1. Introduction
In recent years, animal epidemics have shown a serious public health and safety issue. On the one hand, stopping animal epidemics can directly cut off the disaster overhead that has been hanging over the head of sustainable aquaculture industry development and animal products trade; on the other hand, it is also a great deal to ensure human food safety and restrict local regional economic and social development. For example, outbreaks of diseases such as avian influenza and foot-and-mouth disease have inflicted massive economic losses in many parts of the globe [1].
Vaccines are known to be essential tools for preventing various animal diseases. They are critical for maintaining the health of livestock and pets, which has implications not only for food security but also for public health. Worldwide, the increased incidence of infectious diseases and their societal impact necessitate effective vaccination programs, with benefits extending from animal welfare to the economic stability of the livestock industry [2]. Additionally, vaccines play a key role in preventing zoonotic diseases (diseases that can be transmitted from animals to humans), thereby helping in the overall control of infectious diseases.
This review deals with the research and development progress of vaccines for common animal diseases and successful examples showing achievements in vaccine development. This review article will present an overview and existing challenges in developing new vaccines to cover different conditions affecting animals, as well as the current findings related to those goals.
2. Epidemiology and clinical manifestations of common animal diseases
2.1. Rabies
The rabies virus, compiled from the family Rhabdoviridae and genus Lyssavirus, has a bullet-like appearance, helical symmetry on its nucleocapsid, and an outer envelope. The pathogen is a single-stranded RNA virus responsible for rabies. Rabies is an acute infectious disease caused by the lyssavirus and is predominantly transmitted through bites or scratches from infected animals, usually dogs and cats. After a human has been bitten by an infected animal, the virus replicates and spreads within the muscle tissue at the site of entry, infecting the peripheral nervous system and later invading the nervous system to reach the brain [3]. This invasion causes labyrinthitis and further involves the central respiratory and circulatory centers. Rabies is a serious infectious disease with the following crucial epidemiologic attributes.
The first type is geographical distribution. Rabies is distributed globally, but mainly affects parts of Asia, Africa, and Latin America. This is mainly because some areas in these regions have a higher density of rabies virus carriers and population, increasing the chances of virus transmission[4].Next is the animal host. Rabies is mainly transmitted through dogs, but wild animals such as cats, foxes, raccoons, and bats may also be carriers of the virus. In rural areas, unvaccinated domestic animals are the main source of transmission[4].In addition, seasons and activity patterns are also major epidemiological characteristics of rabies. The incidence rate of rabies is usually high in warm seasons and high humidity areas, which may be related to the activity and exposure frequency of animals.
Rabies clinical symptoms can be divided into four stages. The first stage is the incubation period, which usually lasts 2-3 months but can extend to several years. In this phase, patients remain asymptomatic. The prodromal phase follows, typically lasting 2-4 days, with symptoms like fever, headache, nausea, vomiting, and fatigue. Psychological symptoms, such as anxiety, restlessness, and insomnia, may also occur. The most alarming signs of rabies appear during the excitation period, which usually lasts 2-7 days. During this period, patients may develop hydrophobia (fear of water), photophobia (fear of light), and aerophobia (fear of wind). Additional symptoms can include muscle spasms, difficulty breathing, and an accelerated heartbeat. The paralysis period is the last phase of rabies, lasting up to 1-2 days. At this stage, patients experience muscle paralysis and respiratory failure, ultimately leading to death [5].
2.2. Foot-and-mouth Disease
Foot-and-mouth disease (FMD) is the next common animal disease to be discussed. The foot-and-mouth disease virus (FMDV) is classified into seven serotypes: O, A, C, SAT1, SAT2, SAT3, and Asia1. There is almost no immune protection between different serotypes, so animals infected with one type of FMD can still be infected with another type. FMDV belongs to the family Picornaviridae and the genus Aphthovirus, and is a highly infectious pathogen of cloven hoofed animals. At the center of the virus is a single stranded positive stranded RNA composed of approximately 8500 bases, which is the foundation of infection and genetics. The virus shell is a symmetrical 20hedron. The surrounding proteins determine the antigenicity, immunity, and serological reactivity of the virus[6].
