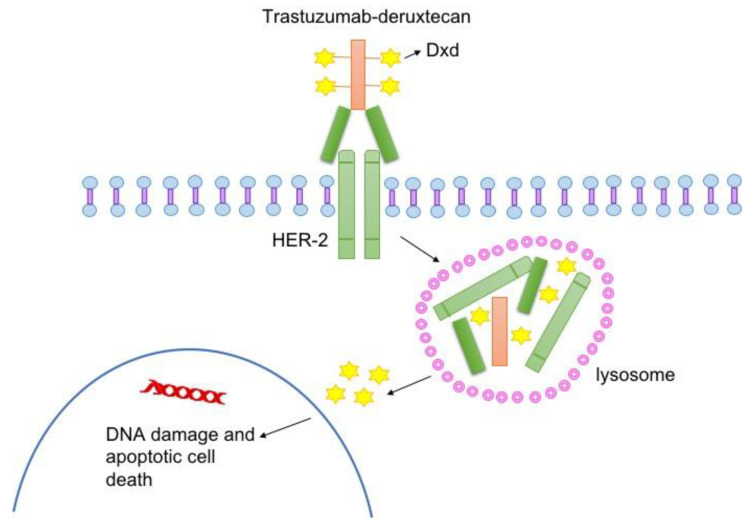
Figure 1. Structure and mechanism of T-DXd [6]
3.2.Development situation
Because of its significant efficacy and superiority in multiple clinical trials, T-DXd has been approved for use by drug regulatory agencies in many countries, and has been included as a recommended treatment in a number of international authoritative guidelines, including the National Cancer Comprehensive Network (NCCN) BC Clinical Practice Guidelines, ESMO Metastatic BC Online Guidelines, etc.
In the DESTINY-Breast03 study, the median progression-free survival (mPFS) of T-DXD-treated patients reached 28.8 months, and the 12-month overall survival (OS) was 94.1% [3]. This demonstrates the groundbreaking efficacy of T-DXd in the second-line treatment of HER2-positive BC.
It is worth mentioning that the specific binding receptor of T-DXd is HER2 receptor, which means that this drug can not only act on HER2-positive BC, but also can act on HER2-positive advanced gastric cancer, HER2 low expression of advanced BC and other indications.
With the continuous advancement of clinical research, it can be expected that the application scope and influence of T-DXd will continue to expand, and the accessibility of its drugs will also be improved.
4.Side effects and safety evaluation
4.1.Side effects
In the development and testing of any drug, side effects are the core content that needs to be paid attention to.
In the treatment process of T-DXd for HER2-positive BC, not only its comprehensive efficacy needs to be considered, but also the side effects caused by the drug are worth exploring. In Gavin P. Dowling's study, six articles were included as qualitative and quantitative synthesis studies through rigorous screening, and meta-analysis was used for complete data extraction, quality assessment and statistical analysis [7]. Common side effects of any grade can be found including nausea, vomiting, constipation, diarrhea, fatigue, anemia, and central granulocytopenia, while anemia and neutropenia have a relatively high incidence of side effects of grade 3 and above [7].
Special attention should be paid to ILD as a potentially serious side effect [7]. This is a heterogeneous disease with a lack of clinical specificity. In some patients, the imaging changes of ILD can be completely reversed by active immunosuppressive therapy, in some patients, the lesions are irreversible but stable for a long time, and in some patients, the lesions continue to progress and eventually develop into end-stage respiratory failure. Among the six articles included in Gavin P. Dowling's study, about 5.22% of patients in Tamura K 2019 had this symptom [8], 13.59% in Modi S 2019 [9], and 14.81% in Modi S 2020 [10]. 6.67% in Bartsch R 2022 [11], 12.1% in Modi S 2022 [12] and 10.5% in Javier Cortes2022 [3]. These data can not be ignored, but also provide new concerns and ideas for future research direction.
4.2.Safety evaluation
In the articles mentioned above, Tamura K 2019, Modi S 2019, Modi S 2020 and Bartsch R 2022 were single-arm experiments, while Javier Cortes2022 and Modi S 2022 were randomized controlled experiments. Figure 2 shows the single-group rate analysis at any level in all single-arm experiments [7], and it can be seen that the heterogeneity (I2) is large except for the analysis of symptoms of constipation and neutropenia. The small number of studies in Bartsch R 2022 (n=15) may be one of the factors contributing to the high heterogeneity.
