1. Introduction
One of the most frequent diseases and major causes of mortality globally is cancer. Despite advances in chemotherapy, radiation and surgery, these approaches are not without their drawbacks, including serious side effects and variable outcomes. Consequently, one of the main areas of focus for medical research now is the quest for novel therapeutic approaches.
Immunotherapy has recently become a very effective clinical treatment. It can effectively strengthen the body's interest-free system to fight disease. Immunotherapy primarily takes three forms: cell treatment, tumor vaccines, and antibody therapy. Clinical therapies for tumors have continued to evolve in recent years and are effective in treating a wide range of clinical conditions including kidney cancer and melanoma. Science magazine recognized tumor immunotherapy as the most significant scientific advance of the year in 2013 due to its exceptional effectiveness and inventiveness [1]. Among them, CAR-T cells can be carried out in the clinical corresponding treatment, and has more significance.
CARs is a protein used in the body that can even be produced by toxic T cells and reencoded. They are created by antigen detection, signaling, and co-stimulatory regions. Through the use of gene transfer technology, CAR-T cell treatment combines the potent cytotoxicity and memory function of T cells with the specificity of antibodies by reprogramming the patient's own T cells to stabilize cell expression. ALL、MM、CLL、AML are the hematological cancers for which CAR-T cells are most frequently used in clinical studies. CAR-T therapy had an overall response rate of over 90% in clinical studies for the treatment of ALL [2,3].
Despite the promising potential of CAR-T immunotherapy, several challenges persist in its clinical application. These issues include the difficulty in identifying ideal target antigens, concerns regarding the durability and potential inactivation of CAR-T cells in vivo, and the lingering problem of overcoming non-tumor toxicity.. At present, the middle point of research is how to improve and optimize CAR-T to achieve the goal of safer and better anti-tumor effects in clinical applications.
2. Principle of treatment
Based on the autoimmune system, cellular immunotherapy is a type of active immunotherapy that targets tumor patients with a more precise, reliable, and long-lasting course of treatment by taking advantage of immune cell death's relative specificity, memory, and persistence.
When it comes to immunotherapy techniques specifically for tumor immunotherapy, they can be broadly classified into two categories: first, which is known as PD-1/PD-L1 immunotherapy, targets the tumor's immune escape mechanism by increasing the tumor's tolerance and shielding effect on the immune system, allowing the immune cells to re-recognize the tumor cells; and second, which uses the antigen produced by the tumor's characteristics (tumor neoantigen/tumor associated antigen) and enable the immune cells to identify and eliminate the tumor.
2.1. Immunotherapy of tumors -- the exploitation of immune escape phenomenon
One of the main areas of application for immunology in translational medicine has always been the fight against cancers using immunological therapy. Tumor cells' immunogenicity due to mutations has been extensively acknowledged with the advent of multiple omics (genomics, proteomics, etc.), which has established the theoretical basis for tumor immunotherapy.
These malignant cells have a large practical impact on the body, including their rapid growth and the rapid avoidance of killing mechanisms that allow them to survive at all stages of life [4,5]. In addition, because the body's immune system is often only able to eliminate the malignant cells have appeared, may appear some cells escape. In 2013, Ira Mellman and Daniel S proposed effective treatments, and the majority of patients' illness was improved; While some patients experienced minor side effects during therapy, overall safety was moderate, and no significant adverse events occurred. Complete remission was achieved in some patients, and the remission continued for a long period. Chen as a way to better comprehend the multi-stage and multi-step intricacy of tumor immunity.
The following seven stages comprise the tumor-immune cycle: 1. Presentation of tumor antigens; 2. Release of tumor antigens; 3. Initiation and stimulation; 4. Transfer to tissue; 5. Beginning of infection; 6. Identification of cells; 7. Removal of related malignant cells. Any deviation from these connections may result in immune escape and the breakdown of the anti-tumor immunological cycle. By creating various linkages between abnormalities, certain tumors can prevent the immune system from properly identifying and eliminating tumor cells, leading to immunological tolerance and potentially encouraging the growth and emergence of new tumors.
