1. Introduction
Konjac glucomannan (KGM) is a natural, high molecular weight, water-soluble polysaccharide derived from konjac. It is extracted from the tubers of the konjac plant and is the primary functional component of konjac. KGM is renowned for its high viscosity, strong water-holding capacity, and excellent gelling properties, making it a dietary fiber highly beneficial to human health.
The distinctive characteristics of KGM are primarily reflected in its unique physicochemical properties. Firstly, it has good water solubility and can form a stable gel-like substance in water. Secondly, KGM exhibits excellent stability; its molecular structure is not easily disrupted, and its physical properties remain stable under both high and low temperature conditions [1-2]. Additionally, KGM can undergo various organic chemical reactions, such as esterification modification, which are used to create new konjac products [3].
The functional activities of KGM in the health domain are mainly demonstrated in the following aspects. Firstly, it promotes the colonization of beneficial bacteria in the gut while inhibiting the proliferation of pathogenic bacteria, thus optimizing the structure of the gut microbiota [4]. Secondly, KGM can slow down the absorption rate of glucose in the intestines, helping to stabilize blood sugar levels and providing an auxiliary treatment effect for diabetic patients [5]. Additionally, it can lower blood lipid and cholesterol levels, preventing the occurrence of cardiovascular diseases.
KGM has a wide range of applications. In the food industry, KGM is used as a thickening agent, stabilizer, and gelling agent to improve the texture and taste of food. In the medical field, KGM is used to treat diseases such as diabetes and constipation and as an ingredient in health foods, it is widely used in the development of health products. Furthermore, KGM can also be used in cosmetics and environmental protection materials. In recent years, with the deepening of research on KGM, its applications in various fields have been continuously expanding.
Currently, scientific research on konjac glucomannan (KGM) is progressing rapidly, covering various areas such as its impact on food quality, food processing performance, preparation techniques for composite films, and applications in medical and health products. For example, Fan Dongcui et al. [6] studied konjac meal replacement powder, which is characterized by strong satiety, low calories, and high fiber. It effectively controls food intake and energy consumption when eaten, achieving nutritional health and weight loss. This aligns with consumer preferences, making it one of the popular meal replacement foods on the market. There have been reviews on related research of KGM and its derivatives, the application prospects of KGM, and the research progress on the food processing performance of KGM. However, a systematic and comprehensive summary of KGM’s properties, various preparation methods, and medical applications is needed. This paper introduces the physicochemical properties, preparation methods, and health benefits of KGM, filling this gap.
This paper starts with the physicochemical properties of KGM, focusing on summarizing its preparation methods and health benefits. It discusses KGM’s unique properties in terms of water solubility, gelation, and organic chemical reactions, and analyzes its application potential and practical effects in the food industry, medical health, and other fields. This provides valuable references for the further development and utilization of KGM.
2. Properties of Konjac Glucomannan
2.1. Molecular Structural Characteristics of Konjac Glucomannan
Konjac glucomannan (KGM) is a polysaccharide primarily composed of glucose and mannose. The molecular chain of KGM is connected by β-(1-4) glycosidic bonds, with alternating glucose and mannose units. This alternating structure allows KGM to form a specific spatial conformation, with a defined molecular size and shape. Reports indicate that the ratio of mannose to glucose is 1.6:1, and acetyl groups are present at the C-6 position of mannose. These acetyl groups control the degree of the molecule’s water solubility. Natural KGM consists of radially arranged micelles and has two types of crystal structures: Mannose I and Mannose II. The former crystal structure does not contain water molecules, while the latter contains crystallization water bound by hydrogen bonds. X-ray diffraction analysis indicates that KGM particles exhibit a nearly amorphous structure [7].