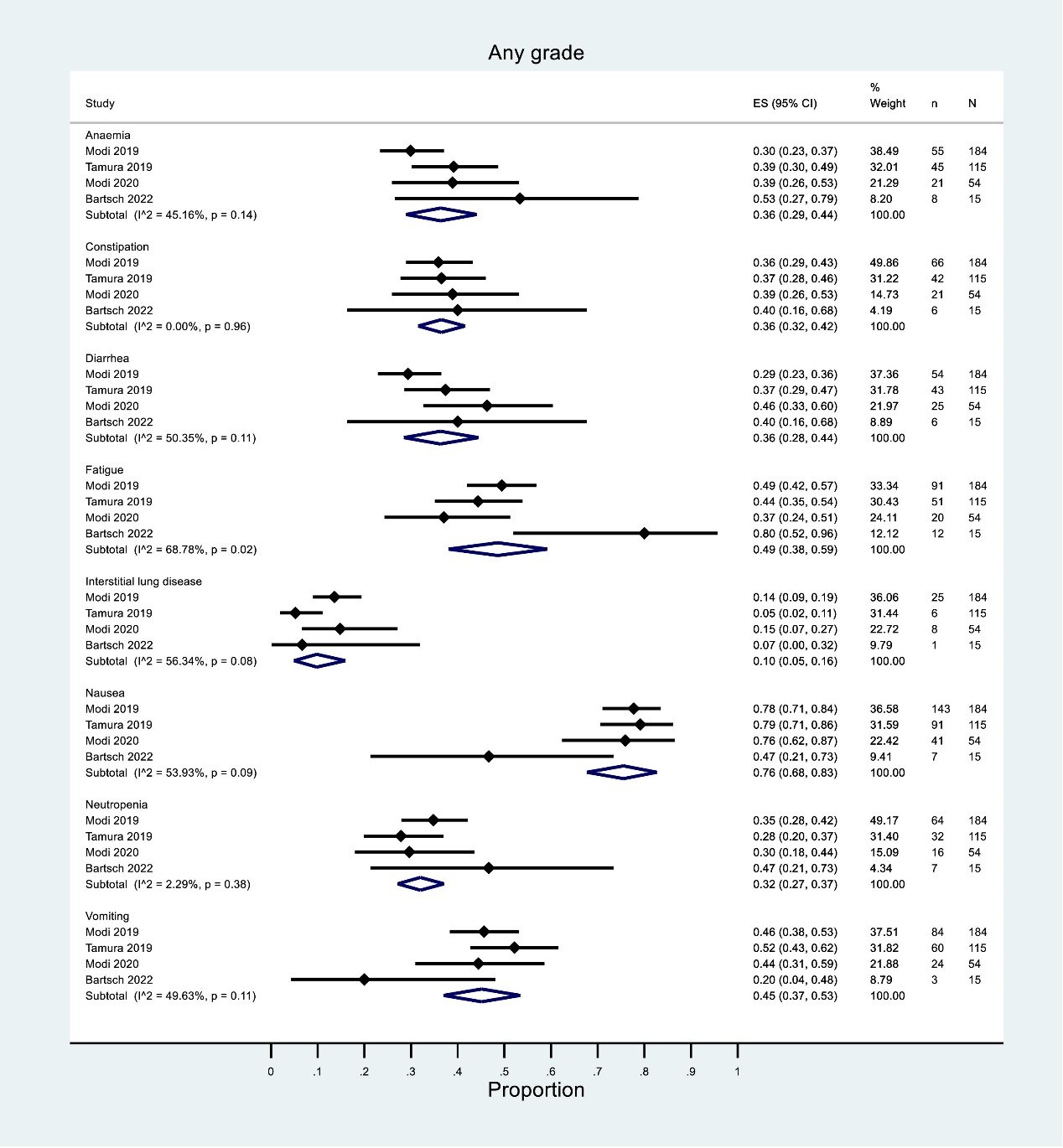
Figure 2. Single-group rate analysis of any level of symptoms in all single-arm experiments [7]
As shown in Figure 3, with a 95% confidence interval, the combined prevalence of nausea was highest (76%), while the combined prevalence of ILD symptoms was lowest (about 10%). The combined prevalence of vomiting and fatigue was higher, neutropenia was lower, and the rest were similar.
Figure 3. Combined prevalence of any level of symptoms in all one-arm trials (funnel plot)
It should be noted in particular that in the analysis of ILD, the OR value was high, indicating that T-DXd drug treatment was clearly correlated with the generation of ILD [7].
In general, as far as the current data is concerned, although the T-DXd drug will bring different degrees of side effects in treatment, it is still a relatively safe drug on the whole. Due to the different conditions of the included trials, such as some patients who had previously received other treatments, individual and regional differences, more clinical studies, especially randomized controlled trials, are needed in the later stage to evaluate the safety of T-DXd more accurately.