In this study, the reactivation and maintenance of immune cycle and the further reconstruction of immune response can effectively promote the health of the body. It is also a very effective treatment to prevent malignant diseases, including vaccines, antibodies and related inhibitors.
2.2. Development process of CAR-T cell therapy Treatment principle
This method based on T cells is widely used in practice, and it is also effective in the diagnosis and treatment of difficult diseases. It can effectively identify and clear malignant cells and inject them into the body.
The following summarizes the basic idea of CAR-T:
Synthetic CARs are made up of co-stimulatory domains, signaling domains, and monoclonal antibodies that are unique to tumors. It can identify a particular antigen on the surface of tumor cells and trigger T cells' ability to kill them, allowing it to realize that tumor cells are under attack. Tumor-associated antigens are not unique to tumor cells, but only vary in quantity during proliferation. Normal cells are also synthesized in trace amounts, hence the name "associated antigens". Tumors of the same tissue type induced by the same carcinogen have the same tumor-associated antigen in different individuals.
CAR-T cell therapy mainly consists of the following steps:
(1) Collection of T cells from the patient: Collection of lymphocytes from peripheral blood.
(2) Genetic engineering modification: In clinical practice, the T cells are designed to be specific antigens of the corresponding cells, thereby activating the corresponding effector cells in the body. The genes in this study were introduced into it, i. e. specific antigens and related genetic material were transferred into it.
(3) Growth of CAR-T: To multiply and proliferate, the altered T cells are cultivated in vitro.
(4) Therapy: Reintroducing the amplified CAR-T cells into the patient enables them to identify and eliminate tumor cells.
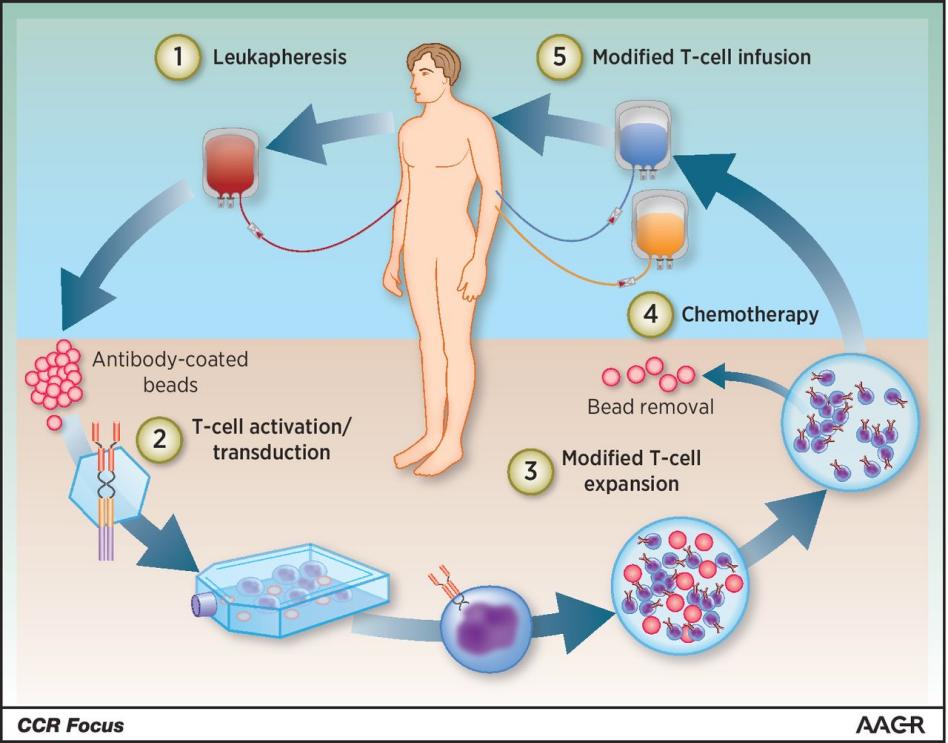
Figure 1. A synopsis of clinical CAR-T cell treatment.
Leukocyte separation is used to extract the patient's T cells, which are subsequently stimulated on antibody-coated beads to form artificial dendritic cells. Following their genetic reprogramming in vitro by transduction employing constructs encoding CAR, the activated T cells were further expanded in vitro to produce CAR-T. When CAR-T is manufactured and quality controlled, patients need to receive the corresponding cell transfusions and in vitro chemotherapy [6].
