1. Introduction
Alzheimer’s disease (AD) is a neurodegenerative disease that is especially prevalent in the elderly. Dementia is a term that refers to a list of diseases that affect an individual’s cognitive abilities, including memory loss [1]. As of current, AD is the leading cause for dementia. AD is characterized by neuronal death, the loss of synapses, the formation of neurofibrillary tangles in the intracellular environment, and the built-up of extracellular amyloid-beta (Aβ) plaques [2]. This results in decline in cognitive abilities, the symptom that characterizes dementia. As of 2020, there are 50 million people affected by dementia, and this number is estimated to triple by 2050 [1]. There are multiple risk factors that can increase the likelihood of an individual contracting AD. AD is especially prevalent in low-middle-income countries due to population aging and more exposure to risk factors [1]. Along with negative impacts on patients and their family members/caretakers, In the global healthcare industry, AD has become a major issue, costing the world an estimated amount of $1 trillion USD annually [1]. By researching potential treatments for AD, researchers could potentially improve the well-being of people affected whilst also reducing the economic burden of dementia.
Data from several studies suggests that dendritic spine loss correlates with the build-up of Aβ-protein deposits. Over the course of AD, the patient can lose as much as 80% of the neurons in their hippocampus [2]. Neuronal cells are critical for communication between brain cells. The number of synapses present within the brain is directly correlated to the person’s cognitive functioning levels. Synaptic loss has a strong correlation with the cognitive decline present in AD, showing that the decrease in the size of the dendritic spines indicates the progression of AD [2]. Furthermore, the loss of neuronal cells leads to long-lasting symptoms like depression, anxiety, decline in memory and attention span [1].
Stem cell therapy is a therapeutic method that repairs or regrows damaged tissues via stem cell’s unique regenerative properties and their ability to differentiate [3]. As AD’s main characteristics involve damaged or destroyed neuronic cells, stem cell therapy can be an effective strategy for combating AD by replacing damaged neuronic cells. Studies have found that induced pluripotent stem cells (iPSCs)-based therapy has the potential to be highly effective at combating AD [4]. Pluripotent stem cells are obtained by reprograming adult cells in vitro, which makes iPSCs technology a safe and effective way to produce pluripotent stem cells [4]. iPSC-based therapy is to produce mature and functioning neurons that can form synaptic circuits [4]. iPSCs can be produced into models which can be utilized for drug testing to evaluate drug efficacy. iPSC-based therapy holds promise for the treatment of AD.
This review summarises the mechanisms and pathology process of AD, the process and key steps of iPSC-based modeling, as well as the application of iPSC-based technology in combating AD.
2. Pathogenesis of AD
AD is a neurodegenerative, progressive disease that is currently the leading cause of dementia. The main symptom of AD consists of the declination of the patient’s cognitive functions, including attention span and memory [1]. In addition to cognitive decline, AD is lethal with no current cure as of writing [4, 5]. These symptoms place major social and emotional burdens upon the patient and caregivers whilst emplacing significant burden on the economy [5]. There are multiple hypothesizes for the cause of AD.
The most widely accepted hypothesis is the cholinergic hypothesis, which correlates the absence of the neurotransmitter acetylcholine (ACh) in the neuromuscular and neuronal regions with AD [4]. This hypothesis is supported by biopsy reports from brain tissues of AD patients, of which indicated a decreased level ACh transferase activity [4].
Phosphorylated tau protein and Amyloid Precursor protein (APP) both play a major role in the formation of AD. The phosphorylation of the tau protein occurs mainly at positions T231, S235 and S265 on the tau protein [4]. This prevents the protein from successfully attaching to microtubules, forming neurofibrillary tangles, leading to the disintegration of the nerve cell’s microtubule network, and blocking the cell from any biochemical communication [4].
Nerve impulse transmissions can be obstructed by the extracellular aggregation of Aβ42. The formation of Aβ42 originates from the mutation of one of the three genes encoding APP [4]. Mutations in any of the above-mentioned genes can cause the APP to have incorrect cleavage, resulting in the formation of Aβ42 instead of Aβ40 [4]. The aggregation of Aβ42 occurs as APOE e4 is unable to break Aβ42 down, which results in the prion protein receptor 9CD230) binding with Aβ42 oligomers, obstructing impulse transmission [4].