FMD is one of the most infectious diseases in animals or humans. FMDV is rapidly replicated and spread in infected animals, susceptible animals and aerosols. Disease signs can appear within 2 to 3 days after exposure and may last for 7 to 10 days. The following are the epidemiological characteristics of foot-and-mouth disease. The first point is the route of transmission. Foot and mouth disease is mainly transmitted through direct contact, droplet transmission, and pollutant transmission. In addition, they are susceptible animals. Ungulates are all susceptible to foot-and-mouth disease virus, with cattle being the most sensitive. Furthermore, another point is seasonality. Foot and mouth disease is usually prevalent in autumn and winter, as animals are more susceptible to the effects of cold and dryness, which reduces their immunity.
2.3. Swine Fever
The pathogen responsible for swine fever is the swine fever virus, which belongs to the family Flaviviridae and the genus Pestivirus. The virus particle is enveloped and slightly circular in shape, with a delicate filamentous structure on its surface. Based on differences in serological characteristics, virulence, antigenicity, and pathogenicity of its strains, the swine fever virus can be classified into two serotype groups. One group encompasses the highly virulent strain and most attenuated strains used in vaccines, while the other group consists primarily of low-virulence strains that cause chronic swine fever. Due to the significant diversity among circulating strains, the swine fever virus can be further divided into two genomes and six gene subgroups.
The occurrence of classical swine fever is not restricted by age, sex and variety, and the incidence rate and mortality rate are high. The main clinical manifestations include high fever retention, loss or reduction of appetite, conjunctivitis, and acute enteritis. There are purple red spots or plaques on the limbs, abdomen, ear tips, tail tips, and other areas. The bleeding around the lymph node section appears as a red and white marble like appearance. The spleen is not enlarged, and there is punctate bleeding on the surface or a wedge-shaped infarction area at the edge. Additionally, punctate bleeding can be observed in the heart, throat, bladder, and gallbladder, along with ulceration in the ileocecal valve, ileum, and colon, which can aid in initial diagnosis.
3. Progress and successful cases of vaccine research and development
3.1. Rabies Vaccine
The development history of rabies vaccines is a long process originating in the late 19th century and pioneered by French scientist Louis Pasteur. The vaccine was first used to treat human bite victims on July 6, 1885. Pasteur successfully inoculated rabbits with the rabies virus using the method of continuous passage to reduce the virus's virulence, enabling them to develop immunity without symptoms. Subsequently, he injected these attenuated viruses into healthy dogs and observed similar effects. This method, later known as the "continuous passage method to reduce viral virulence," became the cornerstone of rabies vaccine development. However, this vaccine was based on the central nervous system tissue of rabbits, and inactivation of RABV required a physical method (drying). Due to the presence of nerve tissue and myelin basic protein, this method carried the risk of residual live viruses and severe allergic reactions [7].
Starting in 1962, the American Wistar Institute conducted research on human diploid cell rabies vaccines and transferred the technology to the Pasteur Merieux Institute in France in 1974. In modern rabies vaccines, China has been developing human diploid cell rabies vaccines since 2004. In 2014, China's first freeze-dried human rabies vaccine with independent intellectual property rights was launched. The refined VERO cell rabies vaccine and refined hamster kidney cell rabies vaccine for human use are both mild turbid white liquids that can be shaken and dispersed over time and contain sulfur-containing mercury preservatives [8].
3.2. Foot-and-Mouth Disease Vaccine
The development history of foot-and-mouth disease (FMD) vaccines is challenging and innovative, reflected in the following aspects. The first is the development of inactivated vaccines, where the earliest vaccines used to prevent and control FMD were based on inactivated viruses. This vaccine inactivates the virus through chemical means (such as formaldehyde treatment) and is then used to immunize animals. The preparation of this vaccine is relatively simple, but it requires regular vaccination to maintain immunity and can sometimes cause injection site reactions[9]. Additionally, there is the subunit vaccine. With the development of molecular biology, researchers have begun to use genetic engineering techniques to develop subunit vaccines. This vaccine contains specific proteins of the virus, such as VP1, rather than the complete virus. This improves the safety of the vaccine, as it does not contain the complete virus and cannot cause disease. Furthermore, there are also attenuated vaccines and recombinant vaccines. To improve immune efficacy and safety, these vaccines reduce the pathogenicity of the virus by altering its genetic composition while maintaining its ability to trigger immune responses [9].
