1. Introduction
In recent years, the morbidity of prostate cancer (PCa) has been significantly rising. According to a report from GLOBOCAN, PCa became the fourth most frequently diagnosed cancer, reaching 1,466,680 cases and occupying 7.3% of all cancers globally in 2022 [1]. Previous PCa therapy can be briefly divided into three types: radiotherapy, chemotherapy, and molecular therapy. Radiotherapy is commonly used in PCa. It can be used on curable patients with localized cancer instead of radical prostatectomy (RP) and shares a similar efficacy with RP in all National Comprehensive Cancer Network risk groups. With the development of science and technology, advanced therapy techniques such as intensity-modulated radiation therapy and volumetric modulated arc therapy have been developed, reducing damage to normal surrounding structures and improving the therapeutic ratio [2]. As for chemotherapy, Docetaxel (DTX) is one of the most commonly used therapies. Other therapies such as Mitoxantrone and Enzalutamide are also used. Molecular therapy is mainly based on the specific features of PCa. For instance, a therapy in clinical trials for BRCA-deficient metastatic castration-resistant prostate cancers (mCRPC) shows that these cancers are significantly sensitive to PARP inhibitors. Thus, an efficient therapy using Olaparib to treat patients is established as Olaparib is the most efficient PARP inhibitor in the clinical curation on mCRPC. Although chemotherapy and radiotherapy have been perfected through clinical practices, severe resistance problems still exist as the majority of patients acquire castration-resistance after treatments [3]. Therefore, finding solutions to resistance problems is of great urgency, and developing more advanced therapeutic targets is necessary. TR4 was discovered to have a significant function in PCa initiation and development. The role TR4 plays in PCa initiation is determined by the presence of PPARγ. The existence of PPARγ allows TR4 to act its function to maintain DNA integrity and prevent PCa initiation while the absence of PPARγ leads to an opposite result. In PCa development, studies show that TR4 may promote PCa metastasis by modulating CCL2, microRNA-373-3p, and the EZH2 gene [4]. In summary, TR4 plays a critical role in PCa. Thus, this article will provide an overview of recent studies on PCa-metastasis-relevant and chemo-resistant and radio-resistant pathways mediated by TR4, and briefly discuss the potential of TR4 to become a target that can be utilized in PCa curation.
2. Nuclear receptor
Nuclear receptors (NRs) are transcription factors that are dependent on ligands and are involved in numerous vital biological processes including as development, metabolism, and reproduction. So far, 48 nuclear receptors have been discovered. NRs, weighing between 66 to 100 kD, have four domains (A/B, C, D, and E) with a conserved functional domain organization, which is one of their defining features [5, 6]. Each domain performs a distinct vital function.
The essential functions of NRs are separated from their overall domain structure. Their functions are played via binding to consensus DNA sequences known as HREs is dependent on their monomeric, homodimeric, or heterodimeric nature. Seven superfamilies, subgroups 0 to subgroup 6, are categorized according to their ligand-binding characteristics of NRs. Members of the same NR superfamily share the same standard structure, although they are different in shape, size, and charges of their activating ligands. Moreover, NRs regulate biological activities in organisms through gene expression by both ligand-dependent and ligand-independent transcriptional repression [5]. Most ligands of nuclear receptors are oleophilic molecules which are small in size. However, roughly over half of NRs do not have any endogenous ligands and are named orphan nuclear receptors. Some orphan receptors, generally found to have low affinity to bind, are modulated by metabolites [6].
3. TR4
TR4, encoded by the NR2C2 gene in humans, is an orphan nuclear receptor. It was first cloned in 1994 from human testis libraries. TR4 has a molecular mass of 67 kD and is widely expressed throughout the body [7].
TR4 is a type of orphan receptor, meaning it does not have endogenous ligands. However, exogenous factors, including some natural molecules, their metabolites, and synthetic compounds, can activate TR4 in place of endogenous ligands. Like many orphan receptors, TR4 participates in numerous physiological processes, including fertility, neuron development and metabolism. Thus, any abnormal expression of TR4 significantly influences various diseases, including cancer. Previous studies showed the critical role that TR4 plays in the repair of DNA damage caused by various factors and participates in various cancers. For instance, TR4 can inhibit the initiation of liver and prostate cancer, but it can also enhance the progression of pituitary corticotroph, liver, and prostate cancers [7]. It also influences cervical, lung, and breast cancers.