A small number of patients (0.1%) developed AD due to genetic inheritance [4]. Inherited patients tend to show symptoms of AD between the age of 30 to 50 [4]. Inherited AD occurs due to the autosomal dominance of one of the three genes encoding for APP, presenilin 1 (PS1) and 2 (PS2), which ultimately results in the formation of senile plaques that kills nerve cells by inducing a pro-inflammatory response from the activation of astrocytes and microglia [4].
3. iPSC technology
iPSCs are pluripotent stem cells derived from reprogramed adult somatic cells. Somatic cells are specialized cells that possess a distinct function in the body [4]. The key defining feature of iPSCs is the ability to differentiate into a wide variety of cell types in vitro, making iPSC technology especially valuable for clinical applications [6].
The first successful generation of iPSCs was developed from mice fibroblasts [6]. Mice fibroblasts, along with human fibroblasts, continue to be the most prevalent in experimental research, whilst other cell types are gradually gaining attention due to their therapeutic potential, availability, or simplicity [6]. However, as the cell type, maturity level, and degree of differentiation influence the efficiency of iPSC generation, there are other cells that can be used as substitutes to greatly enhance the iPSC generation process. Compared to fibroblasts, human primary keratinocytes have the ability to be reprogrammed at double the speed with 100 times greater efficiency [6]. In terms of simplicity, CD133+ cord blood cells are an excellent example with it only requiring SOX2 and OCT4 recombination factors for iPSC generation [6]. Other cell sources like blood and urine samples have also demonstrated to be viable for iPSC generation [6].
There are numerous ways to reprogram somatic cells into iPSCs. As of current, OSKM (also referred to as c-Myc) remains the most widely used transcription factor [6]. There are many alternative methods used to improve the reprogramming process [6]. Some of these methods include introducing new molecules to the reprogramming process and targeting other transcription factor combinations to raise the efficiency of IPSC generations.
Along with OSKM, Klf4 is also a popular transcription factor utilized in the iPSC generation process. OSKM and Klf4 are classified as protooncogenes, carrying possible tumorigenic risks [6]. Researchers have attempted to mitigate this by utilizing substitute molecules, most famously the Oct-4 and Sox2 recombination factors [6]. Additional transcription factors have also been sought after to potentially increase the efficiency of iPSC generation [6]. These transcription factors mainly target genes that are activated during early development that are largely responsible for maintaining the pluripotency of cells that will ultimately form the inner cell mass in the embryo [6]. It has been found that the co-expression of transcription factors can greatly increase the efficiency of iPSC generation. The co-expression of Nanog with OSKM factors halves the time needed for colony appearance compared to utilizing OSKM alone [6].
New innovative approaches employ CRISPR technology to express OSKM factors are utilized to achieve a higher generation rate at a lower cost [6]. This process is carried out by fusing a modified Cas9 enzyme with dCAS9 to repress or activate transcriptional domains. This enables CRISPR-mediated activation to activate individual or specific groups of genes necessary for the generation of iPSC [6].
After the generation of iPSC, the cells will differentiate outside of the human body before being transplanted into the patient [7]. The transplantation of iPSCs still requires additional research to be approved for clinical trials. iPSC also possesses the ability to be developed into models that reflect the patient’s physiologically relevant processes. This model can also be utilised to test the efficacy and safety of drugs to the patient, reducing the risk of unforeseen immunogenicity or lose of drug efficacy due to individual differences [8]. This would have been difficult to achieve with animal models due to the primary differences between species [9].
The process of creating iPSC models involves generating iPSC from extracted somatic cells. The generated iPSC would undergo differentiation, differentiating into the desired cell types [8]. An example of this is differentiating iPSC lines into serotonergic neurons by activating WNT and SHH signalling [8]. Desired cell types can be anatomically organized into miniature, three-dimensional structures called organoids. Organoids will contain multiple cell types to mimic the morphology of the desired human organ [9]. Researchers can utilize brain organoids to study critical features of network properties and brain-specific cytoarchitecture [9].
