1. Introduction
In recent years, the frequent occurrence of international public health emergencies has posed serious public health risks to countries worldwide due to the spread of various diseases. In this context, vaccine development has become particularly important, emerging as a key measure for the prevention and control of infectious diseases. Especially during the COVID-19 pandemic, mRNA vaccines have played a crucial role in disease control, highlighting their status as core technology for the development of new-generation vaccines. The primary mechanism of vaccines is to prevent pathogen invasion by activating the individual's immune response, thereby avoiding disease occurrence. During global public health crises, the development and widespread vaccination of vaccines have significantly controlled morbidity and mortality rates. Vaccine inoculation is based on the principle of herd immunity, achieving community protection against epidemic diseases through widespread immunization, thereby effectively controlling the spread of pathogens within regions. However, due to individual differences, the immune response to the same vaccine may vary among different individuals, but overall, vaccines generally provide effective immune protection.
In the long-term battle between humans and infectious diseases, the emergence of vaccines has provided crucial support in combating highly pathogenic pathogens. For example, the human respiratory syncytial virus has been discovered for over 60 years, and through continuous research and exploration, significant progress has been made in pre-fusion glycoprotein structural vaccines, solving the issue of the lack of available vaccines in past prevention efforts. The practice of immunization can be traced back to the 12th century, when ancient Chinese used variolation to gain immunity against smallpox. The modern beginning of vaccination is usually considered to be in 1798 when Edward Jenner invented the smallpox vaccine by injecting relatively weak cowpox virus into humans to prevent smallpox. The mid-20th century is known as the golden age of vaccine science, where rapid technological advancements enabled scientists to culture cells in controlled laboratory environments, subsequently developing vaccines against various infectious diseases such as poliomyelitis and measles. At the same time, conjugate vaccines combining capsular polysaccharides and protein immunogens were developed, successfully reducing infection-related mortality and alleviating the public healthcare burden. The advent of genetic engineering technology revolutionized the vaccine development process. By the end of the 20th century, researchers were able to produce subunit vaccines in addition to traditional whole-cell vaccines, such as the hepatitis B vaccine, the first subunit vaccine certified through human clinical trials, using viral antigens to initiate immune responses. Through these technological advancements, vaccine science has continuously evolved, providing powerful tools for humans to combat infectious diseases.
2. Basic Principles of Vaccines
2.1. Basic Concepts of the Immune System
The immune system consists of immune organs, immune cells, and immune-active substances, with the primary function of responding to the persistent threat posed by environmental pathogens. The immune system has roles in immune surveillance, immune defense, and immune regulation. In mammals, the immune system is divided into innate immunity (nonspecific immunity) and adaptive immunity (specific immunity). Adaptive immunity is further divided into humoral immunity and cellular immunity, characterized by the complexity and diversity of the antigen recognition system. The innate immune system is the host's first line of defense against invading pathogens, including physical barriers such as skin and mucous membranes. This defense mechanism has no memory function but can immediately recognize and eliminate invading pathogens. The innate immune system initiates immune responses by recognizing common features of pathogens (such as pathogen-associated molecular patterns, PAMPs), primarily involving phagocytes, natural killer cells, and the complement system. The adaptive immune system is the second line of immune defense in the body. This immune response is activated when encountering specific pathogens, responding relatively slowly but with high specificity and memory functions. The adaptive immune system recognizes and eliminates specific pathogens through the actions of T cells and B cells. Humoral immunity is mediated by B cells, which produce antibodies to neutralize and clear pathogens; cellular immunity is mediated by T cells, which respond to infections by directly killing infected cells or regulating the functions of other immune cells. The adaptive immune response is characterized by its memory function, allowing the body to respond quickly and effectively to subsequent encounters with the same pathogen, providing long-lasting immune protection. This memory function is the basis for vaccine efficacy, as vaccines stimulate an adaptive immune response by mimicking pathogen infection, thereby providing rapid and effective protection during actual infection. In summary, the complex structure and multilayered defense mechanisms of the immune system enable it to respond to a wide variety of pathogenic threats, thus maintaining the health of the body.
2.2. Mechanisms of Vaccine Action
Vaccines play a crucial role in disease prevention and control. Common types of vaccines include inactivated vaccines, live attenuated vaccines, subunit vaccines, and nucleic acid vaccines. Inactivated vaccines can be composed of whole viruses or bacteria or their fragmented parts, the latter being called split vaccines. Inactivated vaccines can be protein-based or polysaccharide-based. Protein vaccines include toxoids (inactivated bacterial toxins) and subunit vaccines, while most polysaccharide vaccines consist of purified cell wall polysaccharides from bacteria. Inactivated vaccines primarily induce humoral immune responses, producing antibodies that neutralize and eliminate pathogenic microorganisms and their toxins, offering good protection against extracellular infections. However, they are less effective or ineffective against viruses, intracellular bacteria, and parasites. Inactivated vaccines usually require multiple doses; a single dose typically does not provide protective immunity and merely "primes" the immune system, necessitating a second or third dose for protective immunity. Since inactivated vaccines mainly elicit humoral immunity and rarely induce cellular immunity, antibody titers decline over time, requiring periodic booster vaccinations. Inactivated vaccines are generally not affected by circulating antibodies and can be administered even if antibodies are present in the blood, making them suitable for immunocompromised individuals. Live attenuated vaccines are created by treating pathogens (e.g., with formaldehyde) to retain their antigenicity but reduce their virulence. After vaccination, these pathogens do not cause disease but can elicit an immune response, stimulating the production of specific memory B cells and memory T cells, providing long-term or lifelong protection. Compared to inactivated vaccines, live attenuated vaccines offer stronger immunity and longer duration of action, but their safety poses potential risks, such as the possibility of reverting to virulence through back mutation. Currently clinically used live attenuated vaccines include measles, hepatitis A, rubella, mumps, and oral poliovirus vaccines. Subunit vaccines contain only the antigenic parts of the pathogen that can induce an immune response. Subunit vaccines can be made from dispersed viral particles in cell cultures or recombinant DNA expression, known as recombinant subunit vaccines. Unlike live attenuated or inactivated vaccines, subunit vaccines do not contain the whole pathogen but only the antigenic parts, such as proteins, polysaccharides, or peptides [1-4]. Due to the absence of "live" components of the pathogen, subunit vaccines are safer and more stable, suitable for individuals with compromised immune function [1]. Their drawbacks include relatively complex manufacturing, the potential need for adjuvants and booster shots, and time required to determine the optimal antigen combination [2]. Since subunit vaccines may lack pathogen-associated molecular patterns, the immune response may be weak, usually requiring adjuvants and possibly increased doses and frequency of administration. Nucleic acid vaccines have developed rapidly in recent years, especially achieving significant progress in the prevention and control of COVID-19. DNA vaccines (also known as "naked" DNA vaccines or gene vaccines) and RNA vaccines represent nucleic acid vaccines. DNA vaccines induce strong and long-lasting immune responses by plasmid DNA encoding genes for viral, bacterial, or parasitic antigens, known as third-generation vaccines [5]. DNA vaccines have widespread applications in veterinary medicine. RNA vaccines include self-amplifying RNA (saRNA) vaccines, mRNA vaccines, and circular RNA (circRNA) vaccines. SaRNA vaccines can self-replicate within cells, producing large amounts of antigen proteins from minimal doses; mRNA vaccines are the most mature in research and production; circRNA vaccines have higher stability due to their circular structure [5]. The broad prospects for nucleic acid vaccines make them an important direction for future vaccine development.