In conclusion, the following is an overview of how CAR-Technology operates: Enhance the activity of effector cells, such as T cells or NK cells. Researchers use viral vectors to integrate target genes to achieve stable expression. The transposon or transposase system also inserts foreign DNA into the effector cell genome for stable expression. In the process of gene isolation, researchers extract gene fragments, modify and recombine them by recombinant DNA technology in vitro, and then transfer them into effector cells. Precise editing allows effector cells to respond to specific antigens, enhancing the effect and avoiding misdirected attacks on normal tissue. a cellular immunotherapy technique where effector cells displaying antigen specificity are adoptively transfused back into the patient after being expanded in vitro to therapeutic concentrations.
3. Progress in Clinical application
This section will be based on the treatment of CAR-T applications for the corresponding content of the analysis.
3.1. B-cell lymphoma
Clinically, one of the most prevalent malignant lymphomas is B-cell lymphoma, a tumor formed from B cells that is malignant.
James N. Kochenderfer et al. report that prechemotherapy was used to treat a patient with follicular lymphoma. Genetically engineered T cells can express chimeric antigen receptors (CAR) that recognize CD19 molecules and target malignant B cells with precision. In clinical application, CAR-T cell therapy has shown a strong anti-disease activity, it can effectively clear off B cell precursors and derived cell groups, and significantly reduce lymphoma lesions, so that some patients achieved long-term remission. In addition, the treatment has shown promise for diseases that rely on B cells to produce immunoglobulin. The immunotherapy of T cells by CAR expressing anti-CD19 has opened a new strategy and approach for the treatment of B cell malignant lesions, and brought new hope and potential cure opportunity [7].
The Kymriah expert was first nationally certified in the United States in 2017 to use a display of cell therapies in the clinic.52 (82.5%) of the 63 patients who got Kymriah between April 2015 and August 2016 exhibited improvement, according to data from a trial that Novartis gave to the FDA. But regarding appears in the clinical, the relapse acute leukemia patient, its clinical in cure rate correspondence is high [8]. In addition, in October of the same year, Yescarta researchers also received the authorization of the relevant national institutions, can be effectively applied to clinical practice. According to the most recent information published on the official website of China's National Drug Administration (NMPA) on June 26, 2023, Fosun Kate submitted additional indications for Yescarta injection, which have been formally accepted. With its personalized, one-time therapy, Yescarta has helped over 500 patients with recurrent large B cell, bringing a revolutionary new breakthrough to the field of tumor therapy in China. Its indications are for first-line immunochemotherapy that was ineffective or that recurred within 12 months of r/r LBCL.
3.2. T-cell lymphoma
An aggressive type of lymphoma originating from T cells is called T-cell lymphoma. Currently, the pathophysiology of T-cell lymphoma remains incompletely understood, leading to unsatisfactory therapeutic outcomes. Therefore, this study found that T cells in the clinical diagnosis and treatment of a very promising, promising method.
The interaction between the receptor and the corresponding cell therapy needs to be considered in the design of the corresponding clinical practice. Nonetheless, a number of investigations have demonstrated that CAR-T cell treatment can effectively treat T-cell lymphomas.
3.3. Multiple myeloma
Adult-onset multiple myeloma (MM) is the most prevalent kind of myeloma, a malignant tumor brought on by the proliferation of plasma cells. MM therapy has always been a challenging issue. Hematopoietic stem cell transplantation, chemotherapy, and radiation therapy are examples of conventional therapeutic techniques; nevertheless, their effectiveness is constrained, and drug resistance is a common occurrence. CAR-T cell treatment now primarily targets the BCMA antigen. It can be concluded that the mature antigen corresponding to B cells is a protein produced in the body and plays a vital role in the regulation of the body.
Researcher Cohen AD et al. found that the protein antigens mentioned above can effectively inhibit the activity of the cells. The research team created CAR-T cells that specifically target BCMA and carried out clinical trials to these treatments for multiple myeloma patients. A small number of multiple myeloma patients and were monitored and assessed for effectiveness were included in the clinical trial. The findings demonstrated that in multiple myeloma patients, BCMA CAR-T cells had strong anticancer activity, and the majority of patients' illness was improved; While some patients experienced minor side effects during therapy, overall safety was moderate, and no significant adverse events occurred. Complete remission was achieved in some patients, and the remission continued for a long period [9].
