1. Introduction
Approximately 18.6 million people die annually from cardiovascular diseases (CVD), accounting for 17.4% of global deaths [1]. The issue is particularly severe in low- and middle-income countries, where about a quarter of all CVD deaths occur [2]. In the United States, CVD is the leading cause of death, responsible for 22% of all deaths in 2019. The cost was $311 billion in 2015, with projections reaching $368 billion by 2035 [3,4]. A heart attack occurs every 40 seconds, totaling around 805,000 incidents annually [5]. These statistics underscore the importance of early intervention and effective treatment.
Common risk prediction models include the Cox Proportional Hazards model and the Framingham Risk Score. These models quantify the impact of risk factors, providing evidence for reducing modifiable risks [5,7]. However, they have limitations in accounting for complex or novel risks [8,10,11].
This paper reviews deep learning techniques used in developing CVD detection systems. Deep learning models outperform traditional methods by handling large-scale data, uncovering complex relationships, and making specific predictions. For example, Xu & Liu [19]achieved 87% classification accuracy for 22 types of arrhythmias using a pretrained CNN. Similarly, Lee et al.[25] developed a multimodal model combining DenseNet-169 for retinal fundus images with a neural network for clinical risk factors, using data from Samsung Medical Center and the UK Biobank.
We critically review existing DL paradigms for predicting heart diseases, covering DL model performance on benchmark datasets, major technical challenges, and promising solutions or research directions.
2. Current Research Status on Cardiovascular Disease Prediction
2.1. Prediction Methods Based on Traditional Statistical Learning and Limitations
Cardiovascular disease prediction has long relied on traditional statistical methodologies, such as regression and survival analysis, as foundational tools for assessing prognosis. The Cox Proportional Hazards Model (Cox PH) [5], a statistical model, identifies independent prognostic indicators for time-to-event endpoints. It is widely used in survival statistics for handling censored data and modeling hazard ratios of covariates. The model estimates hazard ratios as a function of covariates and survival time through a baseline hazard function. Coefficients (β) calculate the hazard ratio for each variable, handling right-censored data where event times are unknown due to study termination or incomplete follow-up. However, the Cox PH model is limited to scenarios with no interaction between time and treatment, restricting its use in analyzing time-dependent effects [7]. Additionally, as an additive and linear risk model, it struggles with incorporating complex or novel risk factors, inadequately accounting for nonlinear interactions between covariates, which can affect event risk prediction [8].
The Framingham Risk Score (FRS) [7], developed from the Framingham Heart Study, estimates the 10-year probability of cardiovascular disease based on traditional risk factors like age, sex, blood pressure, cholesterol levels, smoking status, and diabetes. However, it may inaccurately assess risk among non-Caucasian groups and cannot predict changes in lifestyle, medical therapy, or CVD prevalence over time [10,11].
2.2. Prediction Methods Based on Machine Learning and Limitations
Machine learning methods, such as logistic regression, support vector machines (SVM) and random forests etc., excel in handling noisy or high-dimensional data, making them valuable for CVD risk prediction. Support Vector Machines (SVMs) are effective in classification tasks but are computationally intensive and sensitive to parameter changes, requiring careful tuning and substantial computational resources [12,13]. Weng et al. and Dinesh et al. demonstrated high accuracy of SVMs in CVD risk prediction, emphasizing the necessity for extensive parameter tuning [12,13].
Random Forests (RF) [12,13] combine multiple decision trees to reduce overfitting and improve robustness, but they are computationally intensive, especially with large-scale data, resulting in longer training times and higher costs. Dinesh et al. achieved 85% accuracy in CVD risk prediction using RFs but noted the significant computational cost [13]. While ML methods address some limitations of traditional models, clinical application requires overcoming significant challenges. Recent advances in deep learning offer potential solutions to enhance CVD prediction models.
2.3. Development of prediction methods based on deep learning
2.3.1. Early Development and Foundational Research. Although deep learning was conceptualized in the 1980s, substantial progress began only in the early 2000s. The field remained largely dormant until 2006 when it was demonstrated that, with suitable choices of functions and architecture, neural networks outperformed shallow algorithms for certain high-dimensional problems [14]. Convolutional Neural Networks (CNNs), initially researched for digit recognition by LeCun et al. [15], saw significant advancements in image processing, establishing the foundation for modern applications [16].
