1. Introduction
Nitrosamine chemical N-nitrosodimethylamine (NDMA) has attracted vast attention worldwide after it was detected in the Valsartan (Valsartan) category of antihypertensive medicines in 2018 and many medicines have been recalled for its reasons [1-2]. In this regard, further investigations by the U.S. Food and Drug Administration (FDA), the European Medicines Agency (EMA) and other agencies revealed that other nitrosamines such as NEDA also have the potential to be involved in other sartans [3-5]. Random samples of sartans were tested in the laboratory of the German Drug Center and it was found that NDMA was present in the range of 3.7-22.0 micrograms per capsule [4]. Nitrosamines are a type of mutagenic carcinogenic chemicals, which are mainly derived from food, environment and drug synthesis by-products [5-7]. Experimentally, NDMA and other nitrosamines have been demonstrated to cause tumors in almost all organs, and they are carcinogenic to rats, hamsters, guinea pigs, and rabbits regardless of the mode of exposure [5]. Thus, researchers in various countries began to establish analytical controls for the risk evaluation and analysis of nitrosamine byproducts that may occur in current and new drugs [8-9]. In this paper, the common types of nitrosamines, sources, and risk evaluation methods are described by means of review and theoretical analysis. It aims to clarify the effective analytical methods for nitrosamines, thus helping laboratories and pharmaceutical manufacturers in various countries to detect and analyze nitrosamines in finished products.
2. Nitrosamines in drugs
2.1. Source
Nitrosamines are a type of chemical compound with nitroso groups, the occurrence of nitrosames in the drugs can be found under the following six circumstances. Firstly, during the synthesis of Active Pharmaceutical Ingredient (APIs), due to the raw materials containing amine compounds and nitrite ions, these compounds undergo chemical reaction in acidic conditions to form nitrosamine byproducts. Secondly, the API generates nitrosamines under certain conditions, such as pyrolysis. Thirdly, nitrosamine chemicals are derived from impurities in raw materials, solvents, reagents, catalysts and intermediate products. In addition, impurities in water, auxiliaries and facilitators during the production of finished pharmaceuticals are also a source of nitrosamine chemicals. Fifth, certain reaction conditions during the manufacture of the finished pharmaceutical product result in the formation of nitrosamines from the reaction of the precursor substances. Finally, drug packaging systems contain amine and nitrite ions that form nitrosamine impurities [8-9].
The reaction equation for nitrosamine formation is as follows:
H+ + NO2 -+ R1-NH-R2 = R1-N2O-R2 +H2O
The nitrogen in the nitroso group in nitrosamines connects two organic chains, and changes in the length of the two organic chains and functional groups lead to changes in their toxicity [9]. Currently nitrosamines are categorized into two main groups: small molecule nitrosamines and large molecule nitrosamine impurities related to pharmaceutical active substances [8-9].
2.2. Regulation
The U.S. Food and Drug Administration (FDA) specifies seven common small molecule nitrosamines that need to be strictly controlled, as shown in Table 1. The maximum daily intake (MDI) for the seven single nitrosamines is listed in Table 1, and the MDI is the maximum amount that can be consumed per day without presenting a health risk to humans. If nitrosamines are measured in a pharmaceutical product containing more than two of the nitrosamines listed in the table, the total nitrosamine content must not exceed 26.5 ng/day.
