1. Introduction
Due to the massive spread of COVID-19 all over the world, there has been a significant increase in the number of people infected with lung disease. Researchers predict that a large number of people will be infected with lung cancer in the future, and current medical methods are not particularly effective in dealing with lung cancer, so this article introduces gene editing technology. First, gene editing technology is a type of biotechnology that allows scientists to precisely add, remove, or modify the DNA sequence in an organism's genome through specific enzymes. This technology can be used to study gene function, treat genetic diseases, or improve crop yields and resistance, among other things. Currently, the CRISPR-Cas9 system is one of the most well-known gene editing tools, which is derived from the immune mechanism of bacteria and directs the Cas9 enzyme to the target DNA sequence by directing RNA molecules to achieve precise gene editing [1]. The development of gene editing technology has revolutionized biomedical research and applications, but it has also sparked extensive ethical and safety discussions. This article mainly discusses the specific process of gene editing technology to cure lung cancer, and compares it with traditional treatment methods to draw some conclusions. This article aims to provide a reasonable analysis of the future of gene editing technology, and provide a reference for some people who need to adopt gene editing treatment options in the future, while holding reservations about possible side effects, etc.
2. Lung cancer
2.1. Lung cancer introduction
Lung cancer, also known as primary bronchial cancer, is a malignancy that originates in the airways or lung parenchyma. It is one of the most common cancers worldwide and the leading cause of cancer death. According to histopathology, lung cancer is mainly divided into two categories: small cell lung cancer and non-small cell lung cancer, of which non-small cell lung cancer includes squamous cell carcinoma (squamous cell carcinoma), adenocarcinoma, and large cell lung cancer [2]. Among them, small cell lung cancer cells grow and divide faster than other cancer cells, and spread and metastasize relatively early. There are two main types of lung cancer: (1) Small Cell Lung Cancer (SCLC): This type of lung cancer accounts for about 15% of all lung cancers and is usually associated with heavy smoking. Small cell lung cancer grows at a faster rate and can metastasize at an early stage. (2) Non-Small Cell Lung Cancer (NSCLC): This is the more common type of lung cancer, accounting for about 85% of all lung cancers. Non-small cell lung cancer has a relatively slow growth rate and can be further subdivided into several different histological types, including squamous cell carcinoma, adenocarcinoma, and large cell carcinoma.
Causes and risk factors for lung cancer include smoking, passive smoking, occupational exposure (e.g., exposure to carcinogens such as asbestos, arsenic, chromium, and nickel), air pollution, radon exposure, family history, etc. Symptoms may include persistent coughing, coughing up blood, difficulty breathing, chest pain, hoarseness, weight loss, etc. The diagnosis of lung cancer usually involves imaging tests (eg, X-rays, CT scans, PET-CT scans), tissue biopsies, and genetic testing. Treatments may include surgery, radiotherapy, chemotherapy, targeted therapy, immunotherapy, etc., depending on the type of cancer, stage, and the overall health of the patient. The incidence of lung cancer in China in 2022 is 1060600, and the crude death rate is 75.13 per 100,000 [3]. The incidence and mortality rate of lung cancer rank first among all cancers.
2.2. Causes of lung cancer being fatal
At the outset, researchers should know about the lethality of lung cancer, which is the leading cause of cancer-related deaths worldwide. This indicates that lung cancer is aggressive and difficult to treat, posing a serious threat to the lives of patients. In the following description, this article will detail some of the causes of death from lung cancer.
2.3. Histological diversity of lung adenocarcinoma (LUAD)
As the most common histological type of lung cancer, the morphological and genetic diversity of LUAD is closely related to tumor behavior. Different histological features, such as squamous, papillary, acinar, cribriform, micropapillary, and solid patterns, have different effects on tumor aggressiveness and patient prognosis. This makes it difficult for doctors to determine one or more specific treatment options when it comes to treatment, making lung cancer difficult to cure.
2.4. Tumor morphology and disease progression
Morphological features of tumors, such as high-grade patterns (solid, cribriform, and micropapillary), are associated with rapid tumor proliferation and low clonal diversity, which may reflect recent subclonal expansion, which is associated with tumor progression and deterioration. And the progression of the tumor eventually leads to the death of the patient.