2.3.2. Deep learning in medical imaging. The boost in computational power has been crucial in allowing deep learning to scale from impressive ideas to practical deployments. CNNs are particularly advantageous in the pre-processing of images, including medical image analysis. Fully connected networks were applied to analyze X-ray, CT, and MRI images of the heart, outperforming classical methods in predicting cardiovascular diseases [16].
2.3.3. Building Deep Learning Models for Cardiovascular Disease Prediction. Among some of the early applications for deep learning toward cardiovascular disease prediction were those based on electrophysiological data, namely electrocardiographic data. Researchers have developed CNNs to predict heart disease from raw ECG data, capitalizing on the inherent characteristics of the CNN for feature extraction and classification: a simple 1D CNN was applied to a raw ECG and classified the ECG for presence of cardiac illness. Similarly, CNNs have been used to classify arrhythmia signals that contain complex physiological signatures, highlighting utility for problems where order relationships are important. In brief for sequence data, RNNs are particularly useful because of their relative capability to process a temporal sequence better than other models [17].
Table 1. Comparison of Cardiovascular Disease Prediction Methods
Method | Advantages | Disadvantages |
Cox Proportional Hazards Model (Cox PH) | Handles censored data, models hazard ratios | Limited interaction between time and treatment, inadequate for complex risks |
Framingham Risk Score (FRS) | Estimates 10-year CVD probability | Inaccurate for non-Caucasian groups, cannot predict lifestyle changes |
Support Vector Machines (SVM) | Good classification performance, suitable for small datasets | Computationally intensive, sensitive to parameter changes |
Random Forests (RF) | Reduces overfitting, handles complex patterns | High computational cost, less interpretable than single decision trees |
Convolutional Neural Networks (CNN) | Automatic feature extraction, excellent for image data | Requires large computational resources, long training times |
Recurrent Neural Networks (RNN) | Excellent for processing temporal sequences | High computational cost, prone to gradient vanishing issues |
2.3.4. Recent Developments. The growth in the sophistication of new deep learning fitting techniques incorporated into deep learner models over the past years is best reflected by the new iterations of the Inception CNN and the Hybrid ECG model – a CNN-classifier being first connected to an LSTM network, and then to a Naïve Bayes classifier and finally to a Federated Learning model. Such hybrid modelling approaches incorporate the benefits of new techniques while compensating for their shortcomings: the ECG model exploits the model of a CNN to learn spatial features, integrates the LSTM as a strong temporal learning model to help stabilize the spatial learner and adds classical classification algorithms and Federated Learning to compensate for known weaknesses of CNN methods [18].
2.3.5. Summary of Risk Prediction Methods: Current risk prediction methods like the Cox Proportional Hazards (Cox PH) model and the Framingham Risk Score (FRS) provide valuable insights but struggle with complex, heterogeneous data. Machine learning methods, such as Support Vector Machines (SVM) and Random Forests (RF), offer improved performance with high-dimensional data but require significant computational resources. In contrast, deep learning (DL) models, including Convolutional Neural Networks (CNNs) and Recurrent Neural Networks (RNNs), excel at handling large-scale, granular data and uncovering complex relationships among risk factors. DL models enhance prediction accuracy and are well-suited for complex CVD prediction tasks. Consequently, DL models are becoming essential tools for advancing cardiovascular disease detection and prevention(see Table1).
3. Deep learning models
Hierarchical multi-layer neural networks form the foundation of deep learning. Model parameters are adjusted to minimize the loss function using techniques such as back propagation and gradient descent. There are two major types of deep learning technology: CNN and RNN, which are used to handle image data and sequential data respectively.
3.1. Network Structure, and Advantages/Disadvantages of CNNs
CNNs are specifically designed for processing images, unlike generic algorithms used for other machine learning problems [19][20][21]. A CNN consists of multiple layers of neurons that learn features at increasing levels of detail. Convolutional layers generate feature maps through filters applied to input images, using weight sharing and local connectivity to focus on important features, reducing the number of parameters and enhancing efficiency. Fully connected layers link every neuron to the next layer, learning high-level feature combinations to produce the final output (see Figure 1)
CNNs learn features from raw input data, use parameter sharing to reduce weights, and incorporate pooling layers to maintain translations invariance, preserving relevant features. This makes CNNs effective for image data, capable of recognizing multiple patterns. Training deep CNNs with multiple layers is slow and computationally expensive, requiring high-performance computing resources [19]. CNNs demand large volumes of labeled data, challenging to obtain in some domains [20]. The complex inner workings of CNNs make them opaque and difficult to interpret, often referred to as "black boxes." problematic in clinical applications where trust in predictions is crucial [21].