In addition to antigen therapy for BCMA, other effective cell therapies are also abundant in this study.
4. The adverse effects of CAR-T cell therapy
Although CAR-T cell therapy gives the hope of a full recovery for certain patients with advanced cancers, there are some negative effects throughout the treatment that may even lead to death. When using CAR-T treatment, the expected should be examined first.
4.1. cytokine release syndrome (CRS)
Common side effects of this treatment include CRS, which is usually the more cytokines released by these CAR-T cells after activation, and may lead to inflammatory responses in the body. Symptoms of this side effect include hypotension, rash, shortness of breath, and headaches [10]. The amount and activity of the corresponding primordial cells will affect the clustering degree of side effects to some extent, and the more serious side effects may even bring very serious consequences or even death. Therefore, it is crucial to pay attention to the timely and adequate control of CRS during CAR-T cell therapy.
CAR structure, tumor kind and burden, patient gene polymorphism and many other factors are associated with the incidence of CRS. Enforcing stringent limits on the quantity of cells per infusion and developing safe CAR-T cell lines can help prevent CRS. It is possible to lessen death from CRS by using cytokine antagonists and glucocorticoids [11].
4.2. tumor lysis syndrome (TLS)
TLS is a syndrome of short-term lysis of tumor cells and the release of high levels of intracellular metabolites caused by anticancer therapy. Precise editing allows effector cells to respond to specific antigens, enhancing the effect and avoiding misdirected attacks on normal tissue. its main clinical manifestations include acute hyperuricemia, hyperphosphatemia, hypocalcemia and acute renal failure, all of which are associated with rapidly growing tumor cells and tumor cells that decompose after treatment. Renal failure and arrhythmia, ventricular fibrillation and other problems may also occur in more serious cases. a cellular immunotherapy technique where effector cells displaying antigen specificity are adoptively transfused back into the patient after being expanded in vitro to therapeutic concentrations.
Early T cell clinical research has revealed a connection between TLS and both the in vivo cytokines. TLS can be prevented or controlled in the weeks following injection by keeping an eye on and managing T cells promptly [12,13].
4.3. on-target toxicity
Tumor-associated antigen (TAA) refers to antigen molecules that are not specific to a particular tumor and are present in other tumor cells or normal cells. Tumor-associated antigen (TAA) used in clinical diagnosis includes embryonic protein, glycoprotein antigen, squamous cell antigen, etc. Tumor-associated antigens are not unique to tumor cells, but only vary in quantity during proliferation. Normal cells are also synthesized in trace amounts, hence the name "associated antigens". Tumors of the same tissue type induced by the same carcinogen have the same tumor-associated antigen in different individuals.
Due to the high affinity of CAR-T cells to TAA, the toxicity of CAR-T cells to normal tissues after binding with TAA expressed in normal tissues is an off-target effect; This toxicity is mediated by antigen, and antibodies can be applied to block TAA in normal tissue, reduce the number of CAR-T per infusion [14], and construct cross-signaling CAR[T cell activation signal 1(CD3ζ) in CAR structure is not directly linked to the co-stimulatory signal CD28 molecule [15]or by introducing a suicide gene system [16] to prevent and treat this off-target toxicity. For example, B cell deficiency is an off-target toxicity of CAR19-T cell therapy, and B cell deficiency can cause long-term hypogammaglobulinemia, which can be replaced by gamma globulin infusion [17].
4.4. Other Side Effects
Apart from the aforementioned trio of adverse reactions, CAR-T cell therapy may also result in additional side effects, including neurotoxicity (increased intracranial pressure, seizures, consciousness disorders, etc.), toxicity to the hematological system (leukopenia, thrombocytopenia, etc.), infection, liver malfunction, abnormalities in lung function, etc.
5. Conclusion
CAR-T therapy still confronts several problems, such as variable efficacy, high incidence of side effects and expensive cost. Scientists are speeding their efforts to develop the technology of CAR-T treatment.