4. Roles that TR4 played in PCa curation
4.1. TR4 and Prostate cancer (PCa) metastasis
Enhancer of Zeste Homolog 2 (EZH2) is an oncogene linked to prostate cancer metastasis and functions through its downstream genes, which are crucial to cancer metastasis, including NOTCH1, TGFβ1, SLUG, and MMP9. In castration-resistant prostate cancer (CRPC), studies show that TR4 can act as a transcription factor through the attachment to two TR4 response elements (TR4RE) in the EZH2 promoter region causing the dosage-dependent enhancement of EZH2 promoter activity. The first TR4RE in the EZH2 promoter region is necessary for the activity enhancement of the EZH2 promoter. As TR4 promotes the expression of EZH2, the downstream genes of EZH2 are modulated. Consequently, the invasion of stem/progenitor cells (S/P cells) is promoted, leading to a high likelihood of CRPC metastasis. This regulation of prostate cancer by TR4 was confirmed in the study [8].
In PCa, one signaling pathway that influences cancer metastasis led by TR4 is mediated with the help of macrophages. Knocking down TR4 in PCa cells can significantly decrease macrophage recruitment in a PCa-macrophage co-culture system, reducing macrophage infiltration into PCa cells. Tissue inhibitor of metalloproteinases-1 (TIMP-1) is a potent cytokine related to tumor metastasis and can act via binding various cell-surface receptors [9]. Treatment with the culture medium of the co-culture system, which has less macrophage infiltration, causes an increase in TIMP-1 mRNA expression which result in a decrease in MMP2/MMP9 expression in the PCa cells without TR4 knockdown. Therefore, the function of TIMP-1 as a suppressor of MMP2 and MMP9 may be the factor that reduces PCa cell invasion and ultimately suppresses PCa metastasis [10]. Another pathway that shares the same function in PCa is mediated via microRNA. TR4 reduces miR-373-3p expression, which can significantly suppress PCa invasion. The TGFβR2-3'UTR is where MiR-373-3p binds, and it down-regulates both TGFβR2 expression and its downstream gene, p-Smad3, which are associated with PCa metastasis according to the experiments [11].
4.2. TR4 and PCa therapy
Furthermore, chemoresistance and decreased radiosensitivity during cancer treatment have become significant issues. Investigating the role TR4 plays in the process of enhancing treatment sensitivity is also essential. Docetaxel (DTX) is an ordinary chemotherapy for prostate cancer (PCa). However, clinical resistance problem to DTX is in a great urgency to be solved. Increased TR4 expression in PCa cells activates lincRNA-p21 by directly binding to the potential TR4REs which are at the upstream of lincRNA-p21. According to cell viability results, lincRNA-p21 can increase DTX resistance. Upregulation of HIF-1 and VEGF-A is achieved by activating lincRNA-p21. This pathway enhances chemoresistance in DTX-resistant PCa cells, contributing to further PCa progression [12]. Additionally, another signaling pathway influences DTX chemoresistance in PCa. TR4, as a transcription factor, can bind to the TR4RE in the miR-145 promoter region, suppressing miR-145 at the transcriptional level. MiR-145 has direct effects on OCT4 expression by targeting its 3'UTR and reducing it at both transcriptional and translational levels. Consequently, OCT4 expression is upregulated due to the decrease in miR-145. Survivin, a downstream gene of OCT4, is involved, acting as an anti-apoptotic factor in PCa [13]. Thus, TR4-mediated chemoresistance to DTX in PCa is regulated through these pathways.