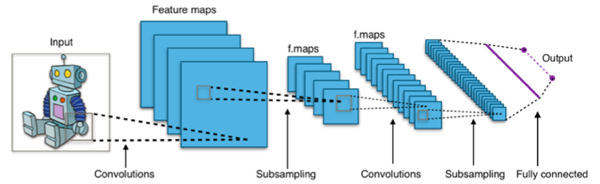
Figure 1. The structure and workflow of a Convolutional Neural Network (CNNs).
3.2. Network Structure, and Advantages/Disadvantages of RNNs
Recurrent Neural Networks (RNNs) excel in processing sequence data such as time series, language, or speech. Unlike traditional neural networks, RNNs have recurrent layers that enable sequential learning and capture temporal dependencies. Multiple hidden layers can be used to extract complex patterns from sequential data, with the final output layer predicting or classifying the processed data (see Figure 2).
RNNs can learn temporal dependencies from sequential data, which is critical for problems such as language modeling or time series prediction [22]. However, RNNs are limited due to their need for sequential activation, making training slow and computationally expensive [23]. Additionally, RNNs suffer from vanishing/exploding gradients, hindering long-term dependency capture. Standard RNNs fail to capture long-term dependencies but advanced variants like Long Short-Term Memory (LSTM) or Gated Recurrent Units (GRU) are helpful [24]. Moreover, RNNs are inherently black box models making them difficult to interpret, which is problematic in scenarios requiring transparency [25].
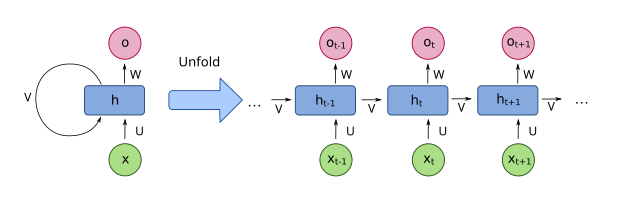
Figure 2. The structure and workflow of a Recurrent Neural Networks (RNNs).
4. Typical application cases
Case 1: Variants of Convolutional Neural Network (CNN) for ECG Arrhythmia Classification. In their model [19], they used Convolutional Neural Network (CNN) for classifying ECG arrhythmia, achieving an accuracy of 99.43% on the MIT-BIH arrhythmia dataset. This high sensitivity was evident when morphological features of ECG were considered, demonstrating the potential of CNNs for accurate clinical classification of arrhythmias.
Jun et al. [20] proposed a 2-D CNN for classifying ECG arrhythmia using 128x128 grayscale images. The CNN model was optimized using Xavier initialization, data augmentation, and batch normalization, achieving an average accuracy of 99.05%, outperforming other CNN models like Alex Net and VGG Net. This shows the utility of deep learning in medical monitoring and diagnosis.
Çınar and Tuncer [21] developed a hybrid deep learning model, incorporating an SVM module and stacking Long Short-Term Memory (LSTM) layers atop Alex Net-SVM. The hybrid system achieved an accuracy of 96.77% for arrhythmias (ARR) and congestive heart failure (CHF) and 100% for normal sinus rhythm (NSR). The precision, recall, and F1-score for arrhythmias were 1.0, 0.95, and 0.97, respectively, indicating robust discriminatory performance.
Case 2: Variants of Recurrent Neural Network (RNN) for Heart Failure Prediction. Another approach is to employ a GRU-based RNN such as the one employed by Chen et al [22] employed a GRU-based RNN on longitudinal EHR data to predict impending heart failure. The RNN model outperformed standard models (logistic regression and random forests) on multiple data domains. For example, the RNN had an AUC of 0.689 in the medication domain, significantly higher than logistic regression (AUC = 0.581) and random forests (AUC = 0.617). Aggregated over all domains, the RNN model achieved an AUC of 0.791, demonstrating the potential of RNNs for early intervention in heart failure.
Kaya et al. [23] proposed a hybrid method combining Angle Transformation (AT) and new LSTM networks for ECG classification, achieving a success rate of 98.97% on a 70-30 train-test split of the MIT-BIH Arrhythmia Database and BIDMC Congestive Heart Failure Database. This method can achieve high accuracy without direct patient contact, relieving clinical physician pressure and optimizing hospital efficiency.