CAR-T cell therapy is an emerging and promising tumor treatment that has shown clear therapeutic effects in clinical trials. Although CAR-T therapy currently presents notable side effects, it is anticipated that ongoing technological advancements and optimization of treatment protocols will effectively address these challenges. As progress continues, CAR-T cell therapy holds the potential to become a key component of cancer treatment, offering promising prospects and improved outcomes for a broader range of patients.
References
[1]. Breakthrough of the year 2013. Notable developments.Science,342(6165),1435-1441.2013.
[2]. Maus, M. V., & June, C. H. (2016). Making better chimeric antigen receptors for adoptive T-cell therapy. Clinical Cancer Research, 22(8), 1875-1884.
[3]. Hu, Y. (2023). Current status and challenges in chimeric antigen receptor-modified T cell immunotherapies for hematological malignancies. Chinese Journal of Clinical Oncology, 50(1), 49-54.
[4]. Jain, M. D., et al. (2021). Tumor interferon signaling and suppressive myeloid cells are associated with CAR-T-cell failure in large B-cell lymphoma. Blood, 137(19), 2621-2633.
[5]. Scholler, N., et al. (2022). Tumor immune contexture is a determinant of anti-CD19 CAR-T cell efficacy in large B cell lymphoma. Nature Medicine, 28(9), 1872-1882.
[6]. Maus, M. V., & June, C. H. (2016). Making better chimeric antigen receptors for adoptive T-cell therapy. Clinical Cancer Research, 22(8), 1875-1884..
[7]. Kochenderfer, J. N., et al. (2010). Eradication of B-lineage cells and regression of lymphoma in a patient treated with autologous T cells genetically engineered to recognize CD19. Blood, 116(20), 4099-4102.
[8]. Philippidis, A. (2017). Kymriah, first CAR-T cancer immunotherapy approved by FDA. Clinical Omics, 4(5), 8-8..
[9]. Cohen, A. D., et al. (2019). B cell maturation antigen-specific CAR-T cells are clinically active in multiple myeloma. Journal of Clinical Investigation, 129(6), 2210-2221.
[10]. Morgan, R. A., et al. (2010). Case report of a serious adverse event following the administration of T cells transduced with a chimeric antigen receptor recognizing ERBB2. Molecular Therapy, 18(4), 843-851.
[11]. Xu, X. J., & Tang, Y. M. (2014). Cytokine release syndrome in cancer immunotherapy with chimeric antigen receptor engineered T cells. Cancer Letters, 343(2), 172-178.
[12]. Porter, D. L., et al. (2011). Chimeric antigen receptor-modified T cells in chronic lymphoid leukemia. New England Journal of Medicine, 365(8), 725-733.
[13]. Kochenderfer, J. N., et al. (2013). Donor-derived CD19-targeted T cells cause regression of malignancy persisting after allogeneic hematopoietic stem cell transplantation. Blood, 122(25), 4129-4139.
[14]. Lamers, C. H., et al. (2013). Treatment of metastatic renal cell carcinoma with CAIX CAR-engineered T cells: Clinical evaluation and management of on-target toxicity. Molecular Therapy, 21(4), 904-912.
[15]. Lanitis, E., et al. (2013). Chimeric antigen receptor T cells with dissociated signaling domains exhibit focused anti-tumor activity with reduced potential for toxicity in vivo. Cancer Immunol Res, 1(1), 43-53.
[16]. Gargett, T., & Brown, M. P. (2014). The inducible caspase-9 suicide gene system as a "safety switch" to limit on-target, off-tumor toxicities of chimeric antigen receptor T cells. Frontiers in Pharmacology, 5, 235.
[17]. Davila, M. L., et al. (2013). CD19 CAR-targeted T cells induce long-term remission and B cell aplasia in an immunocompetent mouse model of B cell acute lymphoblastic leukemia. PLoS One, 8(4), e61338.
Cite this article
Li,Y. (2024). Advances in the application of CAR-T immunotherapy in tumor therapy. Theoretical and Natural Science,61,156-162.
Data availability
The datasets used and/or analyzed during the current study will be available from the authors upon reasonable request.