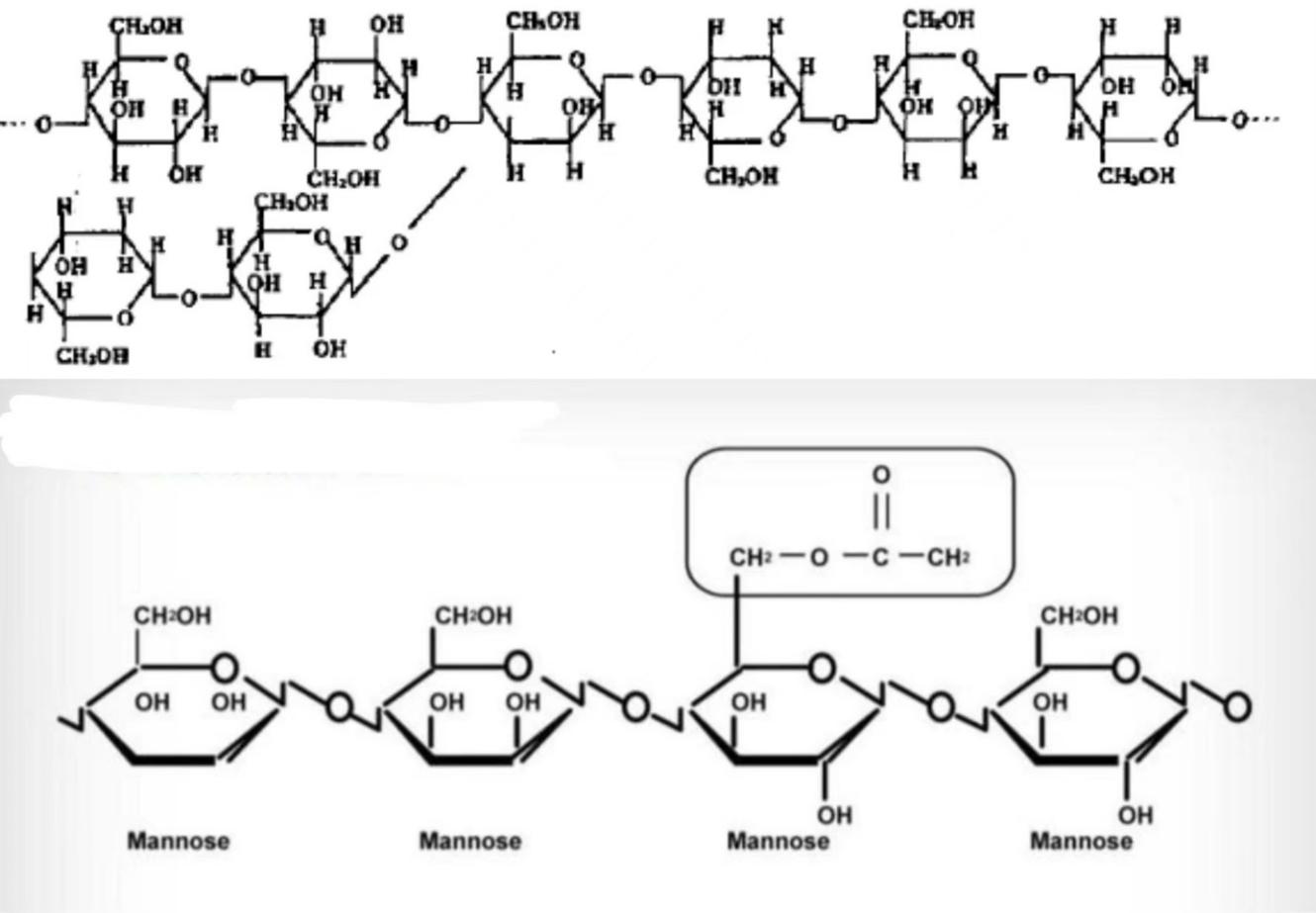
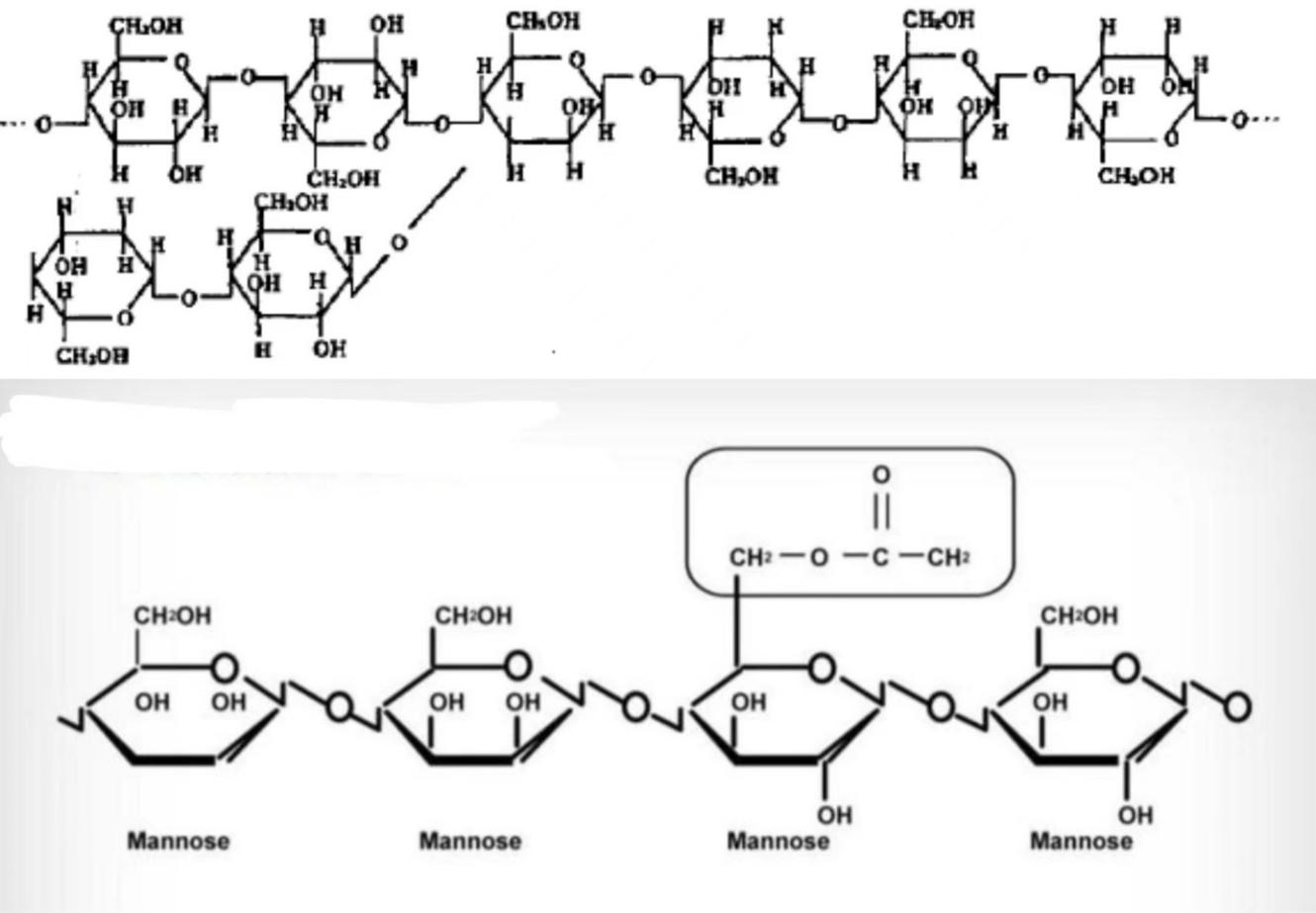
Figure 1. Two Hypothetical Molecular Structures of Konjac Glucomannan [8].
The molecular weight of KGM can reach up to millions of Daltons, and the molecule appears more extended in solution [9]. KGM solutions have high viscosity, excellent hydrophilicity, gelation properties, thickening capacity, and adhesiveness, making it widely used as a thickening and gelling agent in the food industry. However, its large relative molecular mass results in poor rheological properties, strong hydrophilicity, poor stability in aqueous solutions, and susceptibility to degradation, which limit its applications [10].
2.2. Physicochemical Properties of Konjac Glucomannan
Konjac glucomannan (KGM) has good solubility in water, forming colloidal solutions. Natural KGM dissolves easily in water but is insoluble in methanol, ethanol, acetone, ether, and other organic solvents. It can form flexible helices with acetyl groups as delicate conformational stabilizers, resulting in a porous double helix structure. These sugar chains are free-moving and can retain a large amount of water, capable of swelling 80 to 100 times their original volume, exhibiting excellent thickening properties [11]. KGM can form both heat-stable (thermo-irreversible) and heat-unstable (thermo-reversible) gels under different conditions. When heated, the acetyl groups on the glucomannan are removed, forming a stable gel state [12]. The increased viscosity endows KGM solutions with good gelling and adhesive properties, making it widely used as a thickening and gelling agent in the food industry.
KGM demonstrates good thermal stability within a certain temperature range, maintaining relatively stable molecular structure and physical properties under high-temperature conditions. Studies have shown that KGM’s molecular structure remains largely intact at temperatures below 100°C [1-2]. Additionally, KGM exhibits good freeze-thaw stability, retaining its physical properties during freezing and thawing processes.
When KGM sol is dehydrated, it can form smooth, adhesive films that are stable in both hot and cold water as well as in dilute acid and alkali solutions [8]. Due to its unique long-chain structure, KGM has many hydroxyl groups and reactive groups on its main and side chains, allowing it to undergo various chemical reactions such as methylation, esterification, etherification, hydrolysis, degradation, and complexation [13]. For example, acetylation modification utilizes the hydroxyl groups on KGM’s molecular chain to react with acyl chains (carbon atoms 2-12) under certain conditions to produce new acetylated products, a form of esterification modification [3]. Under alkaline conditions, heating KGM leads to thermo-irreversible gelation. In the presence of NaOH, KOH, Na2CO3, or K2CO3, heating causes the acetyl groups on KGM to detach, resulting in a stable gel state [12].