Table 2. Summary of Deep Learning Models for Cardiovascular Disease Prediction
Study | Model Type | Dataset | Accuracy |
Xu and Liu [19] | CNN | MIT-BIH arrhythmia | 99.43% |
Jun et al.[20] | 2-D CNN | MIT-BIH arrhythmia | 99.05% |
Çınar and Tuncer [21] | Hybrid LSTM-CNN-SVM | Arrhythmia,CHF,NSR | Accuracy: 96.77% (ARR, CHF), 100% (NSR) |
Chen et al. [22] | GRU-RNN | Sutter Palo Alto Medical Foundation | AUC: 0.791 (combined domains) AUC: 0.689 (medication domain) Outperformed random forests |
Kaya et al. [23] | Hybrid AT-LSTM | MIT-BIH and BIDMC | 98.97% |
Bagheri et al. [24] | Multimodal BiLSTM | Second Manifestations of ARTerial disease,SMART | AUC 0.847, F1 83.8% |
Lee et al. [25] | Multimodal DenseNet-169 | Samsung Medical Center and UK Biobank | AUROC 0.781 (SMC), 0.872 (UK Biobank) |
Case 3: Multimodal Deep Learning Cardiovascular Disease Prediction. Bagheri et al. [24] used multimodal BiLSTM model to predict major adverse cardiovascular events (MACE) by incorporating structured and unstructured electronic health record(EHR)data respectively. The model using clinical variables and radiology reports had an AUC of 0.847 with a misclassification rate of 14% This situation further highlights the importance of multimodal learning in managing cardiovascular risk and improving patient outcomes.
Lee et al. [25] using a deep learning model that combined structured clinical risk factors and retinal fundus photographs, demonstrated the ability to predict cardiovascular disease A model using DenseNet-169 for fundus image analysis and a five-layer fully connected network for clinical risk factors achieved AUROCs of 0.781 on Samsung Medical Center data, as well as up to 0.872 on UK Biobank data. This illustrates that combining non-invasive imaging with clinical variables provides strong prognostic strength.
Deep learning models, such as CNNs and RNNs, demonstrate high accuracy in processing complex medical data, particularly ECG signals. They are effective in classifying arrhythmias, predicting heart failure, and forecasting major adverse cardiovascular events. Combining various data types in multimodal approaches enhances predictive capability. Despite differences in model structures, datasets, and prediction objectives, deep learning's flexibility and versatility are evident in cardiovascular research. These findings highlight the importance of selecting suitable deep learning architectures and datasets to maximize the potential of this method in improving cardiovascular health outcomes.
5. Challenges, Limitations and Future Directions
5.1. Data-Related Challenges
Data Quantity and Diversity Deep learning models, particularly RNNs and LSTMs, necessitate extensive datasets to achieve statistically stable results. According to Chen et al.[22], data quantity is crucial for model performance. Kaya et al. [23] emphasized the necessity of large and diverse datasets for their LSTM recurrent neural networks.
Data Source and Generalizability Models trained on single-center datasets often lack generalizability. Lee et al.[25] indicated that such models may exhibit geographic bias. Additionally, ECG systems vary based on the recording devices used, which raises concerns about dataset provenance and quality. This presents significant challenges for data generalizability.
5.2. Model-Related Challenges
Computational and Training Complexity The computational resource budget is a major issue for LSTMs and CNNs. Training these models is particularly difficult and time-consuming, especially during the parameter optimization phase [21,23].
Overfitting and Generalization Deep learning models tend to overfit to training data, focusing on noise rather than meaningful patterns. This major pitfall was highlighted by [21]. The importance of generalization was emphasized by Lee et al. [25] and Xu and Liu [19], as models must generalize well to new data to be useful.
Interpretability and Transparency Original deep learning models, including RNNs, function as “black boxes,” making them inherently difficult to interpret. Chen et al.[22] noted that the lack of interpretability is a significant concern for users. Jun et al.[20] emphasized the necessity of developing techniques to enhance model transparency.
5.3. Future Directions
Enhanced Data Representation Future experiments with various data representations will lead to improved preprocessing techniques. Chen et al. [22] indicated that new data sources, such as claims data and geographic information systems, can enhance data representation diversity. Lee et al.[25] also recommended integrating additional sources, such as socioeconomic status and environmental factors, to improve predictive outcomes.