2.3. Immune Memory and Vaccine Efficacy
Immune memory is a key feature of the adaptive immune response. Progenitor T cells derived from the bone marrow develop into naive T cells in the thymus and home to peripheral immune organs. After antigen stimulation, naive T cells differentiate into effector T cells, providing effective immune protection. Most effector T cells undergo apoptosis after clearing the antigen, leaving only a small number of long-lived CD4+ and CD8+ memory T cells to maintain immune memory. Memory T cells rapidly proliferate and produce effector cytokines upon re-encounter with the same antigen, providing immune protection [6]. Unlike naive T cells, which primarily recirculate in peripheral immune organs and peripheral blood, memory T cells can circulate in lymphoid organs, blood, and peripheral tissues (such as lungs, intestines, and skin) to proliferate and respond quickly upon antigen stimulation [7]. Immune memory is typically acquired through infection or vaccination. The immune system can generate a faster and more effective immune response upon the second encounter with a specific antigen, known as a secondary immune response or anamnestic response. Immunological memory cells, when re-stimulated by the same antigen, trigger a stronger antibody production compared to the primary response, evidenced by a significant increase in antibody titers, immunoglobulin class switching (e.g., from IgM to IgG), and enhanced antibody affinity. This indicates not only a quantitative change in the immune system but also a qualitative improvement. The primary function of the immune system is to protect against microbial pathogens. This function is accomplished in two steps: first, generating a specific immune response to control the infection, and then memorizing the pathogen information. When the same pathogen is encountered again, memory T cells and memory B cells can rapidly mount an immune response, either completely preventing the disease or significantly reducing the severity of clinical symptoms. Recent studies have found that memory CD4+ T cells and memory CD8+ T cells can persist without antigens, indicating that memory T cells can maintain their numbers through self-proliferation [8]. Moreover, CD4+ T cells are crucial for the generation of high-quality memory CD8+ T cells [9]. Memory B cells and long-lived plasma cells also play an essential role in maintaining long-term immune protection. Long-lived plasma cells are responsible for the sustained secretion of antibodies, even in the absence of antigen stimulation, maintaining antibody levels [10]. These cells mainly reside in the bone marrow and can survive for long periods while continuously secreting antibodies. In humoral immunity, IgM antibodies appear first during the primary response, followed by IgG antibodies. Although IgG antibodies appear later, they can be maintained for extended periods. During the secondary response, the production of IgG antibodies is significantly faster and at higher levels. This phenomenon of IgM switching to IgG not only reflects a change in antibody quantity but also an improvement in antibody quality. This phenomenon is called antibody affinity maturation, and experiments have shown that the affinity of IgG can increase hundreds of times during the immune response. Vaccine design is based on establishing immune memory, with most vaccines inducing protective immunity by stimulating the production of neutralizing antibodies. However, over time, antibodies in circulation and tissues gradually decrease, and antigen-targeted mutations and rapid pathogen evolution can weaken the protective effect of antibody-mediated humoral immunity. Therefore, long-term immune protection relies on the induction of memory cells. Compared to antibodies, memory T cells can recognize a broader range of pathogen antigens, including highly conserved internal proteins, making them more effective in combating pathogen variants [11-13]. Recent evidence increasingly shows that memory T cells play an important role in immune protection, and inducing memory T cells to mediate long-term immune protection has become a new direction in vaccine design. In 1991, two major subsets of memory T cells, central memory T cells (TCM) and effector memory T cells (TEM), were defined. TCM and TEM are extensively studied due to their abundance in the blood. In 2009, a study first proposed that some memory CD8+ T cells could reside long-term in the skin and dorsal root ganglia without re-entering blood circulation [14].
3. Types of Vaccines
3.1. Inactivated Vaccines
Inactivated vaccines are produced by physically (heat or radiation) or chemically (formaldehyde treatment) killing pathogens directly. Although these inactivated pathogens lose their virulence, they retain their immunogenicity and can still activate the host's immune response. Inactivated vaccines are developed quickly, with simple and mature production processes, and are highly safe because they are dead vaccines, eliminating the risk of infection. However, the production cost of inactivated vaccines is high, involving the cultivation of pathogens and requiring high biosafety levels in production facilities, leading to significant upfront investments. In terms of production capacity, inactivated vaccines have some drawbacks because all pathogens need to be inactivated, which includes the cultivation, inactivation, and testing of pathogens, making the process relatively slow. Additionally, inactivated vaccines have lower immunogenicity, usually requiring multiple doses and are unable to induce cellular immunity, resulting in generally moderate immune effects. Inactivated vaccines typically induce humoral immunity but seldom induce cellular immunity. Common inactivated vaccines include polio vaccine, hepatitis A vaccine, and rabies vaccine [15]. Attenuated live vaccines are generally made by artificially passaging pathogens continuously to selectively screen out those with weakened pathogenicity or by directly screening naturally occurring less virulent or essentially non-virulent pathogens. These live vaccines have reduced virulence but retain their immunogenicity. After being administered to the body, these pathogens usually do not cause disease but can grow and replicate within the host, inducing an immune response. Attenuated live vaccines are the most effective vaccines, providing strong immunity since they are live pathogens that can induce robust and lasting immune responses, usually producing long-term immunity with one or two doses. However, the development of attenuated live vaccines is slow, with a long screening process, high production costs, and lower safety due to the presence of residual virulence, which may cause disease in individuals with weak or deficient immune systems. Additionally, in rare cases, there is a risk of reversion mutation with attenuated live vaccines, potentially causing disease. The storage and transport conditions for attenuated live vaccines are stringent, such as requiring cold chain transport. Common attenuated live vaccines include BCG, polio vaccine, measles vaccine, mumps vaccine, and rotavirus vaccine [16].