Enzalutamide (MDV3100) is an innovative chemotherapy agent that, despite its promise, is not immune to the development of resistance. The transcription factor 4 (TR4) has been linked to resistance mechanisms in prostate cancer (PCa), particularly in relation to MDV3100. Metastasis-associated lung adenocarcinoma transcript 1 (MALAT1) is involveded in the regulation of cell communication, tumorigenesis, cancer progression, and response to therapy [14]. TR4 is able to attach to a particular response element in the 5' promoter region of the MALAT1 gene, which leads to an increase in its expression. This upregulation of MALAT1 is observed to diminish the expression of the androgen receptor variant 7 (AR-V7) in a dose-dependent fashion, culminating in an increased resistance to MDV3100 in PCa [15]. Thus, the interaction between TR4 and MALAT1 is posited to augment the chemoresistance of PCa to MDV3100.
In addition to chemotherapy, radiation therapy is a widely utilized medical method to treat prostate cancer (PCa) which faces a similar challenge of resistance as well. Zinc Finger E-box Binding Homeobox 1 (ZEB1) is a significant transcription factor involved in the epithelial-mesenchal transition (EMT) that is crucial for the progression of cancer metastasis. Studies have indicated that radiation therapy can lead to an increase in the expression of Transcription Factor 4 (TR4). This upregulation of TR4 results in its attachment to the first TR4RE on the 5' promoter region of the QKI gene, thereby enhancing QKI expression. The elevated QKI, in turn, leads to the enhancement of circular RNA ZEB1 (circZEB1) expression. CircZEB1 acts as a sponge for miR-141-3p, preventing it from attaching to the 3'UTR of ZEB1 mRNA. Consequently, ZEB1 protein levels are elevated by this interaction. The overexpression of ZEB1 protein, in turn, upregulates the expression of Checkpoint Kinase 1 (CHK1), a protein associated with the cellular response to radiation. Ultimately, this cascade of events enhances DNA damage repair mechanisms, reducing the time required for PCa cells to complete DNA repair and shielding them from radiation-induced apoptosis, which consequently diminishes the radiosensitivity of PCa cells [16].
5. Discussion
Among all these mechanisms TR4 has participated in, quite a few developments have been established in better cancer curation. The potential of TR4 to act as a target in clinical therapy is obvious. TR4 is mainly discovered to behave in two ways, acting as the target to directly cure cancer or to solve the resistance problem in existed therapies. In PCa metastasis aspect, the increase of TR4 expression in PCa cells can up-regulate the oncogene EZH2 and its downstream genes. TGFβ1 belongs to TGFβ family which are transforming growth factors and can be transduced by its receptor, TGFβR2 [8]. The role of TGFβ1 in cancer cell proliferation, angiogenesis, and apoptosis has been identified to be critical. Thus, both alteration in TGFβ1 expression and TGFβR2 expression can modulate TR4 signaling pathway. According to the study, TR4 decreases the expression of miR-373-3p which down-regulates TGFβR2 expression and its downstream gene, p-Smad3 [11]. Hence, two signaling pathway conducted by TR4 is mediated by TGFβ1/ TGFβR2, and finally modulate PCa metastasis, increase macrophage infiltration, and decrease miR-373-3p. MMP9 that belongs to the downstream genes of EZH2 is also modulated by TIMP-1 [10]. Thus, TR4 can modulate PCa metastasis by modulating MMP9 and TGFβ signaling through four pathways. This indicate the potential of TR4 to act as a high efficient therapeutic target as it can simultaneously modulate multiple targets through various signaling pathways.
Most therapies used in PCa curation have been facing a resistance problem. Common chemotherapy DTX and new chemotherapy MDV3100 also shares the same. In DTX resistance, two pathways that causes the occurrence of the resistance have been developed so far. One pathway is TR4/miR-145/OCT4 pathway, in which TR4 suppresses miR-145 expression, increases OCT4 expression consequently, and leads to survivin expression [13]. The other pathway is TR4/lincRNA-p21/HIF-1α. TR4 expression in DTX-resistant PCa cells increases lincRNA-p21 expression, which in turn up-regulates HIF-1 and VEGF-A. In MDV3100 resistance, only one pathway that leads the occurrence of the resistance have been developed. It is the pathway that increased TR4 expression activate Malat1 expression which decreases AR-v7 [15].