4.3.Pre-scheme for ILD
By comparison, the ILD mortality rate in earlier trials such as Tamura et al. 2019 was more than twice that in the latest trials such as Cortes et al. 2022 [7]. Analysis of T-DXd dosage in each trial, combined with ILD data analysis, showed a strong correlation between symptoms in ILD and T-DXd dosage. The method of reducing drug dosage or intermittent drug use should be considered, and the patient's physical condition and ILD symptoms should be frequently monitored, so as to timely adjust the treatment plan and implement intervention measures as soon as possible [7].
To enhance the safety profile of T-DXd, particularly concerning ILD, future clinical strategies should focus on dose adjustments and intermittent drug administration. Regular monitoring of patients' physical conditions and ILD symptoms is crucial for timely intervention. The observed reduction in ILD mortality rates in recent trials compared to earlier ones highlights the progress in managing this side effect, potentially attributable to improved dosing strategies and better patient management protocols. Continued research, especially through randomized controlled trials, is essential to refine these strategies and further mitigate side effects.
Additionally, exploring biomarkers for early detection of ILD and other severe side effects could significantly enhance patient outcomes. Personalized treatment plans based on individual patient profiles and genetic predispositions may offer a tailored approach, minimizing adverse effects while maximizing therapeutic efficacy. The integration of these strategies into clinical practice will be pivotal in optimizing T-DXd treatment for HER2-positive BC.
5.Conclusion
This paper mainly discusses the mechanism of HER2-positive BC and existing therapeutic means, explores the mechanism of action and application of T-DXd against HER2-positive BC, and analyzes the side effects and safety evaluation of T-DXd targeted therapy for adult female patients with HER2-positive BC. Through the analysis of this paper, it can be seen that although T-DXd is a relatively safe antibody coupling class of drugs, some of its side effects can not be ignored, especially the pulmonary interstitial disease, which needs more attention. In order to reduce the occurrence of diseases such as pulmonary interstitial disease, close monitoring of patients' physical conditions can be considered, and effective adjustment and intervention can be carried out by reducing drug dosage or intermittent drug use. This provides a new idea for future research, which can focus on the principle and solution of side effects, and try to find the safest and effective drug program. Although the safety of T-DXd is evaluated and a pre-solution is proposed in this paper, the supporting data is small, which makes it impossible to conduct a more macro analysis. The heterogeneity of the study is high, and the pre-solution may be biased. In future studies, this problem should be explored more systematically and comprehensively in an attempt to provide more reliable data support and guidance for clinical trials.
References
[1]. Liang X Yan Y & Song G 2022 A round table discussion: clinical landscape of trastuzumab deruxtecan in breast cancer: a retrospective and prospective view Translational Breast Cancer Research: A Journal Focusing on Translational Research in Breast Cancer 3 32
[2]. Yan Y Li Q & Li J 2022 Round table discussion: strategies for the treatment of HER2-positive advanced breast cancer in the rising age of antibody-drug conjugates Translational Breast Cancer Research: A Journal Focusing on Translational Research in Breast Cancer 3 18
[3]. Cortés J Kim S B Chung W P et al 2022 Trastuzumab Deruxtecan versus Trastuzumab Emtansine for Breast Cancer The New England Journal of Medicine 386 12 1143–1154
[4]. Mercogliano M F Bruni S Mauro F L & Schillaci R 2023 Emerging targeted therapies for HER2-positive breast cancer Cancers 15 7 1987
[5]. Marra A Chandarlapaty S & Modi S 2024 Management of patients with advanced-stage HER2-positive breast cancer: current evidence and future perspectives Nature Reviews Clinical Oncology 21 3 185–202
[6]. Indini A Rijavec E & Grossi F 2021 Trastuzumab Deruxtecan: Changing the destiny of HER2 expressing solid tumors International Journal of Molecular Sciences 22 9 4774
[7]. Dowling G P Daly G R Keelan S et al 2023 Efficacy and safety of trastuzumab deruxtecan in breast cancer: A systematic review and meta-analysis Clinical Breast Cancer 23 8 847–855e2
[8]. Tamura K Tsurutani J Takahashi S et al 2019 Trastuzumab deruxtecan DS-8201a in patients with advanced HER2-positive breast cancer previously treated with trastuzumab emtansine: a dose-expansion phase 1 study The Lancet Oncology 20 6 816–826
[9]. Modi S Saura C Yamashita T et al 2020 Trastuzumab Deruxtecan in previously treated HER2-positive breast cancer The New England Journal of Medicine 382 7 610–621
[10]. Modi S Park H Murthy R K et al 2020 Antitumor activity and safety of trastuzumab deruxtecan in patients with HER2-low-expressing advanced breast cancer: Results from a Phase Ib study Journal of Clinical Oncology: Official Journal of the American Society of Clinical Oncology 38 17 1887–1896
[11]. Bartsch R Berghoff A S Furtner J et al 2022 Trastuzumab deruxtecan in HER2-positive breast cancer with brain metastases: A single-arm phase 2 trial Nature Medicine 28 9 1840–1847
[12]. Modi S Jacot W Yamashita T Sohn J et al 2022 Trastuzumab deruxtecan in previously treated HER2-low advanced breast cancer The New England Journal of Medicine 387 1 9–20
Cite this article
Cheng,N. (2024). Evaluation of the safety of T-DXd in the Treatment of HER2-positive breast cancer. Theoretical and Natural Science,59,58-64.