2.5. Genetic and genomic characteristics of tumors
Studies have found that high-grade tumors have a higher tumor mutational burden (TMB) and genome-wide doubling frequency. In addition, mutations in specific genes and copy number changes in chromosomes (e.g., mutations in TP53, KRAS, SMARCA4 genes, and loss of chromosome arms 3p and 3q) are associated with high-grade patterns of tumors. Advanced lung cancer can lead to atmospheric compression, causing symptoms of dyspnea and even respiratory failure, which is life-threatening.
2.6. Clonal evolution of tumors
Clonal evolution analysis has shown that tumors tend to evolve to higher-order morphological patterns, which may be related to the increased aggressiveness and metastatic ability of tumors. Lung cancer leads to malnutrition, cachexia depletion, and in severe cases, it can be life-threatening.
2.7. Risk of tumor recurrence
Studies have shown that certain morphological features of tumors, such as micropapillary patterns and STAS, are associated with intrathoracic recurrence [4]. Solid/cribriform patterns, necrosis, and preoperative detection of ctDNA are associated with extrathoracic recurrence. Recurrence and metastasis are important causes of death from lung cancer.
2.8. Metastasis and spread of tumors
The study also explored the evolution of growth patterns from primary tumors to metastatic tumors, and found that metastatic tumors often exhibit a high-grade pattern, and metastatic regions tend to exhibit higher grades than certain areas of the primary tumor, causing certain impacts [4,5]. For example, lung cancer can cause intracranial metastases, leading to headaches, impaired consciousness, and even coma, which can be life-threatening.
These analyses provide an in-depth understanding of the lethality of lung cancer, including the biological characteristics of the tumor, genetic variation, clonal evolution, recurrence risk, and metastatic capacity, which are all key factors influencing the survival and treatment response of lung cancer patients.
3. Traditional Treatment
3.1. Chemotherapy and radiotherapy
Chemotherapy and radiotherapy (RT) are two common approaches to the treatment of non-small cell lung cancer (NSCLC), especially in locally advanced disease (LA-NSCLC). Chemotherapy is a method of treating cancer using drugs that kill or inhibit the growth of cancer cells. Chemotherapy can be used as first-line treatment or in combination with radiotherapy, surgery, or other treatments. Chemotherapy drugs travel through the bloodstream to all parts of the body, so they can affect cancer cells anywhere in the body. Side effects of chemotherapy may include nausea, vomiting, hair loss, fatigue, low blood counts, etc.
Radiotherapy uses high-energy rays (such as X-rays) or particle beams (such as protons or heavy ions) to destroy the DNA of cancer cells, thereby inhibiting the growth and division of cancer cells. Radiation can be external beam radiation (EBRT), in which rays are emitted from a machine outside the body and penetrate the body to reach the tumor, or internal radiation (brachytherapy), in which radioactive material is placed near or inside the tumor. Radiotherapy can be given as the mainstay or concurrently with chemotherapy (chemoradiotherapy, or CCRT), or sequentially (SCRT, chemotherapy followed by radiotherapy). When treating LA-NSCLC, doctors develop a personalized treatment plan based on the patient's specific situation, such as the type, stage, and location of the tumor, as well as the patient's overall health. The goal of treatment is to control the disease, relieve symptoms, improve quality of life, and prolong survival as much as possible.
3.2. Side effects of radiotherapy and chemotherapy
The above are the treatments for chemotherapy and radiotherapy, and the side effects they cause are described below: The first to bear the brunt of this is lymphopenia and severe lymphopenia (SRL). They are also known as radiation-induced lymphopenia [6]. Chemotherapy and radiation can cause a significant drop in the number of lymphocytes, a condition known as lymphopenia. Radiotherapy-induced lymphopenia (RIL) was found to be associated with a worse prognosis. Severe lymphopenia (SRL) is defined as having an absolute lymphocyte count (ALC) of less than or equal to 0.2×10^3 cells/microlitre [7]. The overall survival (OS) prognosis is worse in LA-NSCLC patients with SRL compared with those without SRL. In addition to this, side effects of radiation therapy may include skin reactions, fatigue, loss of appetite, and inflammation or damage to the treated area.
4. Clustered regularly interspaced short palindromic repeat
4.1. Introduction to CRISPR-Cas9 gene editing technology
CRISPR-Cas9 technology is a revolutionary gene editing technology that is based on the adaptive immune system of bacteria and archaea to defend against the invasion of foreign nucleic acids. In 1987, Y. Ishino first discovered an array of repeats near the alkaline phosphatase gene locus of K12 Escherichia coli [8]. Subsequently, such clustered regular spaced repeats were found to be present in bacteria and archaea [9]. In 2002, this sequence was officially named [clustered regularly interspaced short palindromic repeats (CRISPR)], and CRISPR-associated protein 9 (Cas9) is an endonuclease [10]. This technology is guided by RNA molecules to specifically identify and cleave the target DNA sequence for gene knockout, insertion, or correction (Figure 1).