Model Comparison and Hybridization Analyzing different deep learning models with various hyperparameters allows for selecting the best-fitting models for specific tasks. Xu and Liu [19] employed hybrid models combining CNNs and LSTMs to improve performance, an approach also utilized by Çınar and Tuncer [21] The future use of Auto ML for further model development would be beneficial.
Clinical Application and Explainable AI A primary objective for developing deep learning models is to design and implement robust explainable AI approaches. Bagheri et al.[24] noted that explainable AI would enhance the clinical relevance and utility of disease predictions. Additionally, integrating these models into real-time patient monitoring systems is a future research direction [19].
Extending to Other Diseases The developed techniques and models can be applied to predict the occurrence of other diseases. Common cohorts for different diseases can recalibrate models and facilitate transfer learning. Chen et al. [22] emphasized maximizing the utility of disease-based models as a significant future research direction.
6. Conclusion
Deep learning is now one of the most robust methods to predict the development of cardiovascular disease. To summaries, this review tried to depict the recent advances made with deep learning for CVD risk prediction and to highlight the challenges associated with model usability and applicability. Future research should concentrate on data representations, hybrid models, and improving explainable AI frameworks. Overcoming these challenges may lead to the adoption of deep learning models for use in clinics to improve CVD prediction and further help in patient care.
From my perspective, integrating diverse data sources, such as genetic information and environmental factors, is crucial for refining these models. In addition, data privacy and patient’s security during model development will be crucial aspects to consider. Adopting data efficiency, having a standardized data handling protocol and collaboration among academia, industry and healthcare key players, will enable the innovation and the substantial deal of translation to the real application of these models. By overcoming these challenges and putting more attention and efforts to the data representation, hybrid models and explainable AI frameworks, we can fully explore the potential of deep learning by improving health. This will enhance the whole cardiovascular health scenario, thus enabling more accurate and reliable models of great advantages for patient care.
References
[1]. Global Burden of Cardiovascular Diseases and Risk Factors, 1990–2019. Journal of the American College of Cardiology, 76, 2982 - 3021. https://doi.org/10.1016/j.jacc.2020.11.010 .
[2]. Bowry, A., Lewey, J., Dugani, S., & Choudhry, N. (2015). The Burden of Cardiovascular Disease in Low- and Middle-Income Countries: Epidemiology and Management.. The Canadian journal of cardiology, 31 9, 1151-9 . https://doi.org/10.1016/j.cjca.2015.06.028.
[3]. Mozaffarian, D., Benjamin, E., Go, A., Arnett, D., Blaha, M., Cushman, M., Ferranti, S., Després, J., Fullerton, H., Howard, V., Judd, S., Kissela, B., Lackland, D., Lichtman, J., Lisabeth, L., Liu, S., Mackey, R., Matchar, D., McGuire, D., Mohler, E., Moy, C., Muntner, P., Mussolino, M., Nasir, K., Neumar, R., Nichol, G., Palaniappan, L., Pandey, D., Reeves, M., Rodriguez, C., Sorlie, P., Stein, J., Towfighi, A., Turan, T., Virani, S., Willey, J., Woo, D., Yeh, R., & Turner, M. (2015). Heart Disease and Stroke Statistics – At-a-Glance. . https://doi.org/10.1161/CIR.0000000000000152 .
[4]. Dunbar, S. B., Khavjou, O. A., Bakas, T., Hunt, G., Kirch, R. A., Leib, A. R., Morrison, R. S., Poehler, D. C., Roger, V. L., Whitsel, L. P., & American Heart Association (2018). Projected Costs of Informal Caregiving for Cardiovascular Disease: 2015 to 2035: A Policy Statement from the American Heart Association. Circulation, 137(19), e558–e577. https://doi.org/10.1161/CIR.0000000000000570
[5]. Tsao, C. W., Aday, A. W., Almarzooq, Z. I., Anderson, C. A., Arora, P., Avery, C. L., ... & American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee. (2023). Heart disease and stroke statistics—2023 update: a report from the American Heart Association. Circulation, 147(8), e93-e621.
[6]. Cox, D. R. (1972). Regression models and lifetables (with discussion). Journal of the Royal Statistical Society: Series B (Methodological), 34(2), 187-202.