Data availability
The datasets used and/or analyzed during the current study will be available from the authors upon reasonable request.
Disclaimer/Publisher's Note
The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of EWA Publishing and/or the editor(s). EWA Publishing and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
About volume
Volume title: Proceedings of the 4th International Conference on Biological Engineering and Medical Science
© 2024 by the author(s). Licensee EWA Publishing, Oxford, UK. This article is an open access article distributed under the terms and
conditions of the Creative Commons Attribution (CC BY) license. Authors who
publish this series agree to the following terms:
1. Authors retain copyright and grant the series right of first publication with the work simultaneously licensed under a Creative Commons
Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this
series.
2. Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the series's published
version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial
publication in this series.
3. Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and
during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See
Open access policy for details).
References
[1]. Liang X Yan Y & Song G 2022 A round table discussion: clinical landscape of trastuzumab deruxtecan in breast cancer: a retrospective and prospective view Translational Breast Cancer Research: A Journal Focusing on Translational Research in Breast Cancer 3 32
[2]. Yan Y Li Q & Li J 2022 Round table discussion: strategies for the treatment of HER2-positive advanced breast cancer in the rising age of antibody-drug conjugates Translational Breast Cancer Research: A Journal Focusing on Translational Research in Breast Cancer 3 18
[3]. Cortés J Kim S B Chung W P et al 2022 Trastuzumab Deruxtecan versus Trastuzumab Emtansine for Breast Cancer The New England Journal of Medicine 386 12 1143–1154
[4]. Mercogliano M F Bruni S Mauro F L & Schillaci R 2023 Emerging targeted therapies for HER2-positive breast cancer Cancers 15 7 1987
[5]. Marra A Chandarlapaty S & Modi S 2024 Management of patients with advanced-stage HER2-positive breast cancer: current evidence and future perspectives Nature Reviews Clinical Oncology 21 3 185–202
[6]. Indini A Rijavec E & Grossi F 2021 Trastuzumab Deruxtecan: Changing the destiny of HER2 expressing solid tumors International Journal of Molecular Sciences 22 9 4774
[7]. Dowling G P Daly G R Keelan S et al 2023 Efficacy and safety of trastuzumab deruxtecan in breast cancer: A systematic review and meta-analysis Clinical Breast Cancer 23 8 847–855e2
[8]. Tamura K Tsurutani J Takahashi S et al 2019 Trastuzumab deruxtecan DS-8201a in patients with advanced HER2-positive breast cancer previously treated with trastuzumab emtansine: a dose-expansion phase 1 study The Lancet Oncology 20 6 816–826
[9]. Modi S Saura C Yamashita T et al 2020 Trastuzumab Deruxtecan in previously treated HER2-positive breast cancer The New England Journal of Medicine 382 7 610–621
[10]. Modi S Park H Murthy R K et al 2020 Antitumor activity and safety of trastuzumab deruxtecan in patients with HER2-low-expressing advanced breast cancer: Results from a Phase Ib study Journal of Clinical Oncology: Official Journal of the American Society of Clinical Oncology 38 17 1887–1896
[11]. Bartsch R Berghoff A S Furtner J et al 2022 Trastuzumab deruxtecan in HER2-positive breast cancer with brain metastases: A single-arm phase 2 trial Nature Medicine 28 9 1840–1847
[12]. Modi S Jacot W Yamashita T Sohn J et al 2022 Trastuzumab deruxtecan in previously treated HER2-low advanced breast cancer The New England Journal of Medicine 387 1 9–20