Throughout the research and application history of FMD vaccines, they have gone through a series of historical processes, including inactivated vaccines, live vaccines, and new vaccines. The inactivated vaccine progressed over 20 years from the initial tongue skin virus inactivated by formaldehyde to later cell-cultured virus inactivated by BEI. There are three main ways to produce FMD virus antigens: the Frenkel culture method for bovine tongue skin tissue production, the transfer bottle BHK21 cell monolayer culture method, and the BHK21 cell suspension culture method [10]. The transfer bottle single-layer cultivation method is labor-intensive, and the biological safety of the production process is difficult to control, making it easy to disperse viruses. On the other hand, the suspension cultivation method is a technology-intensive production method that can quickly and conveniently prepare virus antigens and ensure biological safety during production. It is currently the main production method.
3.3. Classical Swine Fever Vaccine
The development history of swine fever vaccines spans decades and involves the persistent efforts of numerous scientists and research institutions. The initial development of swine fever vaccines can be traced back to the 1950s when scientists worldwide developed many traditional swine fever vaccines, mainly inactivated and attenuated vaccines [11]. These traditional vaccines have played an important role in the prevention and control of swine fever. A milestone achievement was the successful development of the attenuated swine fever vaccine, commonly known as the "C strain," in China in 1954 [11]. This vaccine was successfully cultivated by continuously transmitting the strong strain of the swine fever virus in rabbits for hundreds of generations and has been widely spread and applied internationally.
In addition, with the development of molecular biology, significant progress has been made in the research of new vaccines. These new vaccines include genetically engineered subunit vaccines, peptide-based vaccines, nucleic acid vaccines, gene deletion vaccines, and live vector vaccines for viruses. The use of swine fever vaccines is one of the important means of preventing and controlling swine fever, effectively reducing the impact of the disease on the pig industry. The rational application of vaccines can not only ensure animal health but also maintain public health and food safety.
4. Comparison and Analysis
4.1. Comparison of Different Types of Vaccines
Different types of vaccines exhibit varying levels of safety. Firstly, inactivated vaccines are generally considered to have high safety as they inactivate the virus through physical or chemical methods[12]. These vaccines may cause mild side effects, such as redness, swelling, or fever at the injection site, but they typically do not lead to serious adverse reactions. Similarly, subunit vaccines also demonstrate high safety since they only contain partial antigenic components of the virus and do not include complete virus particles. The side effects associated with subunit vaccines are usually limited to local reactions such as pain or swelling at the injection site. However, there are safety concerns regarding attenuated vaccines. Although attenuated vaccines can induce strong immune responses, they may cause disease in some cases, particularly in immunocompromised individuals. Additionally, there is a risk of viral reversion in attenuated vaccines, where the vaccine strain regains pathogenicity, posing a threat to the health of the organism.
From the perspective of immunogenicity, inactivated vaccines have weaker immunogenicity. Since the involved virus or bacteria are killed, adjuvants are often needed to enhance the immune response[9]. Furthermore, inactivated vaccines require multiple doses to maintain and enhance immunity[13]. In contrast, attenuated vaccines possess strong immunogenicity because they mimic natural infections and induce robust cellular and humoral immunity, providing long-lasting immune protection. Subunit vaccines, on the other hand, have limited immunogenicity. As they only contain partial antigens of the pathogen, the immune response they elicit may not be as comprehensive as that caused by the complete pathogen. Like inactivated vaccines, subunit vaccines may also require booster doses to enhance immune responses.
There are significant differences in the production costs of different types of vaccines, which primarily depend on the production process, raw material costs, production scale, and the required technology and equipment. Firstly, inactivated vaccines generally involve cultivating a large amount of virus followed by inactivation treatment. The production process for inactivated vaccines is relatively straightforward, and large-scale production can reduce unit costs. However, strict safety measures and sterile conditions are necessary, resulting in moderate production costs. Due to their widespread application, inactivated vaccines typically enjoy high market acceptance and stable market value. Conversely, the production of attenuated vaccines involves a complex process of attenuation, requiring multiple passages and screenings. This process demands specialized technology and equipment to ensure the safety and effectiveness of the virus strain, leading to high or unstable production costs. Effective attenuated vaccines can reduce disease spread, alleviate the burden on healthcare systems, and enhance population productivity. Lastly, subunit vaccines often require the identification and isolation of specific antigen proteins, a process that involves sophisticated biotechnology and purification steps, resulting in higher costs. The production of subunit vaccines relies heavily on technologies such as recombinant DNA technology or protein engineering and necessitates the use of expensive bioreactors and purification systems. This type of vaccine typically targets specific viral antigens and may have high demand in certain populations, thus achieving good market positioning.