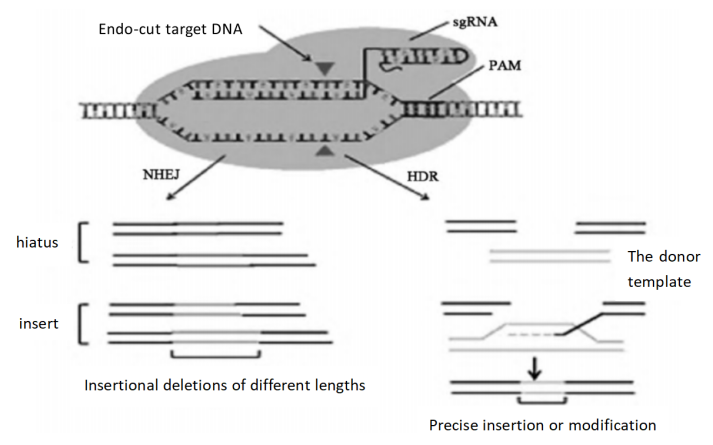
Figure 1. CRISPR-Cas9 fundamentals of gene editing technology [2]
4.2. Some feature and application of CRISPR-Cas9
CRISPR-Cas9 technology has the following features and applications: (1) Highly targeted: CRISPR-Cas9 can achieve precise editing of specific regions of the genome by designing specific sgRNAs (small guide RNAs). (2) High shearing efficiency: Compared with other gene editing technologies, CRISPR-Cas9 has higher efficiency in site-directed genome modification at multiple loci. (3) Easy to operate: The construction of the CRISPR-Cas9 system is relatively simple, and only a specific sgRNA needs to be synthesized to guide the Cas9 protein to target a specific DNA sequence. (4) Wide application: CRISPR-Cas9 technology has a wide range of application prospects in gene function research, gene therapy, transgenic animals and other fields. (5) High efficiency: CRISPR-Cas9 technology can quickly and efficiently realize genome editing, which greatly shortens the experimental cycle compared with traditional gene editing technology. (6) Pluripotency: CRISPR-Cas9 can not only be used for gene knockout, but also for site-directed insertion or mutation of genes through the homology recombination repair (HDR) pathway.
4.3. CRISPR-Cas9 to lung cancer
Before analyzing the method of CRISPR-Cas9 treatment for cancer, researchers need to know the relationship between SET and MYND domain-containing protein 3 (SMYD3) gene and lung cancer: SMYD3 is a histone H3-K4 methylase, which is closely related to the occurrence and development of a variety of malignant tumors. In lung cancer cells, the high expression of SMYD3 is related to the proliferation, migration, apoptosis and other biological behaviors of tumor cells.
In this study, CRISPR/Cas9 technology was used to knock out the SMYD3 gene in human lung cancer cell lines, and the knockout effect was detected by real-time quantitative PCR, and the expression of SMYD3 mRNA after knockout was significantly inhibited in lung cancer cell lines. Studies have shown that after SMYD3 gene knockout, lung cancer cells have reduced proliferation ability, migration and invasion ability, which provides a basis for SMYD3 as a potential target for cancer therapy.
Finally, it can be concluded that CRISPR/Cas9 technology has considerable application prospects: CRISPR/Cas9 technology has broad application prospects in gene function research, gene therapy, transgenic animals and other fields. In terms of cancer treatment, knocking out genes involved in tumorigenesis and progression, such as SMYD3, may provide new strategies for cancer treatment.
Another point to mention is the experimental method and results: the study used a lipid transfection method to transfect the CRISPR/Cas9 vector into lung cancer cells, and the positive cells were screened by flow cytometry. CCK-8 assay and Transwell assay were used to observe the changes in the proliferation, migration and invasion ability of lung cancer cells after knocking out SMYD3 gene [11].