4. iPSC technology in AD
4.1. iPSC model in AD
In terms of iPSC modelling, the initial iPSC model has demonstrated that AD patients secrete an increased amount of Aβ42, reinforcing the PS1 and PS2 mutation hypothesis [9]. Researchers were subsequently able to utilize the model to test drugs that could potentially repair genetic mutations. Abnormal levels of APP expression levels and phosphorylated tau were observed in genetically manipulated neurons derived from iPSC lines created by patients with APP gene mutation [9]. The model was also utilized in drug development where to discover methods of reducing the Aβ load within cultured cells. The researchers tested 1000 compounds on the model, which ultimately concluded with three compounds being combined to improve the anti-Aβ effect (bromocriptine, cromolyn, topiramate) [9].
iPSC modeling can also be utilized in the development of other therapeutic approaches [9]. The hippocampus is involved in emotional functions and new memory formation. The degradation of the hippocampus occurs relatively early in AD [9]. Researchers were able to generate hippocampal spheroid from patient iPSCs [9]. The researchers identified that mutated genes encoding for APP or PS1 exhibited AD symptoms [9]. This allowed the researchers to develop an approach that modulates the expression genes involved in synaptic transmission with gene therapy.
iPSC modeling can be used to determine the inheritance method and the pathological pathways of AD. iPSC models, alongside post-mortem histology and MRI imaging, were used to determine the pathogenicity of the P436S mutation of PS1 [10]. Aβ43, similarly to Aβ42, is an aggregation prone species of Aβ. The iPSC models that were confirmed to possess the P436S mutation displayed a raised Aβ42 to Aβ40 ratio. The models also displayed a significant increase in the Aβ43 to Aβ40 ratio [10]. These findings corroborate the hypothesis that mutation P436S of PS1, like other AD mutations leads to high Aβ43 production and impaired Aβ40 processing.
4.2. iPSC-based therapy, clinical applications in AD
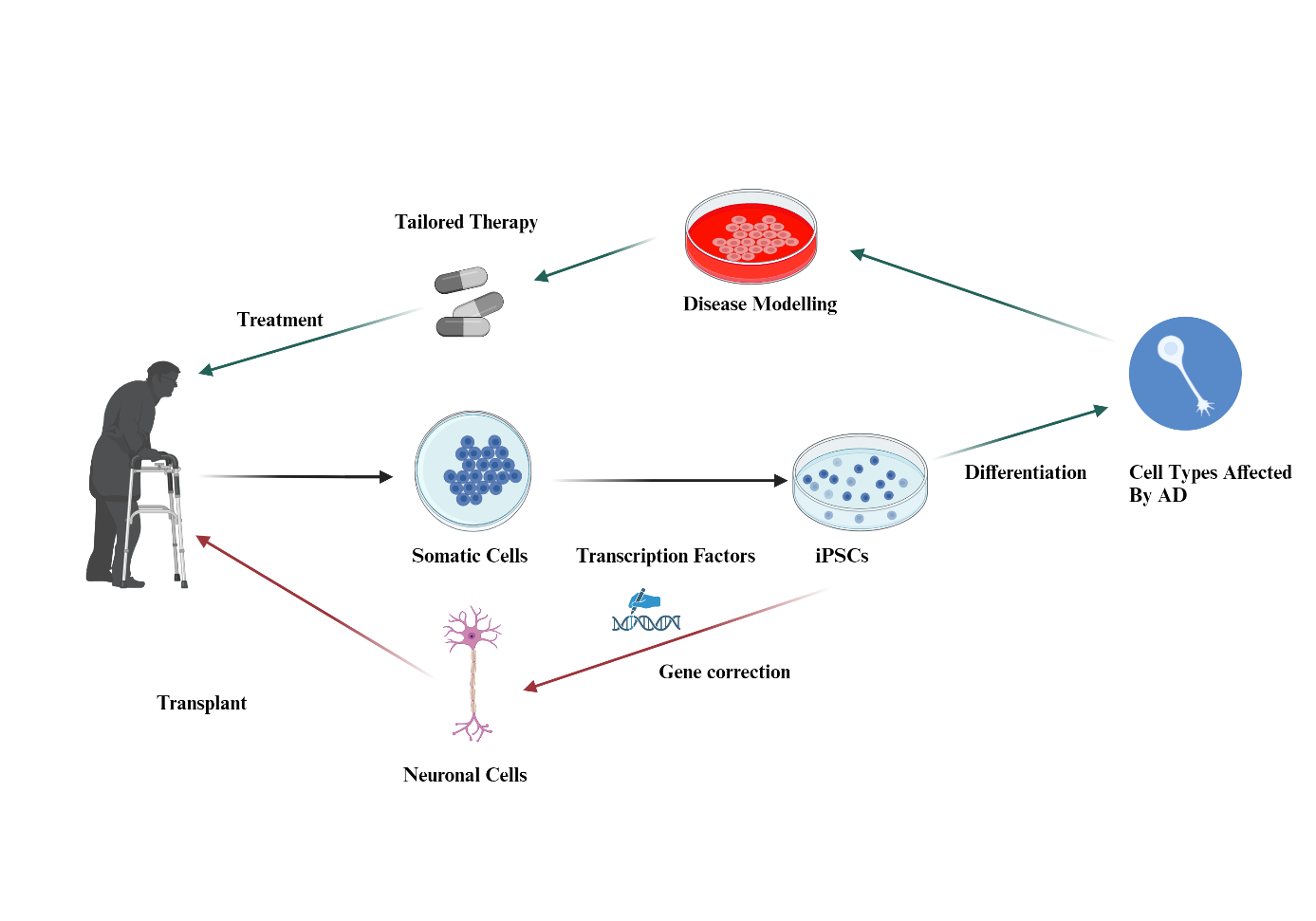
iPSC technology has the potential to be a potent treatment method for AD as shown in Figure 1. iPSC-based cell therapy can be easily produced with fewer ethical disputes compared to other stem cell therapy methods like ESC [4]. iPSC possesses the ability to be expressed in vitro, allowing the development of models that mimic the patient’s physiologically relevant processes [8]. As of current, iPSC-based cell therapy has not been applied in human clinical trials due to safety concerns. The main safety concern for iPSC cell therapy is the tumorigenic potential of iPSC [6]. The specificity aspect of iPSC also hinders its ability to target epigenetic and unpredictable diseases like AD.
| Figure 1. iPSC technology in treating AD. Figure credit: original. Figure made from Biorender.com. |
There are two clinical trials that have been approved to explore the application of iPSC technology in AD. These trials will be beneficial towards exploring the broad potentials of iPSCs in research. The first trial (NCT00874783) focuses on the development of iPSC from donated skin or hair biopsies of up to 10mm in diameter as seen in table 1. The trial will utilize 100 effected donors covering 10 neurological diseases. The trial will also utilize 20 healthy control donors as seen in Table 1. Although the iPSC derived from this trial are not intended for transplant, this trial will aim to develop technology that contributes towards iPSC transplantation therapy research.