Table 1. Seven common small molecule nitrosamines
Title | Abbr. | Molecular Formula | CAS Number | Acceptable Daily Intake (ng/day) |
N-Nitrosodimethylamine | NDMA | C2H6N2O | 62-75-9 | 96 |
N-Nitrosodiethylamine | NDEA | C4H10N2O | 55-18-5 | 26.5 |
N-Nitroso-Di-Iso-Propylamine | NDIPA | C6H14N2O | 601-77-4 | 26.5 |
N-Nitrosoethylisopropylamine | NEIPA | C5H12N2O | 16339-04-1 | 26.5 |
N-Nitrosodibutylamine | NDBA | C8H18N2O | 924-16-3 | N/A |
Nitrosomethylphenylamine | NMPA | C7H8N2O | 614-00-6 | 26.5 |
Nitrosomethylaminobutyric acid | NMBA | C5H10N2O3 | 61445-55-4 | 96 |
Macromolecular nitrosamine impurities related to pharmacologically active substances have also attracted extensive attention following the publication of seven nitrosamine regulations by the U.S. Food and Drug Administration [8]. This impurity is generally found in finished drug products rather than active pharmaceutical ingredients (APIs) and is produced by the reaction of the drug active ingredient with a nitrosating agent (predominantly nitrite ions under acidic conditions) of the co-drug. The drug active substances are generally secondary amines or dimethyl tertiary amines, which undergo nitrosation during the synthesis of the drug product. This type of nitrosamine is similar to the result of the active substance in the drug and is a nitrosamine impurity specific to each drug. In contrast to the seven small-molecule nitrosamines, these nitrosamines lack sufficient data to evaluate its toxicity and are mostly categorized into five classes based on the structure of the chemical compounds, structure-toxicity relationships, as shown in Table 2. Each class has a corresponding Acceptable Daily Intake (ADI) ranging from 26.5-1500 ng/day [10-11].
Table 2. Five toxicity class and prescribed intakes
Toxicity class | Intakes (ng/day) | Clarification |
1 | 26.5 | This established intake level is for the most toxic nitrosamines, such as N-nitrosodiethylamine. The intake is 26.5 ng/day. |
2 | 100 | The toxicity of these nitrosamines is lower than that of N-nitrosodiethylamine and not higher than that of N-nitrosodimethylamine at an intake of 100 ng/day. |
3 | 400 | The structure is characterized by low activity compared to the second toxicity nitrosamines. This type of nitrosamines has a much lower toxicity, which is one-fourth of the toxicity of the second type of nitrosamines. |
4 | 1500 | Low toxicity can be produced by α-carbon hydroxylation, and the intake of such nitrosamines is set at 1500 ng/day. This value is the same as the threshold of toxicological concern specified in ICH M7(R2) [6]. |
5 | 1500 | Low toxicity via α-carbon hydroxylation is not possible, and the intake of such nitrosamines is set at 1500 ng/day. This value is the same as the threshold of toxicological concern specified in ICH M7(R2) [6]. |
3. Control methods
Due to the specific toxicity of nitrosamines, the potential for existence and the measures of control needs to be properly evaluated. Drugs that pose a risk of nitrosamines are subject to the necessary analytical and control measures to ensure that the amount involved is below the established tolerable intake (TDI). The control methodology consists of three steps: a.) Risk assessment of the potential for nitrosamines to be present in the active substance and the drug product; b.) Identification and analysis of drugs with potential for nitrosamine contamination; c.) Proposing improvements to prevent or reduce the presence of nitrosamines in the active substance, new or generic drug.
3.1. Risk assessment
Pharmaceutical manufacturers need to assess the composition of the finished pharmaceutical product to identify whether there is nitrosamine contamination during the manufacturing process and whether the drug components react with each other to produce nitrosamine by-products. Active drug substance synthesis is the main source of nitrosamine generation, as well as auxiliary drugs, drug raw materials and drug production process contamination need to be taken into account. The main process of risk assessment is shown in Figure 1.