3.2. Nucleic Acid Vaccines
Nucleic acid vaccines are a new type of vaccine that has emerged in recent years. By directly injecting DNA or mRNA encoding antigens into humans or animals, these vaccines utilize host cells to express the antigen, triggering a protective immune response for prevention [17]. Compared to the first two generations of vaccines, third-generation vaccine technology is more advanced, with higher specificity, greater efficacy, and shorter development cycles. Nucleic acid vaccines include DNA vaccines and mRNA vaccines [18]. DNA vaccines refer to circular DNA plasmids encoding antigen or antigen epitopes. After entering host cells through specific delivery methods, the carried antigen gene is transcribed, with mRNA then reaching the cytoplasm and translating into antigen proteins, thereby eliciting an immune response [19]. Compared to traditional vaccines, DNA vaccines have simple design and preparation processes, low cost, stable nature, and can be stored long-term at room temperature, continuously expressing natural antigens to induce strong and long-lasting immune responses. However, DNA vaccines require the processes of nuclear entry, transcription, and translation, which delay the onset of action, and there is a risk of DNA plasmids integrating into the host chromosome. Additionally, since DNA vaccines continuously express pathogen antigens in the host, they may lead to immune imbalance, inducing immune tolerance and reducing the immune effect [20]. mRNA vaccines work by directly introducing in vitro synthesized mRNA encoding exogenous antigen genes into host cells to express antigen proteins, thereby inducing an immune response. Currently, two types of RNA are used for vaccine manufacturing: non-replicating mRNA and self-amplifying mRNA. Compared to traditional vaccines, mRNA vaccines have simpler design and synthesis processes, faster development speeds, and lower costs. Compared to DNA vaccines, mRNA translation is faster, does not require nuclear entry, and can be quickly translated in the cytoplasm, with higher safety as mRNA degrades through physiological metabolic pathways, causing minimal side effects and posing no risk of genomic integration [21]. However, mRNA vaccines are prone to degradation, with stringent storage and transport temperature requirements, necessitating modifications to enhance stability, typically using delivery systems such as lipid nanoparticles, which require high technical standards.
3.3. Vector Vaccines
Recombinant vector vaccines, also known as live vector vaccines, are constructed by inserting genes encoding pathogen antigens into the genome of non-virulent or low-virulence vectors using DNA recombinant technology. When recombinant vectors are administered to humans or animals, the pathogen antigens are abundantly expressed along with the proliferation of the recombinant vectors within the body, thereby activating the host's immune response and conferring immunity. Common viral vectors include adenoviruses and poxviruses, while common bacterial vectors include Salmonella, Bacillus subtilis, and Lactobacillus. Recombinant vector vaccines have many advantages, including good immunogenicity, stable long-term antigen protein expression in the body, and the ability to induce durable immune responses, with low production costs suitable for large-scale production. However, recombinant vector vaccines may face issues of pre-existing immunity, necessitating prior evaluation and thorough research to ensure the vector is harmless or minimally harmful to the human body [22]. Additionally, the handling and production of vectors must meet strict requirements. The storage and transport conditions of these vaccines are also stringent. Common vector vaccines include Helicobacter pylori vaccine and Ebola vaccine.
3.4. Protein Subunit Vaccines
Subunit vaccines are produced by breaking down pathogens or generating pathogen antigen proteins with immunological activity based on genetic engineering principles. Compared to the first generation of vaccines, subunit vaccines have the advantages of strong specificity and minimal side effects, including component subunit vaccines and genetically engineered subunit vaccines. Component subunit vaccines are produced by chemically decomposing or hydrolyzing pathogen proteins, isolating and screening antigen proteins with immunological activity. These vaccines have minimal side effects but lower immunogenicity. Compared to whole-cell vaccines, component subunit vaccines usually contain only a few immunogenic antigen proteins, avoiding immune responses triggered by many irrelevant antigens, thereby reducing vaccine side effects. However, the production cost of these vaccines is high, making large-scale production difficult, and the isolated proteins may denature, reducing immunogenicity and requiring combination with adjuvants [23]. Common vaccines include typhoid vaccine and encephalitis vaccine. Recombinant protein vaccines are produced by inserting pathogen antigen genes into expression plasmids using genetic engineering technology, constructing recombinant expression plasmids, and then transforming them into engineering cells to induce antigen protein expression, which is purified for vaccine production. These vaccines have slower initial research and development progress but offer advantages in scalability later. Recombinant protein vaccines are easy to mass-produce, low-cost, with minimal side effects, and non-pathogenic, making them suitable for immunocompromised or chronically ill patients. However, the screening of antigen epitopes requires high technical standards, with lower immunogenicity, needing adjuvants and multiple doses to achieve good immune effects.
3.5. Other Emerging Vaccine Technologies
Studies have found that most viral capsid protein genes can effectively self-assemble into virus-like particles (VLPs) in eukaryotic expression systems and some in prokaryotic expression systems. These recombinant protein vaccines are called virus-like particle vaccines [24]. VLPs range in diameter from 20 to 150 nanometers, lack viral nucleic acids, cannot replicate autonomously, and are non-infectious, thus highly safe. Nevertheless, VLPs are morphologically identical or similar to actual viral particles and can be presented to immune cells through the same pathways as viral infections, effectively inducing immune protection responses. VLPs offer the advantages of structural stability and strong immunogenicity. Additionally, VLPs have relatively mature production processes and can be expressed in various host systems, such as yeast, insect cells, and mammalian cells. This diversified expression system enhances production flexibility and helps reduce production costs. Moreover, VLPs provide high plasticity in vaccine design, allowing the introduction of different antigen epitopes through genetic engineering to enhance immune effects. In the future, with continuous advances in genetic engineering and biomanufacturing technologies, VLP vaccines are expected to play a role in more disease prevention areas. They can be rapidly developed against emerging viruses and achieve breakthroughs in personalized vaccine design and multivalent vaccine development. This will provide more robust tools for public health, helping to address future potential epidemics and infectious disease threats (Table 1).