3. Preparation of Konjac Glucomannan
3.1. Raw Material Selection and Pretreatment
To prepare konjac glucomannan (KGM), konjac tubers must be selected and processed into konjac flour. As the main component of konjac flour, KGM’s yield and application value are significantly affected by the impurity content. Konjac flour, the primary product of rough processing, contains considerable amounts of starch, proteins, minerals, and alkaloids, among other impurities. Therefore, it is necessary to separate and purify KGM from konjac flour. Current methods for KGM separation and purification include microwave and ultrasonic-assisted methods, organic solvent precipitation, chemical reagent purification, enzymatic methods, and acid hydrolysis [14].
3.2. Extraction Process
3.2.1. Acid Hydrolysis Method. Hydrolysis is one of the common methods used to prepare KGM. This method employs dilute acids to hydrolyze impurities in konjac flour to achieve purification. Kumoro et al. reported that acid hydrolysis of konjac flour in 0.5M hydrochloric acid solution at 60°C for 1 hour resulted in a product purity of 90.18% (w/w) and a yield of 66.67% [15]. Compared to the strong corrosiveness and safety risks of hydrochloric and sulfuric acids, citric acid, as an organic acid, has distinct advantages. He Xinlei et al. found that citric acid at pH 5.0 (extraction at 60°C for 1 hour) achieved an SO2 elution rate of 89.89% for KGM, effectively reducing the SO2 content produced during the initial processing and color protection stage of konjac [16]. According to related studies, purifying KGM using the same acid hydrolysis method, citric acid hydrolysis at 60°C is superior to traditional strong acid hydrolysis because it is milder, safer, and can effectively inhibit the browning of KGM gel without compromising the gel strength [17].
3.2.2. Enzymatic Extraction Method. The enzymatic method is another common approach for preparing KGM. By selecting appropriate enzymes, polysaccharides in konjac can be reversibly enzymatically hydrolyzed into glucomannan. Compared to the hydrolysis method, the enzymatic method has relatively relaxed requirements for conditions such as temperature and pH. The KGM prepared by the enzymatic method differs in molecular weight distribution, structural characteristics, and biological activity from that prepared by hydrolysis. Wardhani et al. found that the hydrolytic action of amylase reduced the swelling capacity of pure KGM and lowered the viscosity of its aqueous solution [18]. Researchers can further alter the properties and functions of KGM by adjusting the reaction conditions and types of enzymes used in enzymatic hydrolysis.
3.2.3. Microwave and Ultrasonic-Assisted Extraction Methods. Microwave-assisted extraction is a technique that rapidly increases the internal temperature of cells, accelerating the disruption of cell structures and thereby releasing the active components. Using microwaves to extract KGM results in high yields, saves time, and significantly enhances the viscosity, stability, and antibacterial properties of the product. The ultrasonic-assisted method leverages the cavitation effect, mechanical action, and thermal effects of ultrasound to accelerate the release of intracellular active components, promote the dissolution of these components, and improve the extraction rate. Research indicates that with an ultrasonic power of 207.5W and an ultrasonic time of 20.8 minutes, the KGM extraction rate can exceed 80%, maintaining good reduction capabilities [19-20]. Additionally, high-intensity ultrasonic treatment can reduce the rheological properties of KGM to some extent and disrupt the sol structure of KGM, making it an effective means of degrading KGM.
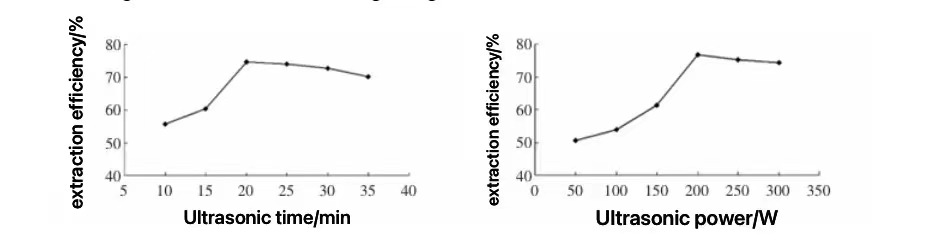
Figures 2. The Effects of Ultrasonic Time and Power on Extraction Efficiency [19].