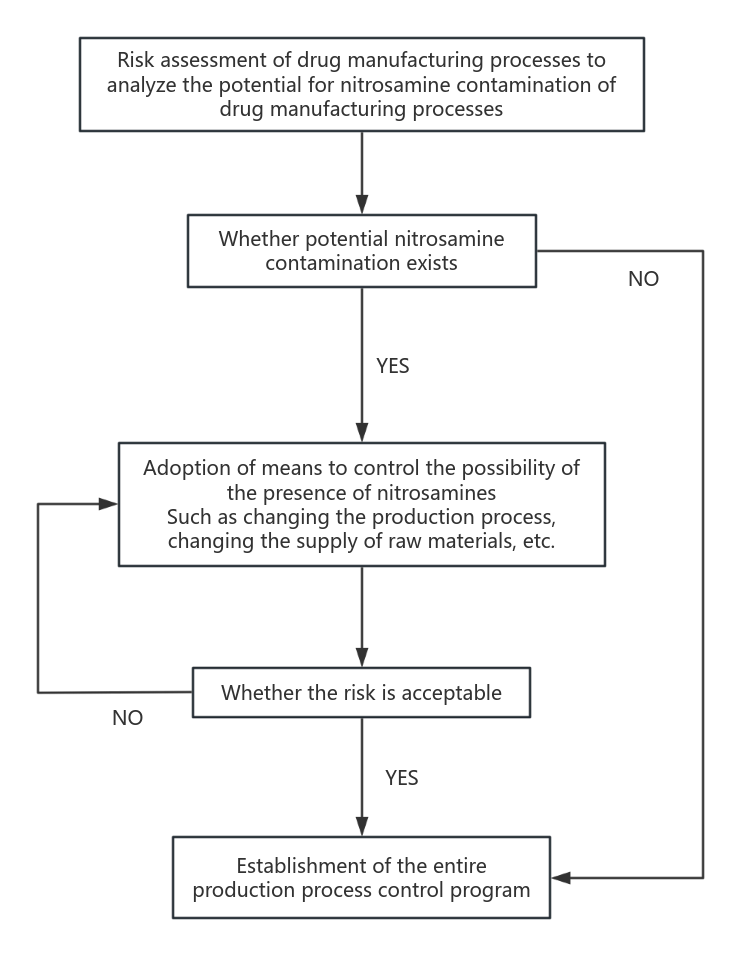
Figure 1. Flow chart for establishing a nitrosamine impurity control program
3.2. Maximum permissible concentration of nitrosamines in drugs
The permitted concentration of nitrosamines in finished pharmaceutical products is determined by the maximum permissible daily intake (MPDA) of nitrosamines and the daily dosage of the drug product. When a pharmaceutical product with a potential risk of nitrosamine contamination is provided with a drug dosage and its potential maximum allowable intake for nitrosamines, the maximum allowable concentration can be calculated according to the following formula.
Permissible level of nitrosamines (ng/g) = Permissible daily intake (ng) / Maximum daily intake of the drug (mg)
The permissible concentrations of nitrosamines for the six known intake levels of nitrosamines (Table 1) at different drug dosing levels, based on the formula above, are listed in Table 3.
Table 3. Relationship between permissible concentrations of nitrosamines and the amount of medication intake
Permissible concentrations of nitrosamines(µg/g) | ||||
50mg | 100mg | 250mg | 1000mg | |
N-Nitrosodimethylamine | 1.920 | 0.960 | 0.384 | 0.096 |
N-Nitrosodiethylamine | 0.530 | 0.265 | 0.106 | 0.0265 |
N-Nitroso-Di-Iso-Propylamine | 0.530 | 0.265 | 0.106 | 0.0265 |
N-Nitrosoethylisopropylamine | 0.530 | 0.265 | 0.106 | 0.0265 |
Nitrosomethylphenylamine | 0.530 | 0.265 | 0.106 | 0.0265 |
Nitrosomethylaminobutyric acid | 1.920 | 0.960 | 0.384 | 0.096 |
Two or more nitrosamines* | 0.530 | 0.265 | 0.106 | 0.0265 |
*If the drug has a risk of multiple nitrosamine contamination, it must not exceed 26.5 ng/day of total nitrosamines.