[7]. Hess, K. R. (1995). Graphical methods for assessing violations of the proportional hazards assumption in Cox regression. Statistics in medicine, 14(15), 1707-1723.
[8]. Heinzl, H., & Kaider, A. (1997). Gaining more flexibility in Cox proportional hazards regression models with cubic spline functions. Computer methods and programs in biomedicine, 54(3), 201-208.
[9]. D’Agostino RB, Vasan RS, Pencina MJ, Wolf PA, Cobain M, Massaro JM, et al. General cardiovascular risk profile for use in primary care: the Framingham Heart Study. Circulation. 2008; 117(6):743–753.
[10]. Brindle, P. M., McConnachie, A., Upton, M. N., Hart, C. L., Smith, G. D., & Watt, G. C. (2005). The accuracy of the Framingham risk-score in different socioeconomic groups: a prospective study. British Journal of General Practice, 55(520), 838-845.
[11]. Lloyd-Jones, D. M., Wilson, P. W., Larson, M. G., Beiser, A., Leip, E. P., D'Agostino, R. B., & Levy, D. (2004). Framingham risk score and prediction of lifetime risk for coronary heart disease. The American journal of cardiology, 94(1), 20-24
[12]. Dinesh, K. G., Arumugaraj, K., Santhosh, K. D., & Mareeswari, V. (2018, March). Prediction of cardiovascular disease using machine learning algorithms. In 2018 International Conference on Current Trends towards Converging Technologies (ICCTCT) (pp. 1-7). IEEE.
[13]. Weng, S., Reps, J., Kai, J., Garibaldi, J., & Qureshi, N. (2017). Can machine-learning improve cardiovascular risk prediction using routine clinical data?. PLoS ONE, 12. https://doi.org/10.1371/journal.pone.0174944.
[14]. Hinton, G. E., Osindero, S., & Teh, Y. W. (2006). A fast learning algorithm for deep belief nets. Neural computation, 18(7), 1527-1554.
[15]. Krizhevsky, A., Sutskever, I., & Hinton, G. E. (2017). ImageNet classification with deep convolutional neural networks. Communications of the ACM, 60(6), 84-90.
[16]. Shin, H. C., Roth, H. R., Gao, M., Lu, L., Xu, Z., Nogues, I., ... & Summers, R. M. (2016). Deep convolutional neural networks for computer-aided detection: CNN architectures, dataset characteristics and transfer learning. IEEE transactions on medical imaging, 35(5), 1285-1298.
[17]. Hannun, A. Y., Rajpurkar, P., Haghpanahi, M., Tison, G. H., Bourn, C., Turakhia, M. P., & Ng, A. Y. (2019). Cardiologist-level arrhythmia detection and classification in ambulatory electrocardiograms using a deep neural network. Nature medicine, 25(1), 65-69.
[18]. Rieke, N., Hancox, J., Li, W., Milletari, F., Roth, H. R., Albarqouni, S., ... & Cardoso, M. J. (2020). The future of digital health with federated learning. NPJ digital medicine, 3(1), 1-7.
[19]. Xu, X., & Liu, H. (2020). ECG heartbeat classification using convolutional neural networks. IEEE access, 8, 8614-8619.
[20]. Jun, T. J., Nguyen, H. M., Kang, D., Kim, D., Kim, D., & Kim, Y. H. (2018). ECG arrhythmia classification using a 2-D convolutional neural network. arXiv preprint arXiv:1804.06812.
[21]. Çınar, A., & Tuncer, S. A. (2021). Classification of normal sinus rhythm, abnormal arrhythmia and congestive heart failure ECG signals using LSTM and hybrid CNN-SVM deep neural networks. Computer methods in biomechanics and biomedical engineering, 24(2), 203-214.
[22]. Chen, R., Stewart, W. F., Sun, J., Ng, K., & Yan, X. (2019). Recurrent neural networks for early detection of heart failure from longitudinal electronic health record data: implications for temporal modeling with respect to time before diagnosis, data density, data quantity, and data type. Circulation: Cardiovascular Quality and Outcomes, 12(10), e005114.
[23]. Kaya, Y., Kuncan, F. & Tekin, R. A New Approach for Congestive Heart Failure and Arrhythmia Classification Using Angle Transformation with LSTM. Arab J Sci Eng 47, 10497–10513 (2022). https://doi.org/10.1007/s13369-022-06617-8
[24]. Bagheri, A., Groenhof, T. K. J., Veldhuis, W. B., de Jong, P. A., Asselbergs, F. W., & Oberski, D. L. (2020). Multimodal learning for cardiovascular risk prediction using EHR data.