4.2. Challenges and Strategies in Vaccine Development
Pathogens, such as viruses and bacteria, exhibit high genetic variability, allowing them to adapt to environmental changes, including immune system attacks and vaccine pressures. The impact of virus mutation can be summarized as follows: Firstly, viral mutations can alter antigenic epitopes, which are recognized and attacked by the immune system, leading to immune escape where existing vaccines fail to recognize new virus strains effectively. For instance, antigen drift and shift in influenza viruses necessitate annual updates of influenza vaccines to combat new strains. Secondly, certain mutations may enhance the virus's transmissibility, and while vaccines can prevent severe disease, they may not stop the spread of new variants. Additionally, mutations can affect the virus's replication rate and viral load in the host, influencing disease severity and vaccine efficacy. Regarding bacterial mutations, they can lead to antibiotic resistance through genetic mutations or horizontal gene transfer, potentially resulting in treatment failure and indirectly affecting vaccine strategies, especially when vaccines are used to reduce antibiotic use. Moreover, changes in bacterial surface antigens can impact the effectiveness of vaccine-induced immune responses, similar to viruses.
There are several major challenges in current vaccine immunogen research, such as unclear theories of immunogen design, insufficient understanding of the intrinsic determinants of immunogens, lack of models and systems for immunogen design, and discrepancies between predictive analysis results and in vivo validation results[14]. Furthermore, significant problems also exist in studying the immune protection mechanisms of vaccines, including unclear mechanisms of vaccine-induced immune response differences, lack of efficient methods for inducing T-cell specific immunity and evaluating T-cell response systems, and the need to clarify the impact of pre-existing immunity on vaccine efficacy[14].
5. Future Development Trends
The development of new vaccine technologies, including recombinant protein vaccines, nucleic acid vaccines, and viral vector vaccines, represents a revolutionary change driven by advancements in molecular biology. Here, we discuss some of these new vaccine technologies.
Firstly, recombinant protein vaccines are highly regarded due to their well-established advantages of safety, stability, and ease of production. Nearly 100 recombinant candidate vaccines are currently in phase I clinical development, representing the largest number among all vaccine technology platforms[15]. Figure 1 shows the distribution ratio of different types of vaccines in the total candidate vaccines and their quantity distribution in each R&D stage. Specifically, recombinant protein and nucleic acid vaccines account for a large proportion, 22% and 18% respectively, and are widely distributed in each R&D stage, especially in the preclinical stage and new drug application stage, reflecting the important position and research investment of these types of vaccines in vaccine development. These vaccines are primarily used in the fields of COVID-19 and influenza, utilizing genetic engineering technology to produce large quantities of antigen proteins that induce an immune response.
Additionally, nucleic acid vaccines, which include RNA and DNA vaccines, offer the flexibility to develop candidate vaccines for pathogens with highly variable target antigens. The successful application of mRNA vaccines in the context of COVID-19 demonstrates the immense potential of this technology. The design and synthesis of mRNA vaccines involve optimizing the mRNA sequence encoding antigens to enhance translation efficiency and reduce the likelihood of immune escape.
Moreover, viral vector vaccines use various types of viral vectors, such as adenoviruses and retroviruses, to induce strong and long-lasting immune responses. To overcome pre-existing immune responses to certain viral vectors, novel viral vectors have been developed, including Ad26, Ad35, and Ad11.