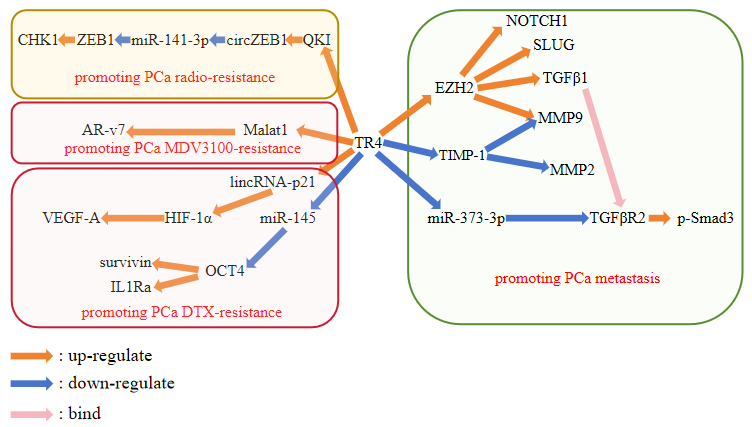
In summary, all mechanism that is illustrated above can be represented as a picture as follow (Figure 1).
|
Figure 1. TR4-mediated pathways in PCa metastasis, radioresistance, and chemoresistance. |
Investigations into small-molecule regulators that target TR4 for use as therapeutic agents in prostate cancer (PCa) treatment have been actively pursued. Several regulators have been identified as potentially effective. One such regulator is the TR4 antagonist bexarotene (BEX). Studies have demonstrated that BEX, via the TR4/lincRNA-p21/HIF-1α pathway, can reduce TR4 transactivation and enhance chemosensitivity in docetaxel (DTX)-resistant PCa cells, thereby contributing to more effective suppression of PCa progression [12]. Additionally, nilotinib (S1033) has emerged as a potent TR4 inhibitor capable of suppressing TR4 transactivation. Comparative experiments have indicated that S1033 exhibits greater efficiency than BEX, which was previously recognized as a TR4 inhibitor in earlier studies [17]. Furthermore, metformin has been reported to enhance radiosensitivity and partially reverse resistance induced by TR4 by slowing down cellular DNA damage repair mechanisms [16].
6. Conclusion
In conclusion, this article provides an overview of seven signaling pathways mediated by TR4 that contribute to prostate cancer (PCa) metastasis, chemoresistance, and radioresistance. TR4 primarily functions as a transcriptional factor, binding to TR4RE in the promoter or UTR of its target genes, thereby exerting both positive and negative regulatory effects. Among the small-molecule regulators identified, bexarotene (BEX), nilotinib (S1033), and metformin have shown potential as new therapeutic agents targeting TR4 in PCa treatment. Bexarotene is relatively advanced in its development, with at least one of its signaling pathways already elucidated. Nilotinib and metformin are more recently identified regulators that are believed to suppress TR4 activity. Given the high incidence rate of PCa and the increasing demand for effective therapies, TR4 has emerged as a promising therapeutic target that is garnering increasing interest among researchers. This review outlines the mechanisms by which TR4 is involved in PCa metastasis and resistance, and briefly discusses the small-molecule inhibitors that could target TR4. It aims to provide foundational theoretical insights for scientists interested in further exploring this area in the future.