[25]. Lee, Y. C., Cha, J., Shim, I., Park, W. Y., Kang, S. W., Lim, D. H., & Won, H. H. (2023). Multimodal deep learning of fundus abnormalities and traditional risk factors for cardiovascular risk prediction. npj Digital Medicine, 6(1), 14.
Cite this article
Wu,Y. (2024). Deep learning for cardiovascular disease prediction: Recent advances, challenges and future directions. Theoretical and Natural Science,62,24-32.
Data availability
The datasets used and/or analyzed during the current study will be available from the authors upon reasonable request.
Disclaimer/Publisher's Note
The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of EWA Publishing and/or the editor(s). EWA Publishing and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
About volume
Volume title: Proceedings of the 4th International Conference on Biological Engineering and Medical Science
© 2024 by the author(s). Licensee EWA Publishing, Oxford, UK. This article is an open access article distributed under the terms and
conditions of the Creative Commons Attribution (CC BY) license. Authors who
publish this series agree to the following terms:
1. Authors retain copyright and grant the series right of first publication with the work simultaneously licensed under a Creative Commons
Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this
series.
2. Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the series's published
version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial
publication in this series.
3. Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and
during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See
Open access policy for details).
References
[1]. Global Burden of Cardiovascular Diseases and Risk Factors, 1990–2019. Journal of the American College of Cardiology, 76, 2982 - 3021. https://doi.org/10.1016/j.jacc.2020.11.010 .
[2]. Bowry, A., Lewey, J., Dugani, S., & Choudhry, N. (2015). The Burden of Cardiovascular Disease in Low- and Middle-Income Countries: Epidemiology and Management.. The Canadian journal of cardiology, 31 9, 1151-9 . https://doi.org/10.1016/j.cjca.2015.06.028.
[3]. Mozaffarian, D., Benjamin, E., Go, A., Arnett, D., Blaha, M., Cushman, M., Ferranti, S., Després, J., Fullerton, H., Howard, V., Judd, S., Kissela, B., Lackland, D., Lichtman, J., Lisabeth, L., Liu, S., Mackey, R., Matchar, D., McGuire, D., Mohler, E., Moy, C., Muntner, P., Mussolino, M., Nasir, K., Neumar, R., Nichol, G., Palaniappan, L., Pandey, D., Reeves, M., Rodriguez, C., Sorlie, P., Stein, J., Towfighi, A., Turan, T., Virani, S., Willey, J., Woo, D., Yeh, R., & Turner, M. (2015). Heart Disease and Stroke Statistics – At-a-Glance. . https://doi.org/10.1161/CIR.0000000000000152 .
[4]. Dunbar, S. B., Khavjou, O. A., Bakas, T., Hunt, G., Kirch, R. A., Leib, A. R., Morrison, R. S., Poehler, D. C., Roger, V. L., Whitsel, L. P., & American Heart Association (2018). Projected Costs of Informal Caregiving for Cardiovascular Disease: 2015 to 2035: A Policy Statement from the American Heart Association. Circulation, 137(19), e558–e577. https://doi.org/10.1161/CIR.0000000000000570
[5]. Tsao, C. W., Aday, A. W., Almarzooq, Z. I., Anderson, C. A., Arora, P., Avery, C. L., ... & American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee. (2023). Heart disease and stroke statistics—2023 update: a report from the American Heart Association. Circulation, 147(8), e93-e621.
[6]. Cox, D. R. (1972). Regression models and lifetables (with discussion). Journal of the Royal Statistical Society: Series B (Methodological), 34(2), 187-202.
[7]. Hess, K. R. (1995). Graphical methods for assessing violations of the proportional hazards assumption in Cox regression. Statistics in medicine, 14(15), 1707-1723.
[8]. Heinzl, H., & Kaider, A. (1997). Gaining more flexibility in Cox proportional hazards regression models with cubic spline functions. Computer methods and programs in biomedicine, 54(3), 201-208.
[9]. D’Agostino RB, Vasan RS, Pencina MJ, Wolf PA, Cobain M, Massaro JM, et al. General cardiovascular risk profile for use in primary care: the Framingham Heart Study. Circulation. 2008; 117(6):743–753.