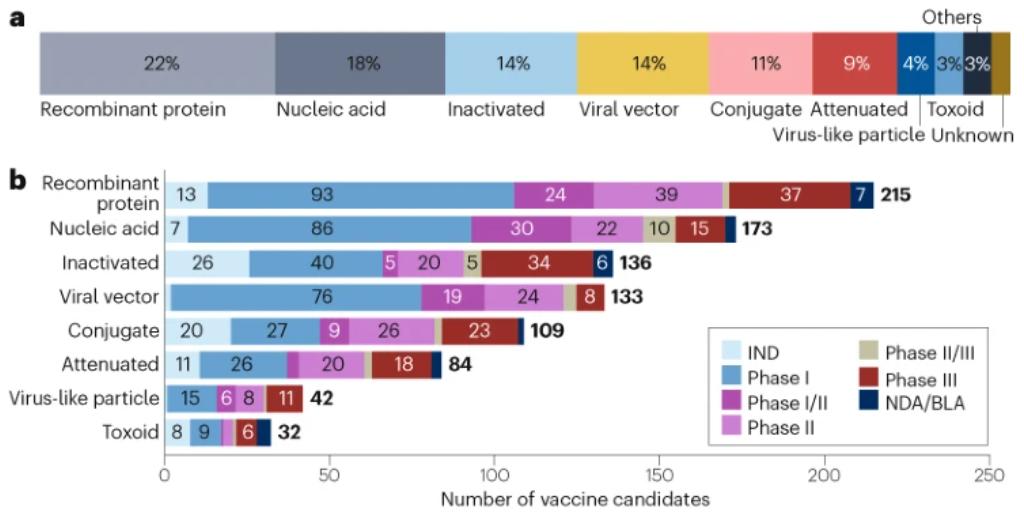
Figure 1. Candidate vaccine landscape divided by technology platform, research and development stage, and disease [15].
Spray vaccines, particularly nasal spray and inhalation vaccines, have emerged as a research hotspot. For example, a nasal spray vaccine has been developed for influenza. Research on spray vaccines also includes the novel coronavirus, with the development of inhalation vaccines such as the inhalation recombinant novel coronavirus vaccine (adenovirus type 5 vector). The advantages of spray vaccines include their ability to directly target the respiratory mucosa, inducing a local immune response and improving overall immune efficacy [16]. Additionally, the administration of spray vaccines is non-invasive, more convenient, and generally more acceptable.
However, there are challenges associated with spray vaccines. Ensuring that the vaccine components are evenly distributed on the respiratory mucosa is crucial to achieving the desired immune effect. Furthermore, the storage and transportation conditions for spray vaccines may be more stringent, presenting additional logistical challenges.
6. Conclusion
The current state and achievements of vaccine research and development encompass several key areas, including the creation of new vaccines, technological advancements, and the promotion of their application. Firstly, with the advent of biotechnology, novel vaccines such as mRNA, DNA, and viral vector vaccines are gradually being developed and implemented. These vaccines offer rapid responses to emerging pathogens and possess advantages such as swift preparation, high safety, and ease of storage and transportation. However, they also face challenges like high costs and stability issues. Additionally, researchers are working towards broad-spectrum vaccines that can provide comprehensive protection against highly variable pathogens, aiming to stimulate immune responses to multiple virus strains and mitigate vaccine failure due to viral mutations. The development of broad-spectrum vaccines is complex, involving challenges in balancing immune responses and covering sufficient viral strains.
Furthermore, international organizations and governments are making concerted efforts to increase vaccine coverage, particularly in low-income countries. Despite these efforts, vaccination rates are still influenced by various factors, including political, economic, cultural, and geographical considerations. In summary, the current landscape and achievements in vaccine research and development are multifaceted, involving technological innovation, disease control, and global cooperation. While numerous challenges remain, the progress in vaccine development continues to bring substantial benefits to human health.
The importance of vaccines in animal disease prevention and control is undeniable. Vaccines not only effectively protect animal health but also indirectly ensure human health and safety. Vaccines stimulate the animal's immune system to produce protective immune responses against specific pathogens, thereby reducing the risk of animal infections. For instance, the use of rabies vaccines has significantly lowered the incidence of rabies in dogs, consequently reducing the risk of humans contracting rabies from infected dogs. In intensive farming environments, vaccination can block disease transmission chains, significantly reducing pathogen spread. In animal husbandry, vaccination reduces production losses due to diseases, improving food production and quality. Healthy livestock translates to faster growth rates, better feed conversion, and lower mortality, enhancing the overall economic benefits of agricultural production. Additionally, effective animal vaccination programs can mitigate the impact of disease outbreaks on international trade, promoting the global trade of animal products.