3.3. Test and Analysis Methods
Nitrosamine analysis belongs to the trace analysis, due to the extremely low content of nitrosamine in pharmaceutical products, generally need to use a high sensitivity and accuracy of the instrument for drug testing. The minimum detection limit of the test needs to reach the maximum permissible level of nitrosamines. A common and reliable analytical method is the combination of chromatographic separation and qualitative and quantitative mass spectrometry [12-18]. The United States Pharmacopeia (USP) proposes four test methods: a.) liquid chromatography high resolution mass spectrometry (LC-HRMS); b.) headspace gas chromatography mass spectrometry (HGC-MS); c.) liquid chromatography tandem mass spectrometry (LC-MS/MS); and d.) gas chromatography tandem mass spectrometry (GC-MS/MS). The specific choice of method is determined by the drug product and the characteristics of the nitrosamines to be determined. The selected method needs to be validated using ICH or USP regulations, including the linearity of concentration and responsivity, analytical intervals, accuracy, precision, robustness of the method and stability of the standards and samples [19-20] .
Abe et al. identified the formation of N-nitrosodimethylamine from ranitidine due to pyrolysis [21]. Since gas chromatography uses high temperature gasification means to analyze samples, it may lead to the production of nitrosamines during the analysis of samples. Therefore, gas chromatography mass spectrometry is not suitable for the analysis of nitrosamines in certain drugs, and liquid chromatography mass spectrometry with ambient temperature analysis is used as much as possible for these drugs. For large molecules of nitrosamines similar to the active substances of drugs, liquid chromatography mass spectrometry (LC-MS) is commonly used for its high molecular weight and poor volatility.
4. Conclusion
Nitrosamines are a type of carcinogenic organisms that can cause genetic mutations, and the potential for occurrence in pharmaceuticals needs to be properly risk evaluated so that the potential for occurrence in pharmaceuticals can be eliminated or reduced to a no-risk range. Determining the maximum daily intake of nitrosamines is a key point in risk evaluation. The U.S. Food and Drug Administration (FDA) has established intake levels for six types of nitrosamines, and other nitrosamines, especially those related to drug-active substances, need to be determined on the basis of structural-toxicity relationships.
Chromatography mass spectrometry is an effective analytical method for trace nitrosamines, mainly gas chromatography mass spectrometry and liquid chromatography. The determination of the analytical method is determined by the characteristics of the drug and the measured nitrosamines, but the analytical method needs to be used after verification. Since N-nitrosodimethylamine is a degradation product of some drugs when the temperature changes, its analysis avoids the use of gas chromatography and uses liquid chromatography. Nitrosamines with large molecules similar to the active substance are mostly analyzed by liquid chromatography in tandem with mass spectrometry.
In addition, this paper still has parts that need to be improved and optimized in the process of analysis, including demonstration through experiments, data demonstration and so on. The article is only based on the analysis and argumentation of the content of the literature, and does not involve the experimental operations. In the future, the topic of the paper will be further argued and researched by combining in the relevant practical ability.
References
[1]. J. Leclerc, Recall of N-Nitrosodimethylamine-Contaminated Pseudogeneric Valsartan: Best Generics Finally No Better Than Others? The Canadian journal of cardiology, 2018 Oct;34(10):1370
[2]. M. Abdel-Tawab, R. Gröner, T. Kopp, J. Meins, J. Wübert, Valsartan: ZL findet NDMA in Tabletten, Pharm. (1856) 158 (2018) 2898–2902.
[3]. U.S. Food & Drug Administration, FDA Provides Update on Its Ongoing Investigation Into Valsartan Products; and Reports on the Finding of an Additional Impurity Identified in One Firm’s Already Recalled Products, 2018.
[4]. EMA/703416/2018, EU Authorities Take Further Action in Ongoing Review of Sartans: Zheijiang Huahai Placed Under Increased Supervision; Aurobindo Pharma Stopped From Supplying Irbesartan to the EU, European Medicines Agency, London, U.K, 2018.
[5]. International Conference for Harmonization of Technical Requirements of Pharmaceuticals for Human Use. ICH M7: Assessment and Control of DNA Reactive (Mutagenic) Impurities in Pharmaceuticals to Limit Potential Carcinogenic Risk, 2017.