[10]. Brindle, P. M., McConnachie, A., Upton, M. N., Hart, C. L., Smith, G. D., & Watt, G. C. (2005). The accuracy of the Framingham risk-score in different socioeconomic groups: a prospective study. British Journal of General Practice, 55(520), 838-845.
[11]. Lloyd-Jones, D. M., Wilson, P. W., Larson, M. G., Beiser, A., Leip, E. P., D'Agostino, R. B., & Levy, D. (2004). Framingham risk score and prediction of lifetime risk for coronary heart disease. The American journal of cardiology, 94(1), 20-24
[12]. Dinesh, K. G., Arumugaraj, K., Santhosh, K. D., & Mareeswari, V. (2018, March). Prediction of cardiovascular disease using machine learning algorithms. In 2018 International Conference on Current Trends towards Converging Technologies (ICCTCT) (pp. 1-7). IEEE.
[13]. Weng, S., Reps, J., Kai, J., Garibaldi, J., & Qureshi, N. (2017). Can machine-learning improve cardiovascular risk prediction using routine clinical data?. PLoS ONE, 12. https://doi.org/10.1371/journal.pone.0174944.
[14]. Hinton, G. E., Osindero, S., & Teh, Y. W. (2006). A fast learning algorithm for deep belief nets. Neural computation, 18(7), 1527-1554.
[15]. Krizhevsky, A., Sutskever, I., & Hinton, G. E. (2017). ImageNet classification with deep convolutional neural networks. Communications of the ACM, 60(6), 84-90.
[16]. Shin, H. C., Roth, H. R., Gao, M., Lu, L., Xu, Z., Nogues, I., ... & Summers, R. M. (2016). Deep convolutional neural networks for computer-aided detection: CNN architectures, dataset characteristics and transfer learning. IEEE transactions on medical imaging, 35(5), 1285-1298.
[17]. Hannun, A. Y., Rajpurkar, P., Haghpanahi, M., Tison, G. H., Bourn, C., Turakhia, M. P., & Ng, A. Y. (2019). Cardiologist-level arrhythmia detection and classification in ambulatory electrocardiograms using a deep neural network. Nature medicine, 25(1), 65-69.
[18]. Rieke, N., Hancox, J., Li, W., Milletari, F., Roth, H. R., Albarqouni, S., ... & Cardoso, M. J. (2020). The future of digital health with federated learning. NPJ digital medicine, 3(1), 1-7.
[19]. Xu, X., & Liu, H. (2020). ECG heartbeat classification using convolutional neural networks. IEEE access, 8, 8614-8619.
[20]. Jun, T. J., Nguyen, H. M., Kang, D., Kim, D., Kim, D., & Kim, Y. H. (2018). ECG arrhythmia classification using a 2-D convolutional neural network. arXiv preprint arXiv:1804.06812.
[21]. Çınar, A., & Tuncer, S. A. (2021). Classification of normal sinus rhythm, abnormal arrhythmia and congestive heart failure ECG signals using LSTM and hybrid CNN-SVM deep neural networks. Computer methods in biomechanics and biomedical engineering, 24(2), 203-214.
[22]. Chen, R., Stewart, W. F., Sun, J., Ng, K., & Yan, X. (2019). Recurrent neural networks for early detection of heart failure from longitudinal electronic health record data: implications for temporal modeling with respect to time before diagnosis, data density, data quantity, and data type. Circulation: Cardiovascular Quality and Outcomes, 12(10), e005114.
[23]. Kaya, Y., Kuncan, F. & Tekin, R. A New Approach for Congestive Heart Failure and Arrhythmia Classification Using Angle Transformation with LSTM. Arab J Sci Eng 47, 10497–10513 (2022). https://doi.org/10.1007/s13369-022-06617-8
[24]. Bagheri, A., Groenhof, T. K. J., Veldhuis, W. B., de Jong, P. A., Asselbergs, F. W., & Oberski, D. L. (2020). Multimodal learning for cardiovascular risk prediction using EHR data.
[25]. Lee, Y. C., Cha, J., Shim, I., Park, W. Y., Kang, S. W., Lim, D. H., & Won, H. H. (2023). Multimodal deep learning of fundus abnormalities and traditional risk factors for cardiovascular risk prediction. npj Digital Medicine, 6(1), 14.