Vaccine research and policy recommendations are crucial for public health protection, global health security, and sustainable development. Effective vaccine research and policy formulation require a comprehensive approach, integrating scientific research, technological development, and policy guidance. By strengthening funding support for vaccine research, enhancing international cooperation and resource sharing, and formulating long-term vaccine development plans, countries can improve the effectiveness of vaccine research and policies, contributing to global public health security and sustainable development.
References
[1]. Fan, X., Han, S., Yan, D., Gao, Y., Wei, Y., Liu, X., & Sun, S. (2018). Foot-and-mouth disease virus infection suppresses autophagy and NF-кB antiviral responses via degradation of ATG5-ATG12 by 3Cpro. Cell Death & Disease, 8(1), Article e2561.
[2]. Smith, Z. K., Anderson, P. T., & Johnson, B. J. (2020). Finishing cattle in all-natural and conventional production systems.
[3]. Neamat-Allah, A. N. F., Ali, A. A., & Mahmoud, E. A. (2020). Jeopardy of Lyssavirus infection in relation to hemato-biochemical parameters and diagnostic markers of cerebrospinal fluid in rabid calves. Comparative Clinical Pathology, 29(2), 553-560.
[4]. Acosta-Jamett, G., Cleaveland, S., Cunningham, A. A., & Bronsvoort, B. D. (2010). Demography of domestic dogs in rural and urban areas of the Coquimbo region of Chile and implications for disease transmission. Preventive Veterinary Medicine, 94(3-4), 272-281.
[5]. Smith, J. S. (1996). New aspects of rabies with emphasis on epidemiology, diagnosis, and prevention of the disease in the United States. Clinical Microbiology Reviews, 9(2), 166-176.
[6]. Grubman, M. J., & Baxt, B. (2004). Foot-and-mouth disease. Clinical Microbiology Reviews, 17(2), 465-493.
[7]. Hicks, D. J., Fooks, A. R., & Johnson, N. (2012). Developments in rabies vaccines. Clinical & Experimental Immunology, 169(3), 199-204.
[8]. Rao, A. K., Briggs, D., Moore, S. M., et al. (2022). Use of a modified preexposure prophylaxis vaccination schedule to prevent human rabies: Recommendations of the Advisory Committee on Immunization Practices — United States, 2022. MMWR. Morbidity and Mortality Weekly Report, 71, 619-627.
[9]. Jeyanathan, M., Afkhami, S., Smaill, F., et al. (2020). Immunological considerations for COVID-19 vaccine strategies. Nature Reviews Immunology, 20(10), 615-632.
[10]. Zhang, L., Li, P., Ren, W., Xie, W., Guo, J., & Song, Z., et al. (2011). Development of foot-and-mouth disease inactivated vaccine. Veterinary Guide.
[11]. Chen, F., Yang, H., & Zhao, Z. (2021). Dilemmas and prospects in the research of African swine fever vaccines. Swine Industry Science, 38(10), 58-62.
[12]. Wood, J. M., & Robertson, J. S. (2004). From lethal virus to life-saving vaccine: Developing inactivated vaccines for pandemic influenza. Nature Reviews Microbiology, 2(10), 842-847.
[13]. Harandi, A. M. (2018). Systems analysis of human vaccine adjuvants. In Seminars in immunology (Vol. 39, pp. 30-34). Academic Press.
[14]. Yue, J., Liu, Y., Zhao, M., Bi, X., Li, G., & Liang, W. (2023). The R&D landscape for infectious disease vaccines. Nature reviews. Drug discovery, 22(11), 867–868.
[15]. Hong, W., Xu, Y. Y., & Sun, R. J. (2020). Key scientific issues in vaccine research for major diseases—Academic review of the 224th Shuangqing Forum. China Science Foundation, 34(5), 11.
[16]. Huang, X. C., Zhuang, C. L., Liu, X. H., Hu, X. W., & Wu, T. (2023). Nasal vaccines for respiratory infectious diseases: Progress and challenges. Chinese Journal of Disease Control, 27(2), 231-237.
Cite this article
Zhang,X. (2024). Research and development of common animal diseases and their vaccines. Theoretical and Natural Science,61,27-34.
Data availability
The datasets used and/or analyzed during the current study will be available from the authors upon reasonable request.