[6]. Wanfeng Wang, Jianwei Yu, Wei An, Min Yang, Occurrence and profiling of multiple nitrosamines in source water and drinking water of China, Science of the Total Environment 551–552 (2016) 489–495.
[7]. Zhao Y., Zhou, W., Ma, K, Sun, T, Guo , C, Luo, X, Advances in the detection of N-nitrosodimethylamine in food and drugs, China Pharmaceuticals, 2021, 30(17):124-127.
[8]. Food and Drug Administration. Control of Nitrosamine Impurities in Human Drugs-Guidance for Industry, 2021.
[9]. USP (1469): Nitrosamine Impurities, 2022.
[10]. Food and Drug Administration. Recommended Acceptable Intake Limits for Nitrosamine Drug Substance Related Impurities (NDSRIs)-Guidance for Industry, August 2023.
[11]. Krista L. Dobo, Michelle O. Kenyon, Olivier Dirat, Maria Engel, Andrew Fleetwood, Matthew Martin, Susan Mattano, Alyssa Musso, James Christopher McWilliams, Alexandros Papanikolaou, Patricia Parris, Jessica Whritenour, Shu Yu, and Amit S., Practical and Science-Based Strategy for Establishing Acceptable Intakes for Drug Product N-Nitrosamine Impurities, Cite This: Chem. Res. Toxicol. 2023, 36, 959−970.
[12]. Maria Kristina Parr, Jan F. Josepha, NDMA impurity in valsartan and other pharmaceutical products: Analytical methods for the determination of N-nitrosamines, Journal of Pharmaceutical and Biomedical Analysis, 164, (2019), 536-549, 2019.
[13]. Sebastian Schmidtsdorff , Alexander H. Schmidt, Simultaneous detection of nitrosamines and other sartan-related impurities in active pharmaceutical ingredients by supercritical fluid chromatography, Journal of Pharmaceutical and Biomedical Analysis, 174 (2019), 151-160.
[14]. Sambasiva Rao TUMMALA, Krishnamanjari Pawar AMGOTH, Development of GC-MS/MS Method for Simultaneous Estimation of Four Nitrosoamine Genotoxic Impurities in Valsartan, Turk J Pharm Sci 2022;19(4):455-461.
[15]. Hyungsoo Kim, Daekwan Sung, Honghyeon Yu, Daeyong Jang, Yeji Koo, Seungha Lee, Kyungmin Lim and Dalwoong Choi, Comparison of EI-GC-MS/MS, APCI-LC-MS/MS, and ESI-LC-MS/MS for the Simultaneous Analysis of Nine Nitrosamines Eluted from Synthetic Resins into Artificial Saliva and Health Risk Assessment, Toxics 2021, 9, 230. https://doi.org/10.3390/toxics9100230.
[16]. Mikhail Khorolskiy, Galina Ramenskaya , Alexander Vlasov, Oleg Perederyaev and Nataliya Maslennikova, Development and Validation of four Nitrosamine Impurities Determination Method in Medicines of Valsartan, Losartan, and Irbesartan with HPLC-MS/MS (APCI), Iranian Journal of Pharmaceutical Research (2021), 20 (3): 541-552.
[17]. Anna B. Witkowska, Joanna Giebułtowicz, Magdalena Dabrowska and Elzbieta U. Stolarczyk, Development of a Sensitive Screening Method for Simultaneous Determination of Nine Genotoxic Nitrosamines in Active Pharmaceutical Ingredients by GC-MS, Int. J. Mol. Sci. 2022, 23, 12125. https://doi.org/10.3390/ijms232012125.
[18]. Jie Liu, Bin Xie, Binliang Mai, Qiang Cai, Rujian He, Dong Guo, Zhifeng Zhang, Jun Fan and Weiguang Zhang, Development of a sensitive and stable GC-MS/MS method for simultaneous determination of four N-nitrosamine genotoxic impurities in sartan substances, Journal of Analytical Science and Technology (2021) 12:3 https://doi.org/10.1186/s40543-020-00254-2.