3.3. Purification Techniques
The ethanol precipitation method is the most commonly used technique for purifying KGM, primarily leveraging KGM’s solubility in water and insolubility in ethanol. Since KGM is a macromolecular substance, its chain-like structure tends to intertwine at room temperature, making it difficult to fully swell and thus challenging to remove entrapped impurities. Therefore, during the purification process, konjac flour is added to hot water and continuously stirred to disperse the molecular chains as much as possible, allowing them to swell rapidly and hasten the precipitation of impurities. KGM forms a gel-like substance when the ethanol concentration is below 50%, and its solubility decreases when the ethanol concentration is above 50%. Thus, KGM can be eluted with ethanol concentrations greater than 50% to remove impurities and produce high-purity konjac refined powder [21].
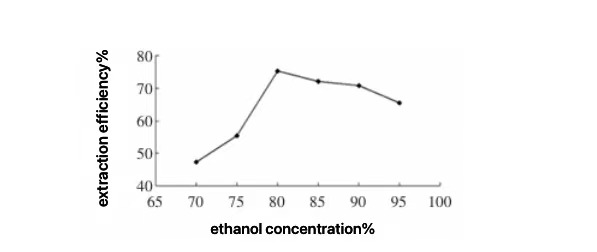
Figure 3. The Effect of Ethanol Concentration on Extraction Efficiency [19].
4. Physiological Activity (Health Benefits) of Konjac Glucomannan
4.1. Promoting Intestinal Health
Konjac glucomannan (KGM) exhibits significant benefits in the realm of intestinal health, serving as a high-quality prebiotic with positive impacts on the microecological balance of the gut. Firstly, it effectively promotes the growth of beneficial bacteria such as bifidobacteria and lactobacilli, which aids in the restoration of the damaged mucosal barrier. This function is crucial for maintaining the homeostasis of the gut microbiota, as it not only inhibits the overgrowth of pathogenic bacteria but also enhances intestinal immune function, thereby positively impacting the alleviation of intestinal diseases [22]. Secondly, KGM can be degraded by specific bacteria in the colon, and its fermentation products provide energy for the body while also serving as precursor substances in the synthesis of nutrients. This characteristic gives KGM a unique advantage in promoting intestinal health. Additionally, KGM and its fermentation products can, through feedback mechanisms, promote the colonization of beneficial bacteria in the gut while inhibiting the proliferation of pathogenic bacteria, further optimizing the structure of the gut microbiota. The production of organic acids significantly lowers the intestinal pH, effectively inhibiting the proliferation of harmful bacteria such as Escherichia coli. Bacteroides can produce β-mannanase, which specifically degrades KGM, aiding its growth and proliferation in the colonic environment [23]. Furthermore, KGM, when dissolved into a colloid, can prevent direct contact between heavy metals and toxic substances with the intestines, effectively reducing the possibility of harmful bacterial infections in the colon and rectum [23].

Figure 5. The Effect of Different Concentrations of KGM on the Viability of NCM460 Cells After 24, 48, and 72 Hours [22].
4.2. Lowering Blood Sugar Levels
When dissolved in water, konjac glucomannan (KGM) exhibits significant swelling properties, which can enhance gastric motility and effectively delay gastric emptying, resulting in a pronounced feeling of satiety. The high-viscosity substance formed after KGM dissolves increases the viscosity of the chyme in the digestive tract, prolonging its retention time in the stomach and forming a protective membrane barrier that effectively inhibits the rise of blood sugar and urine sugar levels. This characteristic further underscores KGM’s potential in maintaining metabolic health [24]. Clinical studies by Zhang Maoyu et al. found that daily intake of a certain amount of glucomannan for diabetic patients over three months resulted in decreased serum cholesterol and blood sugar levels, significantly reducing the patients’ dependence on insulin and hypoglycemic drugs and alleviating the daily burden of taking hypoglycemic medications [25].
4.3. Lowering Blood Lipid Levels
Konjac glucomannan exhibits significant advantages in regulating lipid metabolism. It can effectively inhibit the absorption of cholesterol, bile acids, and other lipid substances in the small intestine, promoting fat metabolism and reducing the entry of fats and cholesterol into the bloodstream, thereby effectively lowering the total amount of triglycerides and cholesterol in the blood. The intake of KGM not only regulates lipid levels by producing short-chain fatty acids (SCFAs) but also forms a gel-like substance on the intestinal surface, which reduces the absorption of fats and cholesterol [25]. Research by Vuksan et al. further revealed the impact of different viscosities of dietary fiber on human cholesterol levels. They found that the reduction in low-density lipoprotein (LDL) was positively correlated with the apparent viscosity of dietary fiber. This finding suggests that high-viscosity dietary fibers, such as KGM, can slow the absorption rate of nutrients in the small intestine by forming a gel-like substance, and in conjunction with the fermentation of KGM by colonic bacteria, ultimately collaborate to lower cholesterol concentrations in the body [26].