Table 1. Common VLPs vaccines and their characteristics | ||
Vaccine type | Advantage | Disadvantage |
Inactivated Vaccine | Good safety performance, easy to transport and preserve, mature production technology. | Many times, cellular immunity is weak, infection causes again antibody dependent enhancement (Antibody-dependent Enhancement, ADE ). |
Attenuated live Vaccine | Fewer vaccinations, inducing strong cellular immune, long duration. | The research and development cycle is long and there is a risk of virus reversion. |
Recombinant protein Vaccine | The antigen is stable, rapid development, high safety, less adverse reactions, and easy to transport. | The immunogenicity was weak, and the conformation of the expressed protein was different from that of the native protein after repeated vaccination. |
Viral vector Vaccine | Fewer vaccinations, gene transfer efficiency is high, the induction of mucosal immune and cell immune. | Viral vector potential risk, easily affected by host antibodies have been established. |
DNA Vaccine | Rapid development, continuous production of antigens, induction of humoral and cellular immunity. | It needs to enter the nucleus, is affected by the expression efficiency, and the inoculation route is complex. |
RNA Vaccine | It has the advantages of rapid research and development, easy mass production, inducing humoral and cellular immunity, and producing immunity rapidly. | mRNA stability is poor, delivery systems, immune tolerance may occur, and transport and storage conditions are stringent. |
4. Recent Vaccine Development Process
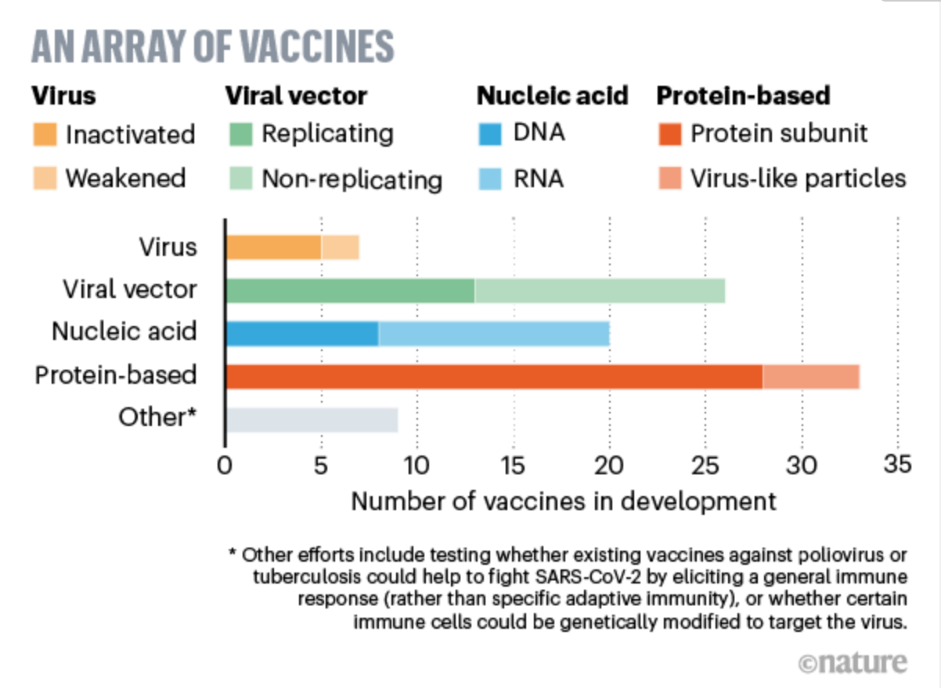
Figure 1. An array of vaccines [25] |
4.1. Basic Process of Development
Typically, it takes at least 8 years, and sometimes more than 20 years, for a vaccine to progress from research and development to market release. The main stages include preclinical research, application for clinical trials, and registration clinical trials (Figure 1). Preclinical research forms the foundation for strain and cell screening, ensuring the safety, efficacy, and continuous supply of vaccines. Depending on the vaccine, animal trials may be conducted using mice, guinea pigs, rabbits, or monkeys. If the preliminary results suggest controlled processes, stable quality, and safety and efficacy, an application can be submitted to the national drug regulatory authority for clinical trials. Preclinical research typically takes 5-10 years.
When applying for clinical trials for preventive vaccines, companies must submit research materials covering pharmaceuticals, pharmacology and toxicology, and clinical aspects to the national drug regulatory authority. Clinical trials are typically divided into three phases: Phase I, Phase II, and Phase III. Each phase of clinical trials has strict standard operating procedures (SOPs) for personnel, sites, and monitoring. Phase I clinical trials: Preliminary assessment of human safety, with typically dozens to a hundred participants. If the results of Phase I clinical trials show good safety, Phase II clinical trials can proceed. Phase II clinical trials: Mainly conduct dose-exploration studies of the vaccine, preliminary efficacy evaluation, and further expanded safety assessments, generally involving hundreds to thousands of participants. Phase III clinical trials: Randomized, blinded, placebo-controlled (or controlled vaccine) design to comprehensively evaluate the efficacy and safety of the vaccine, with typically thousands to tens of thousands of participants. Phase III clinical trials are the basis for the vaccine's market approval. If key Phase III clinical trials achieve the expected clinical protective effects and show good safety, the company can submit the clinical data to the national drug regulatory authority to apply for production. All clinical trials generally take at least 3-8 years, sometimes extending over 10 years. Each phase of the clinical trial has strict safety monitoring and termination criteria. Each vaccine may be halted or even terminated during the clinical phase if it fails to meet preset objectives or expected requirements.
4.2. Focus Areas in Recent Vaccine Development
Taking the COVID-19 vaccine as an example, on January 12, 2020, the World Health Organization officially named the novel coronavirus as 2019-nCoV. Currently, three teams from China National Biotech Group's subsidiaries – Wuhan Institute of Biological Products Co., Ltd., Beijing Institute of Biological Products Co., Ltd., and Beijing Sinovac Biotech Co., Ltd. – have inactivated COVID-19 vaccines that have entered clinical trial stages.
Globally, many companies and research institutions are actively developing COVID-19 vaccines. On April 3, 2020, GSK and Xiamen Innovax Biotech Co., Ltd. (Xiamen Innovax) jointly announced a research collaboration, with GSK providing its vaccine adjuvant system to Xiamen Innovax to evaluate the preclinical effects of a recombinant protein coronavirus candidate vaccine developed by Xiamen Innovax and Xiamen University. On March 16, 2020, the Phase I clinical trial of a recombinant novel coronavirus vaccine (adenovirus vector) developed by Chen Wei, an academician from the Academy of Military Medical Sciences, and CanSino Biologics Inc. began in Wuhan, with the initial 14-day safety observation completed and Phase II clinical trials started on April 12. The Institut Pasteur in France is also progressing with research using a modified measles vaccine strain as a vector to express the coronavirus antigen gene.
The World Health Organization's "COVID-19 Global Research Roadmap" points out that antibody-dependent enhancement (ADE) effects may occur when animals are re-exposed to live viruses after immunization with coronavirus vaccines, leading to more severe infections and disease responses post-vaccination. Key measures to prevent ADE include selecting appropriate target antigens to reduce the induction of non-neutralizing antibody regions. Many research teams choose to target dominant neutralizing antibody regions, such as the RBD region of the antigen. However, smaller antigen molecules may affect immunogenicity. Therefore, optimizing immune strategies is crucial, such as using more effective adjuvants to help antigens produce high-titer neutralizing antibodies to reduce the possibility of ADE [26]. Additionally, COVID-19 vaccine design should consider both humoral and cellular immunity. During animal and clinical trials, monitoring antibody levels and cellular immune responses can help predict potential ADE risks. Future vaccine development should emphasize combining various immune strategies to enhance overall vaccine protection and safety.