5. Conventional treatment regimens (chemotherapy and radiotherapy) versus crispr-cas9 technology
Chemotherapy, radiotherapy, and CRISPR-Cas9 are the three main methods of treating lung cancer, each with its own advantages and disadvantages. Chemotherapy is a systemic treatment for widely metastatic cancer, but it can cause serious side effects, such as nausea and hair loss. It kills cancer cells with drugs, but it can also affect normal cells, sometimes leading to the development of drug resistance.
Radiotherapy is a local treatment that uses high-energy rays to act directly on the tumor, causing less damage to surrounding normal tissue. However, it has limited effect on widely metastatic cancers and may cause local side effects.
CRISPR-Cas9 is an emerging gene editing technology with extreme precision and the ability to edit specific oncogenes. This technology has the potential to treat hereditary cancers, but it is still in the research stage, with off-target risks and ethical issues. In addition, the clinical application of CRISPR-Cas9 faces challenges in terms of technology maturity and delivery systems.
All things considered, chemotherapy and radiotherapy are well-established treatments, but they are accompanied by significant side effects. CRISPR-Cas9 offers a new treatment, although there are still many issues that need to be addressed. The doctor will choose the most appropriate treatment plan based on the patient's specific situation. As technology advances, there may be more personalized treatment options in the future.
6. Conclusion
Derived from the immune mechanism of bacteria and directs the Cas9 enzyme to the target DNA sequence by directing RNA molecules, the CRISPR-Cas9 system is currently one of the most well-known gene editing tools to achieve precise gene editing. The development of gene editing technology has revolutionized biomedical research and applications, but it has also sparked extensive ethical and safety discussions. This article explores the potential of gene editing technologies, especially CRISPR-Cas9, in the treatment of lung cancer and how it compares to conventional therapies. Lung cancer is the leading cancer killer in China, with a high mortality rate and low survival rate. Gene editing technology allows for precise modification of DNA sequences, and the CRISPR-Cas9 system has attracted attention for its high efficiency and ease of operation. Although chemotherapy and radiotherapy are routine treatments for NSCLC, they come with serious side effects. CRISPR-Cas9 technology provides a new strategy for cancer treatment by knocking out key genes, such as SMYD3, which shows the ability to reduce lung cancer cell proliferation and metastasis. However, the technology still faces challenges such as off-target risks, ethical issues, and technological maturity. The article argues that with the advancement of technology, there may be more personalized treatment options in the future.
References
[1]. Wen, M., Yongqin, Y., Meitao, S., Xiaoqian, D., Zefang, Y., Xiaojuan, Z., & Wei, X. Research progress of CRISPR-Cas9 gene editing technology in tumor gene therapy. [J] Journal of Pingdingshan University, 33(2), 61-66 (2017).
[2]. Yang, Z., & Lin, C. (2018). Present Situation and Progress of Lung Cancer Therapy. [J] Advances in Clinical Medicine, 8(9), 27497. https://doi.org/10.12677/ACM.2018.89140.
[3]. ]Han, B., Zheng, R., Zeng, H., Wang, S., Sun, K., Chen, R., Li, L., Wei, W., & He, J. Cancer incidence and mortality in China, 2022. [J] Journal of the National Cancer Center, 4(1), 47-53 (2024). https://doi.org/10.1016/j.jncc.2024.01.006.
[4]. Karasaki, T., Moore, D. A., & Others. Evolutionary characterization of lung adenocarcinoma morphology in TRACERx. [J] Nature Medicine, 29, 833–845 (2023). https://doi.org/10.1038/s41591-023-02230-w.
[5]. Lastwika, K. J. et al. Control of PD-L1 expression by oncogenic activation of the AKT–mTOR pathway in non-small cell lung cancer. [J] Cancer Res. 76, 227–238 (2016).
[6]. Zhao, Q., Li, T., Chen, G., Zeng, Z., & He, J. Prognosis and risk factors of radiation-induced lymphopenia in early-stage lung cancer treated with stereotactic body radiation therapy. [J] Frontiers in Oncology, 9, 1488 (2019). https://doi.org/10.3389/fonc.2019.01488
[7]. Li, Y., Fan, X., Pei, Y., Yu, Q., Lu, R., Jiang, G., & Wu, K. The impact of different modalities of chemoradiation therapy and chemotherapy regimens on lymphopenia in patients with locally advanced non-small cell lung cancer. [J] Translational Lung Cancer Research, 13(6), 1190-1200 (2024). https://dx.doi.org/10.21037/tlcr-24-60
[8]. Yoshikawa, S., Kawakami, K., & Zhao, X. C. G2R Cre Reporter Transgenic Zebrafish. PubMed Central, 237(9), 2460-2465 (2008).