4.4. Immunomodulatory Effects
Konjac glucomannan covers the intestinal wall surface, where invading pathogens are recognized and attached by exogenous lectins on the cell surface, thereby filtering and eliminating pathogens and toxins, and enhancing the body’s immunity [12]. The fermentation products of KGM in the colon, SCFAs, can regulate the chemotaxis of immune cells and exhibit anti-inflammatory effects by releasing cytokines and reactive oxygen species (ROS) [22]. As a unique dietary fiber, KGM contains abundant mannose residues in its structure. Under the action of intestinal microorganisms, these mannose residues are degraded and released as mannose monomers. These mannose monomers exhibit strong immunomodulatory capabilities through the mannose receptor pathway. They not only inhibit infections by certain harmful bacteria but also promote the wound healing process, demonstrating excellent anti-inflammatory effects in the body [26].
5. Conclusion
Konjac glucomannan (KGM) is a high molecular weight, non-ionic, water-soluble polysaccharide extracted from the tuber of the konjac plant, and is a major component of konjac. This polysaccharide is primarily composed of glucose and mannose linked by β-(1-4) glycosidic bonds, featuring an extremely high molecular weight and unique gelling properties. It is the natural polymer with the highest viscosity among known plant polysaccharides. Abundant in nature, konjac glucomannan not only has excellent value in the food industry but also shows broad application potential in pharmaceuticals, cosmetics, and environmental protection. It is commonly used as a source of dietary fiber, a raw material for skincare products, and in wastewater treatment.
Konjac glucomannan is a high molecular weight heteropolysaccharide polymerized through β-1,4 glycosidic bonds. It possesses various unique physicochemical properties, including hydrophilicity, gelling ability, thickening capacity, and thermal stability, and can undergo various reactions such as methylation, esterification, etherification, and hydrolysis. The preparation of konjac glucomannan involves dissolving konjac powder, purifying it, and extracting and concentrating it through methods like acid hydrolysis, enzymatic extraction, microwave cleavage, or ultrasonic extraction, ultimately yielding high-purity konjac glucomannan. The purification process includes several steps such as ethanol precipitation. Konjac glucomannan offers multiple health benefits, including promoting gut health, lowering blood sugar and lipid levels, and immune modulation. As a natural soluble dietary fiber, it reduces glucose absorption by forming a gel-like substance on the intestinal surface, thereby improving gut microbiota.
The future applications of konjac glucomannan are vast. With the development of the pharmaceutical and food industries, its applications in food, cosmetics, medicine, and environmental protection will continue to expand. This will lead to the development of more high-quality, functional konjac products, meeting diverse market demands and supporting the rapid growth of the konjac industry.
References
[1]. Xu, S., & Yang, L. (1990). Properties of konjac glucomannan and the quality of konjac flour. Journal of Wuxi Institute of Light Industry, 9(3), 26-32.
[2]. Xu, S., & Qian, H. (1991). Chemical structure and rheological properties of konjac glucomannan. Journal of Wuxi Institute of Light Industry, 10(1), 1-12.
[3]. Liao, Q., & Zhang, H. (2022). Functional modification and application of konjac glucomannan. Journal of Shaanxi Normal University, 50(5), 64-80.
[4]. Li, X., Cao, Y., Tang, X., et al. (2023). Effect of konjac glucomannan on genomic stability in human colon cell lines. Journal of Food and Biotechnology, 42(11), 11-18.
[5]. Wang, Y. (2021). Structure and health benefits of konjac glucomannan. Modern Food, (07), 113-115. https://doi.org/10.16736/j.cnki.cn41-1434/ts.2021.07.031
[6]. Fan, D., Qiu, L., Chen, J., et al. (2024). Functional characteristics of konjac fruit and vegetable health meal replacement powder. Journal of Food and Biotechnology, 43(3), 85-93.
[7]. Ogawa, K., Yui, T., & Mizuno, T. (1991). X-ray diffraction study of glucomannans and their acetates. Agricultural and Biological Chemistry, 55(8).
[8]. Chen, Y., & Hou, Z. (2006). Mechanism and practice of konjac gum (konjac glucomannan, KGM) in the food additive industry. Food Industry Technology, 27(01), 155-157.