References
[1]. Bray, F., Laversanne, M., Sung, H., Ferlay, J., Siegel, R. L., Soerjomataram, I., & Jemal, A. (2024). Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: a cancer journal for clinicians, 74(3), 229–263. https://doi.org/10.3322/caac.21834
[2]. Mattes M. D. (2024). Overview of Radiation Therapy in the Management of Localized and Metastatic Prostate Cancer. Current urology reports, 25(8), 181–192. https://doi.org/10.1007/s11934-024-01217-5
[3]. Posdzich, P., Darr, C., Hilser, T., Wahl, M., Herrmann, K., Hadaschik, B., & Grünwald, V. (2023). Metastatic Prostate Cancer-A Review of Current Treatment Options and Promising New Approaches. Cancers, 15(2), 461. https://doi.org/10.3390/cancers15020461
[4]. Lin, S. J., Yang, D. R., Li, G., & Chang, C. (2015). TR4 Nuclear Receptor Different Roles in Prostate Cancer Progression. Frontiers in endocrinology, 6, 78. https://doi.org/10.3389/fendo.2015.00078
[5]. Sar P. (2023). Nuclear receptor: Structure and function. Progress in molecular biology and translational science, 196, 209–227. https://doi.org/10.1016/bs.pmbts.2022.07.014
[6]. Frigo, D. E., Bondesson, M., & Williams, C. (2021). Nuclear receptors: from molecular mechanisms to therapeutics. Essays in biochemistry, 65(6), 847–856. https://doi.org/10.1042/EBC20210020
[7]. Lin, S. J., Yang, D. R., Yang, G., Lin, C. Y., Chang, H. C., Li, G., & Chang, C. (2017). TR2 and TR4 Orphan Nuclear Receptors: An Overview. Current topics in developmental biology, 125, 357–373. https://doi.org/10.1016/bs.ctdb.2017.02.002
[8]. Zhu, J., Yang, D. R., Sun, Y., Qiu, X., Chang, H. C., Li, G., Shan, Y., & Chang, C. (2015). TR4 Nuclear Receptor Alters the Prostate Cancer CD133+ Stem/Progenitor Cell Invasion via Modulating the EZH2-Related Metastasis Gene Expression. Molecular cancer therapeutics, 14(6), 1445–1453. https://doi.org/10.1158/1535-7163.MCT-14-0971
[9]. Schoeps, B., Frädrich, J., & Krüger, A. (2023). Cut loose TIMP-1: an emerging cytokine in inflammation. Trends in cell biology, 33(5), 413–426. https://doi.org/10.1016/j.tcb.2022.08.005
[10]. Ding, X., Yang, D. R., Xia, L., Chen, B., Yu, S., Niu, Y., Wang, M., Li, G., & Chang, C. (2015). Targeting TR4 nuclear receptor suppresses prostate cancer invasion via reduction of infiltrating macrophages with alteration of the TIMP-1/MMP2/MMP9 signals. Molecular cancer, 14(1), 16. https://doi.org/10.1186/s12943-014-0281-1
[11]. Qiu, X., Zhu, J., Sun, Y., Fan, K., Yang, D. R., Li, G., Yang, G., & Chang, C. (2015). TR4 nuclear receptor increases prostate cancer invasion via decreasing the miR-373-3p expression to alter TGFβR2/p-Smad3 signals. Oncotarget, 6(17), 15397–15409. https://doi.org/10.18632/oncotarget.3778
[12]. Hu, L., Sun, Y., Luo, J., He, X., Ye, M., Li, G., Zhang, Y., Bai, J., Zhang, D., & Chang, C. (2020). Targeting TR4 nuclear receptor with antagonist bexarotene increases docetaxel sensitivity to better suppress the metastatic castration-resistant prostate cancer progression. Oncogene, 39(9), 1891–1903. https://doi.org/10.1038/s41388-019-1070-5
[13]. Zhu, J., Qin, P., Cao, C., Dai, G., Xu, L., & Yang, D. (2021). Use of miR‑145 and testicular nuclear receptor 4 inhibition to reduce chemoresistance to docetaxel in prostate cancer. Oncology reports, 45(3), 963–974. https://doi.org/10.3892/or.2021.7925
[14]. Imran, M., Abida, Eltaib, L., Siddique, M. I., Kamal, M., Asdaq, S. M. B., Singla, N., Al-Hajeili, M., Alhakami, F. A., AlQarni, A. F., Abdulkhaliq, A. A., & Rabaan, A. A. (2024). Beyond the genome: MALAT1's role in advancing urologic cancer care. Pathology, research and practice, 256, 155226. https://doi.org/10.1016/j.prp.2024.155226