[9]. Zeng, H. M., & Chen, W. Q. Current status of cancer epidemiology and prevention research in China. Chemical Progress, 25(9), 1415-1420 (2013).
[10]. Wang, D. Y., Ma, N., & Hui, Y. Application of CRISPR/Cas9 genome-editing technology in cancer research. Hereditas (Beijing), 38(1), 1-8 (2016).
[11]. Lu, Y. R. The effect of CRISPR/Cas9-mediated SMYD3 gene knockout on human lung cancer cells. Inner Mongolia University (2016).
Cite this article
Lou,L. (2024). The treatment of lung cancer by gene editing technology and its comparison with traditional therapies. Theoretical and Natural Science,60,58-63.
Data availability
The datasets used and/or analyzed during the current study will be available from the authors upon reasonable request.
Disclaimer/Publisher's Note
The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of EWA Publishing and/or the editor(s). EWA Publishing and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
About volume
Volume title: Proceedings of the 4th International Conference on Biological Engineering and Medical Science
© 2024 by the author(s). Licensee EWA Publishing, Oxford, UK. This article is an open access article distributed under the terms and
conditions of the Creative Commons Attribution (CC BY) license. Authors who
publish this series agree to the following terms:
1. Authors retain copyright and grant the series right of first publication with the work simultaneously licensed under a Creative Commons
Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this
series.
2. Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the series's published
version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial
publication in this series.
3. Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and
during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See
Open access policy for details).
References
[1]. Wen, M., Yongqin, Y., Meitao, S., Xiaoqian, D., Zefang, Y., Xiaojuan, Z., & Wei, X. Research progress of CRISPR-Cas9 gene editing technology in tumor gene therapy. [J] Journal of Pingdingshan University, 33(2), 61-66 (2017).
[2]. Yang, Z., & Lin, C. (2018). Present Situation and Progress of Lung Cancer Therapy. [J] Advances in Clinical Medicine, 8(9), 27497. https://doi.org/10.12677/ACM.2018.89140.
[3]. ]Han, B., Zheng, R., Zeng, H., Wang, S., Sun, K., Chen, R., Li, L., Wei, W., & He, J. Cancer incidence and mortality in China, 2022. [J] Journal of the National Cancer Center, 4(1), 47-53 (2024). https://doi.org/10.1016/j.jncc.2024.01.006.
[4]. Karasaki, T., Moore, D. A., & Others. Evolutionary characterization of lung adenocarcinoma morphology in TRACERx. [J] Nature Medicine, 29, 833–845 (2023). https://doi.org/10.1038/s41591-023-02230-w.
[5]. Lastwika, K. J. et al. Control of PD-L1 expression by oncogenic activation of the AKT–mTOR pathway in non-small cell lung cancer. [J] Cancer Res. 76, 227–238 (2016).
[6]. Zhao, Q., Li, T., Chen, G., Zeng, Z., & He, J. Prognosis and risk factors of radiation-induced lymphopenia in early-stage lung cancer treated with stereotactic body radiation therapy. [J] Frontiers in Oncology, 9, 1488 (2019). https://doi.org/10.3389/fonc.2019.01488
[7]. Li, Y., Fan, X., Pei, Y., Yu, Q., Lu, R., Jiang, G., & Wu, K. The impact of different modalities of chemoradiation therapy and chemotherapy regimens on lymphopenia in patients with locally advanced non-small cell lung cancer. [J] Translational Lung Cancer Research, 13(6), 1190-1200 (2024). https://dx.doi.org/10.21037/tlcr-24-60
[8]. Yoshikawa, S., Kawakami, K., & Zhao, X. C. G2R Cre Reporter Transgenic Zebrafish. PubMed Central, 237(9), 2460-2465 (2008).
[9]. Zeng, H. M., & Chen, W. Q. Current status of cancer epidemiology and prevention research in China. Chemical Progress, 25(9), 1415-1420 (2013).
[10]. Wang, D. Y., Ma, N., & Hui, Y. Application of CRISPR/Cas9 genome-editing technology in cancer research. Hereditas (Beijing), 38(1), 1-8 (2016).
[11]. Lu, Y. R. The effect of CRISPR/Cas9-mediated SMYD3 gene knockout on human lung cancer cells. Inner Mongolia University (2016).