[9]. Li, B., & Xie, B. (2003). Study on the molecular chain morphology and chain parameters of konjac glucomannan. Acta Pharmaceutica Sinica, (11), 838-842. https://doi.org/10.16438/j.0513-4870.2003.11.010
[10]. Hou, Z. (2008). Chemical modification and property study of konjac glucomannan (Doctoral dissertation). Wuhan University of Technology.
[11]. Wang, Y., Zhang, S., & Wei, F. (2011). Effects of modification treatments on the properties of konjac glucomannan. Grain Science and Technology, 36(02), 49-51+56. https://doi.org/10.16465/j.gste.2011.02.019
[12]. Zhang, Y. (2023). Physicochemical properties and application prospects of konjac glucomannan. Food Safety Guide, (18), 148-150.
[13]. Xu, Q., & Pang, J. (2003). Characteristics of konjac glucomannan and its application in food. Grain and Oil, 16(07), 46-47.
[14]. Kumoro, A. C., Yuganta, T. H. A., Ratnawati, R., et al. (2016). Effect of catalyst concentration and reaction time on the extraction of glucomannan from porang ( Amorphophallus oncophyllus ) flour via acid hydrolysis. IOP Conference Series: Materials Science and Engineering, 162(1), 012020.
[15]. He, X., Wen, C., & Chen, Q. (2008). Discussion on the acid purification of konjac polysaccharides. Guangxi Tropical Agriculture, (01), 3-5.
[16]. Teng, S., Hui, L., Razak, M. A., et al. (2022). Improvement of Storage Stability of Zein-Based Pickering Emulsions by the Combination of Konjac Glucomannan and L-Lysine. Frontiers in Nutrition, 9, 1520.
[17]. Wardhani, D. H., Aryanti, N. R., & Amdani, A. D., et al. (2017). Swelling Capacity of Glucomannan from Amorphophallus oncophyllus Purified with Enzymatic Hydrolysis. Advanced Science Letters, 23(6), 5623-5625.
[18]. Zhang, Y. (2015). Extraction of konjac glucomannan and its antioxidant activity. Food Research and Development, 36(14), 87-91.
[19]. Yang, M. (2014). Optimization of konjac glucomannan extraction technology and development of sports drinks. Food Industry, 35(10), 28-31.
[20]. Nie, Y. (2016). Purification of konjac glucomannan and its application in rice (Doctoral dissertation). Jilin University.
[21]. Li, X., Cao, Y., Tang, X., et al. (2023). Effect of konjac glucomannan on genomic stability in human colon cell lines. Journal of Food and Biotechnology, 42(11), 11-18.
[22]. Li, Y. (2021). Microwave and enzymatic degradation preparation of konjac glucomannan and its probiotic activity (Doctoral dissertation). Nanchang University. https://doi.org/10.27232/d.cnki.gnchu.2021.002310
[23]. Zhang, M., Huang, C., Hong, J., et al. (1989). Study on the effects of konjac food on human lipid metabolism. Journal of Nutrition, (1), 25-30.
[24]. Chen, H., Nie, Q., Hu, J., et al. (2019). Hypoglycemic and Hypolipidemic Effects of Glucomannan Extracted from Konjac on Type 2 Diabetic Rats. Journal of Agricultural and Food Chemistry, 67(18), 5278-5288.
[25]. Vuksan, V., Sievenpiper, J. L., Owen, R., et al. (2000). Beneficial effects of viscous dietary fiber from Konjac-mannan in subjects with the insulin resistance syndrome results of a controlled metabolic trial. Diabetes Care, 23(1), 9-14.
[26]. Yeh, S. L., Lin, M. S., & Chen, H. L. (2010). Partial hydrolysis enhances the inhibitory effects of konjac glucomannan from Amorphophallus konjac C. Koch on DNA damage induced by fecal water in Caco-2 cells Food Chemistry, 119(2), 614-618.
Cite this article
Feng,Y. (2024). Konjac Glucomannan: Properties, Preparation, and Health Effects. Theoretical and Natural Science,62,123-130.
Data availability
The datasets used and/or analyzed during the current study will be available from the authors upon reasonable request.
Disclaimer/Publisher's Note
The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of EWA Publishing and/or the editor(s). EWA Publishing and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
About volume
Volume title: Proceedings of the 4th International Conference on Biological Engineering and Medical Science
© 2024 by the author(s). Licensee EWA Publishing, Oxford, UK. This article is an open access article distributed under the terms and
conditions of the Creative Commons Attribution (CC BY) license. Authors who
publish this series agree to the following terms:
1. Authors retain copyright and grant the series right of first publication with the work simultaneously licensed under a Creative Commons
Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this
series.
2. Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the series's published
version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial
publication in this series.
3. Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and
during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See
Open access policy for details).