The second active clinical trial (NCT06372587) involves testing genetically encoded engineered proteins (GEEP) in human iPSCs to investigate the effects of GEEP on AD-related synaptic pathway complications. GEEPs are utilized to potentially overcome the limitations of traditional AD treatments. The experiment aims to preserve essential brain functions in AD by boosting synaptic plasticity via GEEPs. The current estimation for the number of participants in this trial is 14. As of June 2024, the trial currently open for recruitment as shown in Table 1.
Table 1. Clinical trials of iPSC technology in AD and other neurological diseases
Trial reference number | Aim of the trial | Number of participants | Methodology | Study Start and estimated completion | Results | NCT00874783 | To conduct basic research and disease modelling through the use of human iPSC cells deriving from skin biopsies cell cultures. | 120 Donors covering 10 different neurodegenerative disorders with 20 healthy control donors | Case-control observational study involving donors donating a skin specimen of up to 10mm in diameter. | 2009-04 to 2025-12 | N/A, trial is currently ongoing | NCT06372587 | To investigate the effects of genetically encoded engineered proteins (GEEPS) in vitro in order to obtain an inducible control of their activity in human neurons preventing dendric loss | 14 (estimated) | To compare the effects of engineered neurons derived from AD patient skin biopsies (4mm punch) compared to the effects of engineered proteins in human neurons derived from skin biopsies of healthy participants (4mm punch) | 2023-12-19 to 2027-02-28 | N/A, trial is currently recruiting |
5. Conclusion
AD is a fatal disease that needs to be eliminated. AD can be characterized by the gradual degradation of neuronal structures. This can be attributed to multiple factors including a reduced level of Ach and mutations of genes encoding for APP, PS1, and PS2. Although the demographic affected by inheritance is small compared to other factors, AD can be inherited in the form of an autosomal dominant gene. iPSC technology, whilst being relatively new, is an invaluable tool for AD treatment. Host cell selections are critical for the iPSC production as the generation process is heavily dependent on the maturity, degree of differentiation, and cell type. The iPSC generation process also heavily relies upon the transcription factor utilized. iPSC technology is constantly being innovated upon, with CRISPR technology potentially playing a great role in the advancement of iPSC technology. iPSC cell therapy is currently not eligible for clinical trials due to safety concerns. However, there are clinical trials actively gathering data in order to improve the safety and efficacy of iPSC cell therapy. iPSC modeling provides an invaluable tool for the treatment of AD, allowing researchers to test the efficacy of other treatment methods in vitro before testing on the patient. However, there is a lack of direct evidence of iPSC cell therapy on AD. Further development on iPSC technology is required in order to potentially administer iPSC cell therapy to patients.