[19]. USP (36): Validation of Compendial Procedures (1225), 2012.
[20]. International Conference for Harmonization of Technical Requirements of Pharmaceuticals for Human Use. ICH Q2 (R1): Validation of Analytical Procedure: Text and Methodology, November 2005.
[21]. Yasuhiro Abe, Eiichi Yamamoto, Hiroyuki Yoshida, Akiko Usui, Naomi Tomita, Hitomi Kanno, Sayaka Masada, Hidetomo Yokoo, Genichiro Tsuji, Nahoko Uchiyama, Takashi Hakamatsuka, Yosuke Demizu, Ken-ichi Izutsu, Yukihiro Goda, and Haruhiro Okuda, Temperature-Dependent Formation of N-Nitrosodimethylamine during the Storage of Ranitidine Reagent Powders and Tablets, Chem. Pharm. Bull. 68, 1008–1012, 2020.
Cite this article
Zhang,S. (2024). Risk assessment and quality control for nitrosamines occurrence in drug product. Theoretical and Natural Science,61,149-155.
Data availability
The datasets used and/or analyzed during the current study will be available from the authors upon reasonable request.
Disclaimer/Publisher's Note
The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of EWA Publishing and/or the editor(s). EWA Publishing and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
About volume
Volume title: Proceedings of the 4th International Conference on Biological Engineering and Medical Science
© 2024 by the author(s). Licensee EWA Publishing, Oxford, UK. This article is an open access article distributed under the terms and
conditions of the Creative Commons Attribution (CC BY) license. Authors who
publish this series agree to the following terms:
1. Authors retain copyright and grant the series right of first publication with the work simultaneously licensed under a Creative Commons
Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this
series.
2. Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the series's published
version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial
publication in this series.
3. Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and
during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See
Open access policy for details).
References
[1]. J. Leclerc, Recall of N-Nitrosodimethylamine-Contaminated Pseudogeneric Valsartan: Best Generics Finally No Better Than Others? The Canadian journal of cardiology, 2018 Oct;34(10):1370
[2]. M. Abdel-Tawab, R. Gröner, T. Kopp, J. Meins, J. Wübert, Valsartan: ZL findet NDMA in Tabletten, Pharm. (1856) 158 (2018) 2898–2902.
[3]. U.S. Food & Drug Administration, FDA Provides Update on Its Ongoing Investigation Into Valsartan Products; and Reports on the Finding of an Additional Impurity Identified in One Firm’s Already Recalled Products, 2018.
[4]. EMA/703416/2018, EU Authorities Take Further Action in Ongoing Review of Sartans: Zheijiang Huahai Placed Under Increased Supervision; Aurobindo Pharma Stopped From Supplying Irbesartan to the EU, European Medicines Agency, London, U.K, 2018.
[5]. International Conference for Harmonization of Technical Requirements of Pharmaceuticals for Human Use. ICH M7: Assessment and Control of DNA Reactive (Mutagenic) Impurities in Pharmaceuticals to Limit Potential Carcinogenic Risk, 2017.
[6]. Wanfeng Wang, Jianwei Yu, Wei An, Min Yang, Occurrence and profiling of multiple nitrosamines in source water and drinking water of China, Science of the Total Environment 551–552 (2016) 489–495.
[7]. Zhao Y., Zhou, W., Ma, K, Sun, T, Guo , C, Luo, X, Advances in the detection of N-nitrosodimethylamine in food and drugs, China Pharmaceuticals, 2021, 30(17):124-127.
[8]. Food and Drug Administration. Control of Nitrosamine Impurities in Human Drugs-Guidance for Industry, 2021.
[9]. USP (1469): Nitrosamine Impurities, 2022.
[10]. Food and Drug Administration. Recommended Acceptable Intake Limits for Nitrosamine Drug Substance Related Impurities (NDSRIs)-Guidance for Industry, August 2023.