[15]. Chen X. D. (2018). The role of TR4 on Enzalutamide resistance in castration resistant prostate cancer. Zhejiang University. https://kns.cnki.net/kcms2/article/abstract?v=xEDmK2-VgJgBY2gFw2YA8EABeRLGqFApCvDAjMfk-xbMF3XpAvmryboDZTXtjprh38Iu1eamf2kvhYtBkmFgbjV6BNaWFSUGI3nu-YasQmfbF7s9iEis9QOQ2ni2B-810oRADEPEK09qSDCoZPwKsa1WV-R-sUcR7jLdGfcSUbKjK_-7n6_WUbDFRyOG9dhVPxvdJNhPOdc=&uniplatform=NZKPT&language=CHS
[16]. Chen, D., Chou, F. J., Chen, Y., Tian, H., Wang, Y., You, B., Niu, Y., Huang, C. P., Yeh, S., Xing, N., & Chang, C. (2020). Targeting the radiation-induced TR4 nuclear receptor-mediated QKI/circZEB1/miR-141-3p/ZEB1 signaling increases prostate cancer radiosensitivity. Cancer letters, 495, 100–111. https://doi.org/10.1016/j.canlet.2020.07.040
[17]. Xia, L., Shen, D., Wang, H., Ren, L., Chen, Y., & Li, G. (2020). Identification of Small-Molecule Regulators of Testicular Receptor 4 via a Drug Repurposing Screening. ACS omega, 5(47), 30625–30632. https://doi.org/10.1021/acsomega.0c04623
Cite this article
Zhang,S. (2024). The mechanism of TR4 participate in prostate cancer metastasis and therapy. Theoretical and Natural Science,61,21-26.
Data availability
The datasets used and/or analyzed during the current study will be available from the authors upon reasonable request.
Disclaimer/Publisher's Note
The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of EWA Publishing and/or the editor(s). EWA Publishing and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
About volume
Volume title: Proceedings of the 4th International Conference on Biological Engineering and Medical Science
© 2024 by the author(s). Licensee EWA Publishing, Oxford, UK. This article is an open access article distributed under the terms and
conditions of the Creative Commons Attribution (CC BY) license. Authors who
publish this series agree to the following terms:
1. Authors retain copyright and grant the series right of first publication with the work simultaneously licensed under a Creative Commons
Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this
series.
2. Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the series's published
version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial
publication in this series.
3. Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and
during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See
Open access policy for details).
References
[1]. Bray, F., Laversanne, M., Sung, H., Ferlay, J., Siegel, R. L., Soerjomataram, I., & Jemal, A. (2024). Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: a cancer journal for clinicians, 74(3), 229–263. https://doi.org/10.3322/caac.21834
[2]. Mattes M. D. (2024). Overview of Radiation Therapy in the Management of Localized and Metastatic Prostate Cancer. Current urology reports, 25(8), 181–192. https://doi.org/10.1007/s11934-024-01217-5
[3]. Posdzich, P., Darr, C., Hilser, T., Wahl, M., Herrmann, K., Hadaschik, B., & Grünwald, V. (2023). Metastatic Prostate Cancer-A Review of Current Treatment Options and Promising New Approaches. Cancers, 15(2), 461. https://doi.org/10.3390/cancers15020461
[4]. Lin, S. J., Yang, D. R., Li, G., & Chang, C. (2015). TR4 Nuclear Receptor Different Roles in Prostate Cancer Progression. Frontiers in endocrinology, 6, 78. https://doi.org/10.3389/fendo.2015.00078
[5]. Sar P. (2023). Nuclear receptor: Structure and function. Progress in molecular biology and translational science, 196, 209–227. https://doi.org/10.1016/bs.pmbts.2022.07.014