Disclaimer/Publisher's Note
The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of EWA Publishing and/or the editor(s). EWA Publishing and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
About volume
Volume title: Proceedings of the 4th International Conference on Biological Engineering and Medical Science
© 2024 by the author(s). Licensee EWA Publishing, Oxford, UK. This article is an open access article distributed under the terms and
conditions of the Creative Commons Attribution (CC BY) license. Authors who
publish this series agree to the following terms:
1. Authors retain copyright and grant the series right of first publication with the work simultaneously licensed under a Creative Commons
Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this
series.
2. Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the series's published
version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial
publication in this series.
3. Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and
during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See
Open access policy for details).
References
[1]. Breakthrough of the year 2013. Notable developments.Science,342(6165),1435-1441.2013.
[2]. Maus, M. V., & June, C. H. (2016). Making better chimeric antigen receptors for adoptive T-cell therapy. Clinical Cancer Research, 22(8), 1875-1884.
[3]. Hu, Y. (2023). Current status and challenges in chimeric antigen receptor-modified T cell immunotherapies for hematological malignancies. Chinese Journal of Clinical Oncology, 50(1), 49-54.
[4]. Jain, M. D., et al. (2021). Tumor interferon signaling and suppressive myeloid cells are associated with CAR-T-cell failure in large B-cell lymphoma. Blood, 137(19), 2621-2633.
[5]. Scholler, N., et al. (2022). Tumor immune contexture is a determinant of anti-CD19 CAR-T cell efficacy in large B cell lymphoma. Nature Medicine, 28(9), 1872-1882.
[6]. Maus, M. V., & June, C. H. (2016). Making better chimeric antigen receptors for adoptive T-cell therapy. Clinical Cancer Research, 22(8), 1875-1884..
[7]. Kochenderfer, J. N., et al. (2010). Eradication of B-lineage cells and regression of lymphoma in a patient treated with autologous T cells genetically engineered to recognize CD19. Blood, 116(20), 4099-4102.
[8]. Philippidis, A. (2017). Kymriah, first CAR-T cancer immunotherapy approved by FDA. Clinical Omics, 4(5), 8-8..
[9]. Cohen, A. D., et al. (2019). B cell maturation antigen-specific CAR-T cells are clinically active in multiple myeloma. Journal of Clinical Investigation, 129(6), 2210-2221.
[10]. Morgan, R. A., et al. (2010). Case report of a serious adverse event following the administration of T cells transduced with a chimeric antigen receptor recognizing ERBB2. Molecular Therapy, 18(4), 843-851.
[11]. Xu, X. J., & Tang, Y. M. (2014). Cytokine release syndrome in cancer immunotherapy with chimeric antigen receptor engineered T cells. Cancer Letters, 343(2), 172-178.
[12]. Porter, D. L., et al. (2011). Chimeric antigen receptor-modified T cells in chronic lymphoid leukemia. New England Journal of Medicine, 365(8), 725-733.
[13]. Kochenderfer, J. N., et al. (2013). Donor-derived CD19-targeted T cells cause regression of malignancy persisting after allogeneic hematopoietic stem cell transplantation. Blood, 122(25), 4129-4139.
[14]. Lamers, C. H., et al. (2013). Treatment of metastatic renal cell carcinoma with CAIX CAR-engineered T cells: Clinical evaluation and management of on-target toxicity. Molecular Therapy, 21(4), 904-912.
[15]. Lanitis, E., et al. (2013). Chimeric antigen receptor T cells with dissociated signaling domains exhibit focused anti-tumor activity with reduced potential for toxicity in vivo. Cancer Immunol Res, 1(1), 43-53.
[16]. Gargett, T., & Brown, M. P. (2014). The inducible caspase-9 suicide gene system as a "safety switch" to limit on-target, off-tumor toxicities of chimeric antigen receptor T cells. Frontiers in Pharmacology, 5, 235.
[17]. Davila, M. L., et al. (2013). CD19 CAR-targeted T cells induce long-term remission and B cell aplasia in an immunocompetent mouse model of B cell acute lymphoblastic leukemia. PLoS One, 8(4), e61338.