[11]. Krista L. Dobo, Michelle O. Kenyon, Olivier Dirat, Maria Engel, Andrew Fleetwood, Matthew Martin, Susan Mattano, Alyssa Musso, James Christopher McWilliams, Alexandros Papanikolaou, Patricia Parris, Jessica Whritenour, Shu Yu, and Amit S., Practical and Science-Based Strategy for Establishing Acceptable Intakes for Drug Product N-Nitrosamine Impurities, Cite This: Chem. Res. Toxicol. 2023, 36, 959−970.
[12]. Maria Kristina Parr, Jan F. Josepha, NDMA impurity in valsartan and other pharmaceutical products: Analytical methods for the determination of N-nitrosamines, Journal of Pharmaceutical and Biomedical Analysis, 164, (2019), 536-549, 2019.
[13]. Sebastian Schmidtsdorff , Alexander H. Schmidt, Simultaneous detection of nitrosamines and other sartan-related impurities in active pharmaceutical ingredients by supercritical fluid chromatography, Journal of Pharmaceutical and Biomedical Analysis, 174 (2019), 151-160.
[14]. Sambasiva Rao TUMMALA, Krishnamanjari Pawar AMGOTH, Development of GC-MS/MS Method for Simultaneous Estimation of Four Nitrosoamine Genotoxic Impurities in Valsartan, Turk J Pharm Sci 2022;19(4):455-461.
[15]. Hyungsoo Kim, Daekwan Sung, Honghyeon Yu, Daeyong Jang, Yeji Koo, Seungha Lee, Kyungmin Lim and Dalwoong Choi, Comparison of EI-GC-MS/MS, APCI-LC-MS/MS, and ESI-LC-MS/MS for the Simultaneous Analysis of Nine Nitrosamines Eluted from Synthetic Resins into Artificial Saliva and Health Risk Assessment, Toxics 2021, 9, 230. https://doi.org/10.3390/toxics9100230.
[16]. Mikhail Khorolskiy, Galina Ramenskaya , Alexander Vlasov, Oleg Perederyaev and Nataliya Maslennikova, Development and Validation of four Nitrosamine Impurities Determination Method in Medicines of Valsartan, Losartan, and Irbesartan with HPLC-MS/MS (APCI), Iranian Journal of Pharmaceutical Research (2021), 20 (3): 541-552.
[17]. Anna B. Witkowska, Joanna Giebułtowicz, Magdalena Dabrowska and Elzbieta U. Stolarczyk, Development of a Sensitive Screening Method for Simultaneous Determination of Nine Genotoxic Nitrosamines in Active Pharmaceutical Ingredients by GC-MS, Int. J. Mol. Sci. 2022, 23, 12125. https://doi.org/10.3390/ijms232012125.
[18]. Jie Liu, Bin Xie, Binliang Mai, Qiang Cai, Rujian He, Dong Guo, Zhifeng Zhang, Jun Fan and Weiguang Zhang, Development of a sensitive and stable GC-MS/MS method for simultaneous determination of four N-nitrosamine genotoxic impurities in sartan substances, Journal of Analytical Science and Technology (2021) 12:3 https://doi.org/10.1186/s40543-020-00254-2.
[19]. USP (36): Validation of Compendial Procedures (1225), 2012.
[20]. International Conference for Harmonization of Technical Requirements of Pharmaceuticals for Human Use. ICH Q2 (R1): Validation of Analytical Procedure: Text and Methodology, November 2005.
[21]. Yasuhiro Abe, Eiichi Yamamoto, Hiroyuki Yoshida, Akiko Usui, Naomi Tomita, Hitomi Kanno, Sayaka Masada, Hidetomo Yokoo, Genichiro Tsuji, Nahoko Uchiyama, Takashi Hakamatsuka, Yosuke Demizu, Ken-ichi Izutsu, Yukihiro Goda, and Haruhiro Okuda, Temperature-Dependent Formation of N-Nitrosodimethylamine during the Storage of Ranitidine Reagent Powders and Tablets, Chem. Pharm. Bull. 68, 1008–1012, 2020.