[6]. Frigo, D. E., Bondesson, M., & Williams, C. (2021). Nuclear receptors: from molecular mechanisms to therapeutics. Essays in biochemistry, 65(6), 847–856. https://doi.org/10.1042/EBC20210020
[7]. Lin, S. J., Yang, D. R., Yang, G., Lin, C. Y., Chang, H. C., Li, G., & Chang, C. (2017). TR2 and TR4 Orphan Nuclear Receptors: An Overview. Current topics in developmental biology, 125, 357–373. https://doi.org/10.1016/bs.ctdb.2017.02.002
[8]. Zhu, J., Yang, D. R., Sun, Y., Qiu, X., Chang, H. C., Li, G., Shan, Y., & Chang, C. (2015). TR4 Nuclear Receptor Alters the Prostate Cancer CD133+ Stem/Progenitor Cell Invasion via Modulating the EZH2-Related Metastasis Gene Expression. Molecular cancer therapeutics, 14(6), 1445–1453. https://doi.org/10.1158/1535-7163.MCT-14-0971
[9]. Schoeps, B., Frädrich, J., & Krüger, A. (2023). Cut loose TIMP-1: an emerging cytokine in inflammation. Trends in cell biology, 33(5), 413–426. https://doi.org/10.1016/j.tcb.2022.08.005
[10]. Ding, X., Yang, D. R., Xia, L., Chen, B., Yu, S., Niu, Y., Wang, M., Li, G., & Chang, C. (2015). Targeting TR4 nuclear receptor suppresses prostate cancer invasion via reduction of infiltrating macrophages with alteration of the TIMP-1/MMP2/MMP9 signals. Molecular cancer, 14(1), 16. https://doi.org/10.1186/s12943-014-0281-1
[11]. Qiu, X., Zhu, J., Sun, Y., Fan, K., Yang, D. R., Li, G., Yang, G., & Chang, C. (2015). TR4 nuclear receptor increases prostate cancer invasion via decreasing the miR-373-3p expression to alter TGFβR2/p-Smad3 signals. Oncotarget, 6(17), 15397–15409. https://doi.org/10.18632/oncotarget.3778
[12]. Hu, L., Sun, Y., Luo, J., He, X., Ye, M., Li, G., Zhang, Y., Bai, J., Zhang, D., & Chang, C. (2020). Targeting TR4 nuclear receptor with antagonist bexarotene increases docetaxel sensitivity to better suppress the metastatic castration-resistant prostate cancer progression. Oncogene, 39(9), 1891–1903. https://doi.org/10.1038/s41388-019-1070-5
[13]. Zhu, J., Qin, P., Cao, C., Dai, G., Xu, L., & Yang, D. (2021). Use of miR‑145 and testicular nuclear receptor 4 inhibition to reduce chemoresistance to docetaxel in prostate cancer. Oncology reports, 45(3), 963–974. https://doi.org/10.3892/or.2021.7925
[14]. Imran, M., Abida, Eltaib, L., Siddique, M. I., Kamal, M., Asdaq, S. M. B., Singla, N., Al-Hajeili, M., Alhakami, F. A., AlQarni, A. F., Abdulkhaliq, A. A., & Rabaan, A. A. (2024). Beyond the genome: MALAT1's role in advancing urologic cancer care. Pathology, research and practice, 256, 155226. https://doi.org/10.1016/j.prp.2024.155226
[15]. Chen X. D. (2018). The role of TR4 on Enzalutamide resistance in castration resistant prostate cancer. Zhejiang University. https://kns.cnki.net/kcms2/article/abstract?v=xEDmK2-VgJgBY2gFw2YA8EABeRLGqFApCvDAjMfk-xbMF3XpAvmryboDZTXtjprh38Iu1eamf2kvhYtBkmFgbjV6BNaWFSUGI3nu-YasQmfbF7s9iEis9QOQ2ni2B-810oRADEPEK09qSDCoZPwKsa1WV-R-sUcR7jLdGfcSUbKjK_-7n6_WUbDFRyOG9dhVPxvdJNhPOdc=&uniplatform=NZKPT&language=CHS
[16]. Chen, D., Chou, F. J., Chen, Y., Tian, H., Wang, Y., You, B., Niu, Y., Huang, C. P., Yeh, S., Xing, N., & Chang, C. (2020). Targeting the radiation-induced TR4 nuclear receptor-mediated QKI/circZEB1/miR-141-3p/ZEB1 signaling increases prostate cancer radiosensitivity. Cancer letters, 495, 100–111. https://doi.org/10.1016/j.canlet.2020.07.040
[17]. Xia, L., Shen, D., Wang, H., Ren, L., Chen, Y., & Li, G. (2020). Identification of Small-Molecule Regulators of Testicular Receptor 4 via a Drug Repurposing Screening. ACS omega, 5(47), 30625–30632. https://doi.org/10.1021/acsomega.0c04623