In summary, COVID-19 vaccine development is not only a current global scientific focus but also represents future vaccine development directions and challenges. By continuously optimizing vaccine design, improving production processes, and deeply understanding virus-host interaction mechanisms, scientists hope to develop safer and more effective vaccines, providing robust protection for global public health.
4.3. Challenges in Vaccine Development
Current issues in vaccine immunogen research include unclear theories on immunogen design, insufficient understanding of intrinsic determinants of immunogens, lack of models and systems for immunogen design, lack of original immunogen screening techniques, and discrepancies between predictive analysis results and in vivo validation results. These issues are particularly prominent in analyzing immunogen characteristics and structural information and exploring intrinsic determinants of immunogenicity. For example, determining the immunogenicity of different epitopes, quantifying the interactions between epitopes, and enhancing the immunogenicity of protective antigen epitopes are all pressing issues.
Using new technologies to identify natural immunogens, reverse vaccinology, and systems vaccinology to screen protective antigens can significantly improve vaccine development efficiency and efficacy. Structural vaccinology and informatics vaccinology help enhance the immunogenicity of weak immunogen epitopes and further explore broadly protective immune epitopes. The design of vaccine immunogens is a manifestation of interdisciplinary integration and new technology fusion, including the application of emerging disciplines such as bioinformatics, structural biology, and antibody engineering, greatly enriching immunogen design strategies. Multidisciplinary cooperation is crucial for vaccine development, combining the roles of germinal center B cells and follicular T-like cells in effective antibody responses and antibody response mechanisms, focusing on mucosal immune effects and mechanisms, and the relationship between pre-existing immunity and vaccine efficacy. In researching vaccine immune memory mechanisms, strengthening basic immunology research to analyze the nature and mechanisms of vaccine-induced immune memory and induce long-term immune protection is key. Mechanisms needing research include the origin, differentiation, and maintenance mechanisms of immune memory cells, and the mechanisms of regional persistent immune memory responses in organs and tissues.
Moreover, modern vaccine development faces a series of technical and operational challenges. First, vaccine development requires efficient and precise antigen screening techniques, including both laboratory screening and the combination of high-throughput computational screening and in vivo validation. Second, the production and manufacturing process of vaccines must comply with strict quality control and biosafety standards, especially during large-scale production, to ensure the consistency of efficacy and safety in each batch of vaccines. Third, vaccine transportation and storage conditions are stringent, particularly for temperature-sensitive vaccines such as mRNA vaccines, which require cold chain transport and storage, adding complexity and cost to logistics and management.
Finally, the clinical trial phase of vaccines is also challenging. Clinical trials require substantial funding and time and must overcome ethical and regulatory obstacles. The efficacy and safety of vaccines in different populations need to be validated through large-scale clinical trials, a complex and time-consuming process. Post-marketing, continuous monitoring and research are necessary to assess long-term effects and potential side effects. In general, vaccine development is a complex and arduous task involving interdisciplinary cooperation and continuous technological innovation. Despite many challenges, advancements in science and technology and strengthened global cooperation are expected to improve the efficiency and success rate of vaccine development, providing stronger safeguards for human health.
5. Advances in Vaccine Technology
5.1. Development of mRNA Vaccine Technology
As a revolutionary bioproduct, mRNA vaccines have shown great potential in the field of infectious disease prevention and treatment. Compared to novel DNA vaccines, mRNA vaccines avoid the risk of genome integration; compared to widely used protein vaccines, their cell-free production method significantly speeds up research and development, enhancing production efficiency. mRNA vaccines can encode multiple antigens, adapt to various pathogen variants, and have the dual function of encoding antigens and adjuvants, effectively stimulating a strong immune response. However, the stability, immunogenicity, and safety of mRNA need further optimization, and improving the efficiency of delivery systems (especially the application of lipid nanoparticles) remains a key challenge in the development of this technology.
Quality control is crucial in the development of mRNA vaccines. For example, in the in vitro transcription process, the removal of dsRNA, template DNA, proteins, and salt ions; during capping, the removal of excess caps and uncapped RNA; and during encapsulation, the control of particle size need to be considered. Among these, the purification of template plasmids and mRNA products are among the most critical aspects of quality control. The purity of template plasmids determines the quality of the template and mRNA activity; the purity of mRNA significantly impacts its protein expression levels and safety. Particularly, the by-product dsRNA produced during in vitro transcription can reduce mRNA transfection efficiency and protein expression levels while causing severe inflammatory and immune stress responses [27, 28]. On the other hand, balancing immunogenicity and the expression level of the target antigen requires in-depth exploration. Numerous studies have shown that pseudouridine modifications can significantly reduce the natural immunogenicity of mRNA and improve expression efficiency. Recent studies indicate that unmodified mRNA has higher target protein expression levels compared to modified mRNA [29]. Furthermore, issues such as the dosage and administration schedule of mRNA vaccines in clinical phases need further discussion. As a new star in the vaccine field, mRNA has a promising future but still faces many challenges. We look forward to more breakthroughs in related research to further open and lead the era of mRNA vaccines.
5.2. Other Technological Advances
Exposed mRNA is unstable in the body and easily degraded by ribonucleases. Additionally, large mRNA molecules with negative charges find it challenging to penetrate similarly negatively charged host cell membranes, leading to low cell permeability. Lipid nanoparticles (LNPs) are recognized as the most promising mRNA delivery system because they can alter properties through controllable and simple chemical synthesis, enhancing the loading capacity and delivery efficiency of mRNA.
LNP lipid components generally include ionizable lipids or cationic lipids, helper lipids, cholesterol, and PEG lipids. These components promote the formation of nanoparticles, improve nanoparticle stability, achieve efficient nucleic acid encapsulation, and facilitate cellular uptake. At the same time, LNPs encapsulate nucleic acid drugs, preventing nuclease degradation until nucleic acids are transported to the cytoplasm, and facilitate endosomal escape of nucleic acid drugs. mRNA LNP vaccines elicit immune responses by transfecting antigen-presenting cells. After mRNA vaccines are endocytosed by antigen-presenting cells and escape from endosomes into the cytoplasm, the mRNA is translated into proteins by ribosomes, and the translated antigen proteins can stimulate the immune system in various ways. Intracellular antigens are broken down into smaller fragments by proteasomes, presented on the cell surface via major histocompatibility complex (MHC) class I molecules, and presented to cytotoxic T cells. Activated cytotoxic T cells kill infected cells by secreting perforin and granzymes. Additionally, antigen fragments can be presented to helper T cells via MHC class II molecules, which stimulate B cells to produce neutralizing antibodies and promote the clearance of circulating pathogens.To achieve preventive or therapeutic effects, mRNA must produce sufficient encoded proteins within target cells. Therefore, the efficacy of mRNA is closely related to the specific administration route and target organs/cells.Another strategy involves using cell-specific ligands.