Disclaimer/Publisher's Note
The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of EWA Publishing and/or the editor(s). EWA Publishing and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
About volume
Volume title: Proceedings of the 4th International Conference on Biological Engineering and Medical Science
© 2024 by the author(s). Licensee EWA Publishing, Oxford, UK. This article is an open access article distributed under the terms and
conditions of the Creative Commons Attribution (CC BY) license. Authors who
publish this series agree to the following terms:
1. Authors retain copyright and grant the series right of first publication with the work simultaneously licensed under a Creative Commons
Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this
series.
2. Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the series's published
version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial
publication in this series.
3. Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and
during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See
Open access policy for details).
References
[1]. Fan, X., Han, S., Yan, D., Gao, Y., Wei, Y., Liu, X., & Sun, S. (2018). Foot-and-mouth disease virus infection suppresses autophagy and NF-кB antiviral responses via degradation of ATG5-ATG12 by 3Cpro. Cell Death & Disease, 8(1), Article e2561.
[2]. Smith, Z. K., Anderson, P. T., & Johnson, B. J. (2020). Finishing cattle in all-natural and conventional production systems.
[3]. Neamat-Allah, A. N. F., Ali, A. A., & Mahmoud, E. A. (2020). Jeopardy of Lyssavirus infection in relation to hemato-biochemical parameters and diagnostic markers of cerebrospinal fluid in rabid calves. Comparative Clinical Pathology, 29(2), 553-560.
[4]. Acosta-Jamett, G., Cleaveland, S., Cunningham, A. A., & Bronsvoort, B. D. (2010). Demography of domestic dogs in rural and urban areas of the Coquimbo region of Chile and implications for disease transmission. Preventive Veterinary Medicine, 94(3-4), 272-281.
[5]. Smith, J. S. (1996). New aspects of rabies with emphasis on epidemiology, diagnosis, and prevention of the disease in the United States. Clinical Microbiology Reviews, 9(2), 166-176.
[6]. Grubman, M. J., & Baxt, B. (2004). Foot-and-mouth disease. Clinical Microbiology Reviews, 17(2), 465-493.
[7]. Hicks, D. J., Fooks, A. R., & Johnson, N. (2012). Developments in rabies vaccines. Clinical & Experimental Immunology, 169(3), 199-204.
[8]. Rao, A. K., Briggs, D., Moore, S. M., et al. (2022). Use of a modified preexposure prophylaxis vaccination schedule to prevent human rabies: Recommendations of the Advisory Committee on Immunization Practices — United States, 2022. MMWR. Morbidity and Mortality Weekly Report, 71, 619-627.
[9]. Jeyanathan, M., Afkhami, S., Smaill, F., et al. (2020). Immunological considerations for COVID-19 vaccine strategies. Nature Reviews Immunology, 20(10), 615-632.
[10]. Zhang, L., Li, P., Ren, W., Xie, W., Guo, J., & Song, Z., et al. (2011). Development of foot-and-mouth disease inactivated vaccine. Veterinary Guide.
[11]. Chen, F., Yang, H., & Zhao, Z. (2021). Dilemmas and prospects in the research of African swine fever vaccines. Swine Industry Science, 38(10), 58-62.
[12]. Wood, J. M., & Robertson, J. S. (2004). From lethal virus to life-saving vaccine: Developing inactivated vaccines for pandemic influenza. Nature Reviews Microbiology, 2(10), 842-847.
[13]. Harandi, A. M. (2018). Systems analysis of human vaccine adjuvants. In Seminars in immunology (Vol. 39, pp. 30-34). Academic Press.
[14]. Yue, J., Liu, Y., Zhao, M., Bi, X., Li, G., & Liang, W. (2023). The R&D landscape for infectious disease vaccines. Nature reviews. Drug discovery, 22(11), 867–868.
[15]. Hong, W., Xu, Y. Y., & Sun, R. J. (2020). Key scientific issues in vaccine research for major diseases—Academic review of the 224th Shuangqing Forum. China Science Foundation, 34(5), 11.
[16]. Huang, X. C., Zhuang, C. L., Liu, X. H., Hu, X. W., & Wu, T. (2023). Nasal vaccines for respiratory infectious diseases: Progress and challenges. Chinese Journal of Disease Control, 27(2), 231-237.