References
[1]. Livingston G, et al. 2020 Lancet 396 413-446
[2]. Kocahan S and Doğan Z. 2017 Clin Psychopharmacol Neurosci. 15 1-8
[3]. Hoang DM, et al. 2022 Signal Transduct. Target Ther. 7 272
[4]. Srivastava S, Ahmad R and Khare SK. 2021 Eur. J. Med. Chem. 216 113320
[5]. Scheltens P, De Strooper B, Kivipelto M, Holstege H, Chételat G, Teunissen CE, Cummings J and van der Flier WM. 2021 Lancet 397 1577-1590
[6]. Pazzin DB, Previato TTR, Budelon Gonçalves JI, Zanirati G, Xavier FAC, da Costa JC and Marinowic DR. 2024 Cells 13 745
[7]. CiRA, What are iPS cells? (2024), Available online at: https://www.cira.kyotou.ac.jp/e/faq/faq_ips.html.
[8]. Zivko C, Sagar R, Xydia A, Lopez-Montes A, Mintzer J, Rosenberg PB, Shade DM, Porsteinsson AP, Lyketsos CG and Mahairaki V. 2024 Mol. Psychiatry.
[9]. Beghini DG, Kasai-Brunswick TH and Henriques-Pons A. 2024 Int J Mol Sci. 25 2392
[10]. Arber C, et al. 2024 Alzheimers Dement.
Cite this article
Guo,Y. (2024). Applications of iPSC technology in Alzheimer's disease. Theoretical and Natural Science,59,215-220.
Data availability
The datasets used and/or analyzed during the current study will be available from the authors upon reasonable request.
Disclaimer/Publisher's Note
The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of EWA Publishing and/or the editor(s). EWA Publishing and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
About volume
Volume title: Proceedings of the 4th International Conference on Biological Engineering and Medical Science
© 2024 by the author(s). Licensee EWA Publishing, Oxford, UK. This article is an open access article distributed under the terms and
conditions of the Creative Commons Attribution (CC BY) license. Authors who
publish this series agree to the following terms:
1. Authors retain copyright and grant the series right of first publication with the work simultaneously licensed under a Creative Commons
Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this
series.
2. Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the series's published
version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial
publication in this series.
3. Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and
during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See
Open access policy for details).
References
[1]. Livingston G, et al. 2020 Lancet 396 413-446
[2]. Kocahan S and Doğan Z. 2017 Clin Psychopharmacol Neurosci. 15 1-8
[3]. Hoang DM, et al. 2022 Signal Transduct. Target Ther. 7 272
[4]. Srivastava S, Ahmad R and Khare SK. 2021 Eur. J. Med. Chem. 216 113320
[5]. Scheltens P, De Strooper B, Kivipelto M, Holstege H, Chételat G, Teunissen CE, Cummings J and van der Flier WM. 2021 Lancet 397 1577-1590
[6]. Pazzin DB, Previato TTR, Budelon Gonçalves JI, Zanirati G, Xavier FAC, da Costa JC and Marinowic DR. 2024 Cells 13 745
[7]. CiRA, What are iPS cells? (2024), Available online at: https://www.cira.kyotou.ac.jp/e/faq/faq_ips.html.
[8]. Zivko C, Sagar R, Xydia A, Lopez-Montes A, Mintzer J, Rosenberg PB, Shade DM, Porsteinsson AP, Lyketsos CG and Mahairaki V. 2024 Mol. Psychiatry.
[9]. Beghini DG, Kasai-Brunswick TH and Henriques-Pons A. 2024 Int J Mol Sci. 25 2392
[10]. Arber C, et al. 2024 Alzheimers Dement.