6. Future Prospects and Research Directions
Strengthening frontier research in immunology and deeply integrating new technologies with vaccine research and translation are key to future vaccine development.
6.1. Other Technological Advances
Immunogens are the core of vaccine research, determining the specificity of vaccine-activated immune responses. Future research needs to focus on analyzing the characteristics and structural information of immunogens, exploring intrinsic determinants of immunogenicity. For example, determining the immunogenicity of different epitopes, quantifying the interactions between epitopes, enhancing the immunogenicity of protective antigen epitopes, and identifying natural immunogens using new technologies. Protective antigens can be screened using reverse vaccinology and systems vaccinology; weak immunogenic epitopes can be enhanced using structural vaccinology and informatics vaccinology. Designing vaccine immunogens requires interdisciplinary cooperation, with bioinformatics, structural biology, and antibody engineering greatly enriching immunogen design strategies.
6.2. Other Technological Advances
Adjuvants and delivery systems determine the strength and type of immune responses. Future research needs to analyze the functions of adjuvants, their impact on the immune system, and the mechanisms of inducing protective immunity. Discovering novel adjuvants that effectively activate antibody and T cell responses by focusing on innate immune signaling pathways, adaptive immune co-stimulatory signaling pathways, and lymph node microenvironments is crucial. Exploring the effects of new adjuvant components and combinations on adjuvant function, such as enhancing adjuvant effects through nanoization, controllability, and targeting. Developing new delivery systems, combining different carriers, and deeply modifying commonly used carriers are also necessary.
6.3. Other Technological Advances
The goal of vaccination is to induce long-lasting protective immune memory. Therefore, strengthening basic immunology research to analyze the nature and mechanisms of vaccine-induced immune memory and inducing long-term immune protection is essential. Future research should explore the origin, differentiation, and maintenance mechanisms of immune memory cells; analyze regional, persistent immune memory responses in organs and tissues; and establish evaluation systems for vaccine immune memory function.
In researching vaccine immune protection mechanisms, the main issues include unclear mechanisms of vaccine-induced immune response differences and the lack of efficient methods to induce T cell immunity and evaluate T cell responses. Improving vaccinology theories is necessary to guide future vaccinology development. Future research should analyze key factors affecting vaccine efficacy and explore measures to regulate vaccine effects. Starting from existing successful vaccines, researchers should study the mechanisms of vaccine immune protection and their effectors. Focusing on positive and negative immune regulatory factors in the microenvironment and their regulatory mechanisms, discovering adjuvant technologies and target molecules that remove negative regulatory factors and enhance vaccine immune effects. Studying the roles of germinal center B cells and follicular T-like cells in effective antibody responses and antibody response mechanisms. Investigating mucosal immune effects and mechanisms, the relationship between pre-existing immunity and vaccine efficacy, etc.
Conducting in-depth systematic vaccine epidemiology research to understand pre-existing immunity caused by antigens, baseline herd immunity, and their impact on vaccine efficacy, focusing on vaccine research for adults and the elderly, studying the mechanisms of immune aging regulating immune protection responses in the elderly [30]. Investigating changes in pathogen spectra of existing vaccines, new characteristics of epidemic trends; the impact of pre-existing immunity induced by vectors and antigens on vaccine protection; the relationship between pre-existing immunity and severe influenza infection; the relationship between low-level neutralizing antibodies induced by vaccination and pathogen variation, etc.
Establishing vaccine immune evaluation systems is an important step in identifying vaccines. It is necessary to strengthen the integration of basic and applied research to form new technologies, methods, and standards for vaccine evaluation. Exploring cell models and highly humanized animal models suitable for evaluating vaccine efficacy to enhance the value of efficacy data in animals. Finding alternative vaccine clinical evaluation methods through systems biology and other means [31]. Addressing major infectious diseases like influenza and AIDS requires discovering and determining immune epitopes that can induce broad-spectrum protection [32]. For new emerging infectious diseases, new strategies and systems for vaccine development are needed, focusing on establishing universal rapid vaccine platforms for quick preparation and application [33]. For persistent infections and tumors, developing therapeutic vaccines, exploring strategies to break immune tolerance and repair immune damage exhaustion, researching strategies for efficiently inducing T cell immune responses, and immunotherapy strategies for combined vaccine use. Researching new concept vaccines, such as polysaccharide vaccines and superbug vaccines, etc. [34].
7. Conclusion
Currently, original research on vaccines is limited, and the foundation for solving core technology issues is weak. Therefore, it is necessary to further refine scientific questions and strengthen basic research on vaccines. Based on the difficulties and challenges faced in vaccine research, future research should focus on the following directions: First, it is necessary to clarify the mechanisms of forming advantageous epitopes and protective epitopes of immunogens. This is crucial for improving the targeting and effectiveness of vaccines. At the same time, new adjuvants and carriers should be developed, and their mechanisms of action on immune responses should be elucidated. Exploring the nature of immune memory responses and the techniques for inducing long-term immune responses, particularly focusing on mucosal immunity and regional immune protection, to enhance immune responses at pathogen invasion sites or disease occurrence sites. Second, key technological breakthroughs in the evaluation system of immune protective effects of vaccines are necessary. Deepening vaccine epidemiology research to clarify the influence of individual factors, pre-existing immunity, and ecological environments, and strengthening joint research between clinical vaccines and immunology to improve the practical application effects of vaccines.
To ensure the feasibility and evaluation efficiency of vaccine evaluation data, establishing vaccine evaluation platforms and maintaining corresponding mechanisms is essential. This includes establishing primate and highly humanized animal models and clinical GLP laboratories. Additionally, building vaccine research resource banks and establishing mechanisms for connecting vaccine research with the industry to promote vaccine translation and application. Strengthening the development of vaccines for major diseases (such as viruses, bacteria, tumors, metabolic diseases), vaccines for specific target populations (such as vaccines for the elderly), superbug control vaccines, and rapid emergency vaccines is also necessary. This will help improve the application effects of vaccines in different diseases and populations, comprehensively advancing vaccine technology development.
References
[1]. GAVI. (2021). What are protein subunit vaccines and how could they be used against COVID-19? Archived from the original.
[2]. World Health Organization. (2021). Module 2 - Subunit vaccines. WHO Vaccine Safety Basics e-learning course. Archived from the original.
[3]. Francis, M. J. (2018). Recent Advances in Vaccine Technologies. The Veterinary Clinics of North America. Small Animal Practice, Vaccines and Immunology, 48(2), 231–241.
[4]. Lidder, P., & Sonnino, A. (2012). Biotechnologies for the management of genetic resources for food and agriculture. Advances in Genetics, 78, 1–167.
[5]. Innis, B. L., Snitbhan, R., Kunasol, P., et al. (1994). Protection Against Hepatitis A by an Inactivated Vaccine. JAMA, 271(17), 1328–1334.
[6]. Osman, M., Park, S.L., & Mackay, L.K. (2023). Tissue-resident memory T (TRM) cells: Front-line workers of the immune system. European Journal of Immunology, 1(4), e2250060.
[7]. Gebhardt, T., Wakim, L.M., Eidsmo, L., et al. (2009). Memory T cells in nonlymphoid tissue that provide enhanced local immunity during infection with herpes simplex virus. Nature Immunology, 10(5), 524-530.
[8]. Mueller, S.N., & Mackay, L.K. (2016). Tissue-resident memory T cells: Local specialists in immune defence. Nature Reviews Immunology, 16(2), 79-89.
[9]. Ariotti, S., Hogenbirk, M.A., Dijkgraaf, F.E., et al. (2014). T cell memory, Skin-resident memory CD8+ T cells trigger a state of tissue-wide pathogen alert. Science, 346(6205), 101-105.
[10]. Nguyen, Q.P., Deng, T.Z., Witherden, D.A., et al. (2019). Origins of CD4+ circulating and tissue-resident memory T-cells. Immunology, 157(1), 3-12.
[11]. Mori, T., Shugo, S., et al. (2021). Tertiary lymphoid structures show infiltration of effective tumor-resident T cells in gastric cancer. Cancer Science, 112(5), 1746-1757.
[12]. Nizard, M., Hélène, R., & Eric, T. (2016). Resident memory T cells as surrogate markers of the efficacy of cancer vaccines. Clinical Cancer Research, 22(3), 530-532.
[13]. Chang, J.T., Wherry, E.J., & Goldrath, A.W. (2014). Molecular regulation of effector and memory T cell differentiation. Nature Immunology, 15(12), 1104-1115.
[14]. Rapharl, I., Joern, R.R., & Forsthuber, T.G. (2020). Memory CD4(+) T cells in immunity and autoimmune diseases. Cells, 9(3), 531.
[15]. Khoshnood, S., Arshadi, M., Akrami, S., et al. (2022). An overview on inactivated and live-attenuated SARS-CoV-2 vaccines. Journal of Clinical Laboratory Analysis, 36(5), e24418.
[16]. Wang, Y., Yang, C., Song, Y., et al. (2021). Scalable live-attenuated SARS-CoV-2 vaccine candidate demonstrates preclinical safety and efficacy. Proceedings of the National Academy of Sciences USA, 118(29), e2102775118.
[17]. Heine, A., Juranek, S., & Brossart, P. (2021). Clinical and immunological effects of mRNA vaccines in malignant diseases. Molecular Cancer, 20(1), 52.
[18]. Chaudhary, N., Weissman, D., & Whitehead, K.A. (2021). mRNA vaccines for infectious diseases: principles, delivery and clinical translation. Nature Reviews Drug Discovery, 20(11), 817-838.
[19]. Klein, N.P., Lewis, N., Goddard, K., et al. (2021). Surveillance for adverse events after COVID-19 mRNA vaccination. JAMA, 326(14), 1390-1399.
[20]. Barda, N., Dagan, N., Ben-Shlomo, Y., et al. (2021). Safety of the BNT162b2 mRNA Covid-19 vaccine in a nationwide setting. New England Journal of Medicine, 385(12), 1078-1090.
[21]. Boettler, T., Csernalabics, B., Sali, E.H., et al. (2022). SARS-CoV-2 vaccination can elicit a CD8 T-cell dominant hepatitis. Journal of Hepatology, 77(3), 653-659.
[22]. Tseng, C.T., Sbrana, E., Iwata-Yoshikawa, N., et al. (2012). Immunization with SARS coronavirus vaccines leads to pulmonary immunopathology on challenge with the SARS virus. PLOS One, 7(4), e35421.
[23]. Yadav, T., Srivastava, N., Mishra, G., et al. (2020). Recombinant vaccines for COVID-19. Human Vaccines & Immunotherapeutics, 16(12), 2905-2912.
[24]. Lundstrom, K. (2021). Viral vectors for COVID-19 vaccine development. Viruses, 13(2), 317.
[25]. Callaway, E. (2020). The race for coronavirus vaccines: a graphical guide. Nature, 580(7805), 576-577.
[26]. Kang, Z., & Tang, M. (2020). Progress and analysis on the development of 2019-nCoV vaccine. Sheng Wu Yi Xue Gong Cheng Xue Za Zhi, 37(3), 373-379. Chinese.
[27]. Hartmann, G. (2017). Nucleic acid immunity. Advances in Immunology, 133, 121-169.
[28]. Feng, X., Su, Z.G., Cheng, Y., et al. (2023). Messenger RNA chromatographic purification: advances and challenges. Journal of Chromatography A, 1707, 464321.
[29]. Thess, A., Grund, S., Mui, B.L., et al. (2015). Sequence-engineered mRNA without chemical nucleoside modifications enable effective protein therapy in large animals. Molecular Therapy, 23(9), 1456-1464.
[30]. Keener, A. (2019). Tailoring vaccines for older people and the very young. Nature, 575(7784), S48-S50.
[31]. Cotugno, N., Ruggiero, A., Santilli, V., et al. (2019). OMIC Technologies and vaccine development: from the identification of vulnerable individuals to the formulation of invulnerable vaccines. Journal of Immunology Research, 2019, 8732191.
[32]. Burton, D.R. (2019). Advancing an HIV vaccine; advancing vaccinology. Nature Reviews Immunology, 19(2), 77-78.
[33]. Gilbert, S.C., & Warimwe, G.M. (2017). Rapid development of vaccines against emerging pathogens: the replication-deficient simian adenovirus platform technology. Vaccine, 35(35 Part A), 4461-4464.
[34]. Jansen, K.U., Knirsch, C., & Anderson, A.S. (2018). The role of vaccines in preventing bacterial antimicrobial resistance. Nature Medicine, 24(1), 10-19.
Cite this article
Yan,N. (2024). Advances in Vaccine Development: A Comprehensive Review of Technological Progress and Challenges. Theoretical and Natural Science,61,45-58.
Data availability
The datasets used and/or analyzed during the current study will be available from the authors upon reasonable request.
Disclaimer/Publisher's Note
The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of EWA Publishing and/or the editor(s). EWA Publishing and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
About volume
Volume title: Proceedings of the 4th International Conference on Biological Engineering and Medical Science
© 2024 by the author(s). Licensee EWA Publishing, Oxford, UK. This article is an open access article distributed under the terms and
conditions of the Creative Commons Attribution (CC BY) license. Authors who
publish this series agree to the following terms:
1. Authors retain copyright and grant the series right of first publication with the work simultaneously licensed under a Creative Commons
Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this
series.
2. Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the series's published
version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial
publication in this series.
3. Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and
during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See
Open access policy for details).
References
[1]. GAVI. (2021). What are protein subunit vaccines and how could they be used against COVID-19? Archived from the original.
[2]. World Health Organization. (2021). Module 2 - Subunit vaccines. WHO Vaccine Safety Basics e-learning course. Archived from the original.
[3]. Francis, M. J. (2018). Recent Advances in Vaccine Technologies. The Veterinary Clinics of North America. Small Animal Practice, Vaccines and Immunology, 48(2), 231–241.
[4]. Lidder, P., & Sonnino, A. (2012). Biotechnologies for the management of genetic resources for food and agriculture. Advances in Genetics, 78, 1–167.
[5]. Innis, B. L., Snitbhan, R., Kunasol, P., et al. (1994). Protection Against Hepatitis A by an Inactivated Vaccine. JAMA, 271(17), 1328–1334.
[6]. Osman, M., Park, S.L., & Mackay, L.K. (2023). Tissue-resident memory T (TRM) cells: Front-line workers of the immune system. European Journal of Immunology, 1(4), e2250060.
[7]. Gebhardt, T., Wakim, L.M., Eidsmo, L., et al. (2009). Memory T cells in nonlymphoid tissue that provide enhanced local immunity during infection with herpes simplex virus. Nature Immunology, 10(5), 524-530.
[8]. Mueller, S.N., & Mackay, L.K. (2016). Tissue-resident memory T cells: Local specialists in immune defence. Nature Reviews Immunology, 16(2), 79-89.
[9]. Ariotti, S., Hogenbirk, M.A., Dijkgraaf, F.E., et al. (2014). T cell memory, Skin-resident memory CD8+ T cells trigger a state of tissue-wide pathogen alert. Science, 346(6205), 101-105.
[10]. Nguyen, Q.P., Deng, T.Z., Witherden, D.A., et al. (2019). Origins of CD4+ circulating and tissue-resident memory T-cells. Immunology, 157(1), 3-12.
[11]. Mori, T., Shugo, S., et al. (2021). Tertiary lymphoid structures show infiltration of effective tumor-resident T cells in gastric cancer. Cancer Science, 112(5), 1746-1757.
[12]. Nizard, M., Hélène, R., & Eric, T. (2016). Resident memory T cells as surrogate markers of the efficacy of cancer vaccines. Clinical Cancer Research, 22(3), 530-532.
[13]. Chang, J.T., Wherry, E.J., & Goldrath, A.W. (2014). Molecular regulation of effector and memory T cell differentiation. Nature Immunology, 15(12), 1104-1115.
[14]. Rapharl, I., Joern, R.R., & Forsthuber, T.G. (2020). Memory CD4(+) T cells in immunity and autoimmune diseases. Cells, 9(3), 531.
[15]. Khoshnood, S., Arshadi, M., Akrami, S., et al. (2022). An overview on inactivated and live-attenuated SARS-CoV-2 vaccines. Journal of Clinical Laboratory Analysis, 36(5), e24418.
[16]. Wang, Y., Yang, C., Song, Y., et al. (2021). Scalable live-attenuated SARS-CoV-2 vaccine candidate demonstrates preclinical safety and efficacy. Proceedings of the National Academy of Sciences USA, 118(29), e2102775118.
[17]. Heine, A., Juranek, S., & Brossart, P. (2021). Clinical and immunological effects of mRNA vaccines in malignant diseases. Molecular Cancer, 20(1), 52.
[18]. Chaudhary, N., Weissman, D., & Whitehead, K.A. (2021). mRNA vaccines for infectious diseases: principles, delivery and clinical translation. Nature Reviews Drug Discovery, 20(11), 817-838.
[19]. Klein, N.P., Lewis, N., Goddard, K., et al. (2021). Surveillance for adverse events after COVID-19 mRNA vaccination. JAMA, 326(14), 1390-1399.
[20]. Barda, N., Dagan, N., Ben-Shlomo, Y., et al. (2021). Safety of the BNT162b2 mRNA Covid-19 vaccine in a nationwide setting. New England Journal of Medicine, 385(12), 1078-1090.
[21]. Boettler, T., Csernalabics, B., Sali, E.H., et al. (2022). SARS-CoV-2 vaccination can elicit a CD8 T-cell dominant hepatitis. Journal of Hepatology, 77(3), 653-659.
[22]. Tseng, C.T., Sbrana, E., Iwata-Yoshikawa, N., et al. (2012). Immunization with SARS coronavirus vaccines leads to pulmonary immunopathology on challenge with the SARS virus. PLOS One, 7(4), e35421.
[23]. Yadav, T., Srivastava, N., Mishra, G., et al. (2020). Recombinant vaccines for COVID-19. Human Vaccines & Immunotherapeutics, 16(12), 2905-2912.
[24]. Lundstrom, K. (2021). Viral vectors for COVID-19 vaccine development. Viruses, 13(2), 317.
[25]. Callaway, E. (2020). The race for coronavirus vaccines: a graphical guide. Nature, 580(7805), 576-577.
[26]. Kang, Z., & Tang, M. (2020). Progress and analysis on the development of 2019-nCoV vaccine. Sheng Wu Yi Xue Gong Cheng Xue Za Zhi, 37(3), 373-379. Chinese.
[27]. Hartmann, G. (2017). Nucleic acid immunity. Advances in Immunology, 133, 121-169.
[28]. Feng, X., Su, Z.G., Cheng, Y., et al. (2023). Messenger RNA chromatographic purification: advances and challenges. Journal of Chromatography A, 1707, 464321.
[29]. Thess, A., Grund, S., Mui, B.L., et al. (2015). Sequence-engineered mRNA without chemical nucleoside modifications enable effective protein therapy in large animals. Molecular Therapy, 23(9), 1456-1464.
[30]. Keener, A. (2019). Tailoring vaccines for older people and the very young. Nature, 575(7784), S48-S50.
[31]. Cotugno, N., Ruggiero, A., Santilli, V., et al. (2019). OMIC Technologies and vaccine development: from the identification of vulnerable individuals to the formulation of invulnerable vaccines. Journal of Immunology Research, 2019, 8732191.
[32]. Burton, D.R. (2019). Advancing an HIV vaccine; advancing vaccinology. Nature Reviews Immunology, 19(2), 77-78.
[33]. Gilbert, S.C., & Warimwe, G.M. (2017). Rapid development of vaccines against emerging pathogens: the replication-deficient simian adenovirus platform technology. Vaccine, 35(35 Part A), 4461-4464.
[34]. Jansen, K.U., Knirsch, C., & Anderson, A.S. (2018). The role of vaccines in preventing bacterial antimicrobial resistance. Nature Medicine, 24(1), 10-19.