References
[1]. Xu, S., & Yang, L. (1990). Properties of konjac glucomannan and the quality of konjac flour. Journal of Wuxi Institute of Light Industry, 9(3), 26-32.
[2]. Xu, S., & Qian, H. (1991). Chemical structure and rheological properties of konjac glucomannan. Journal of Wuxi Institute of Light Industry, 10(1), 1-12.
[3]. Liao, Q., & Zhang, H. (2022). Functional modification and application of konjac glucomannan. Journal of Shaanxi Normal University, 50(5), 64-80.
[4]. Li, X., Cao, Y., Tang, X., et al. (2023). Effect of konjac glucomannan on genomic stability in human colon cell lines. Journal of Food and Biotechnology, 42(11), 11-18.
[5]. Wang, Y. (2021). Structure and health benefits of konjac glucomannan. Modern Food, (07), 113-115. https://doi.org/10.16736/j.cnki.cn41-1434/ts.2021.07.031
[6]. Fan, D., Qiu, L., Chen, J., et al. (2024). Functional characteristics of konjac fruit and vegetable health meal replacement powder. Journal of Food and Biotechnology, 43(3), 85-93.
[7]. Ogawa, K., Yui, T., & Mizuno, T. (1991). X-ray diffraction study of glucomannans and their acetates. Agricultural and Biological Chemistry, 55(8).
[8]. Chen, Y., & Hou, Z. (2006). Mechanism and practice of konjac gum (konjac glucomannan, KGM) in the food additive industry. Food Industry Technology, 27(01), 155-157.
[9]. Li, B., & Xie, B. (2003). Study on the molecular chain morphology and chain parameters of konjac glucomannan. Acta Pharmaceutica Sinica, (11), 838-842. https://doi.org/10.16438/j.0513-4870.2003.11.010
[10]. Hou, Z. (2008). Chemical modification and property study of konjac glucomannan (Doctoral dissertation). Wuhan University of Technology.
[11]. Wang, Y., Zhang, S., & Wei, F. (2011). Effects of modification treatments on the properties of konjac glucomannan. Grain Science and Technology, 36(02), 49-51+56. https://doi.org/10.16465/j.gste.2011.02.019
[12]. Zhang, Y. (2023). Physicochemical properties and application prospects of konjac glucomannan. Food Safety Guide, (18), 148-150.
[13]. Xu, Q., & Pang, J. (2003). Characteristics of konjac glucomannan and its application in food. Grain and Oil, 16(07), 46-47.
[14]. Kumoro, A. C., Yuganta, T. H. A., Ratnawati, R., et al. (2016). Effect of catalyst concentration and reaction time on the extraction of glucomannan from porang ( Amorphophallus oncophyllus ) flour via acid hydrolysis. IOP Conference Series: Materials Science and Engineering, 162(1), 012020.
[15]. He, X., Wen, C., & Chen, Q. (2008). Discussion on the acid purification of konjac polysaccharides. Guangxi Tropical Agriculture, (01), 3-5.
[16]. Teng, S., Hui, L., Razak, M. A., et al. (2022). Improvement of Storage Stability of Zein-Based Pickering Emulsions by the Combination of Konjac Glucomannan and L-Lysine. Frontiers in Nutrition, 9, 1520.
[17]. Wardhani, D. H., Aryanti, N. R., & Amdani, A. D., et al. (2017). Swelling Capacity of Glucomannan from Amorphophallus oncophyllus Purified with Enzymatic Hydrolysis. Advanced Science Letters, 23(6), 5623-5625.
[18]. Zhang, Y. (2015). Extraction of konjac glucomannan and its antioxidant activity. Food Research and Development, 36(14), 87-91.
[19]. Yang, M. (2014). Optimization of konjac glucomannan extraction technology and development of sports drinks. Food Industry, 35(10), 28-31.
[20]. Nie, Y. (2016). Purification of konjac glucomannan and its application in rice (Doctoral dissertation). Jilin University.
[21]. Li, X., Cao, Y., Tang, X., et al. (2023). Effect of konjac glucomannan on genomic stability in human colon cell lines. Journal of Food and Biotechnology, 42(11), 11-18.
[22]. Li, Y. (2021). Microwave and enzymatic degradation preparation of konjac glucomannan and its probiotic activity (Doctoral dissertation). Nanchang University. https://doi.org/10.27232/d.cnki.gnchu.2021.002310
[23]. Zhang, M., Huang, C., Hong, J., et al. (1989). Study on the effects of konjac food on human lipid metabolism. Journal of Nutrition, (1), 25-30.
[24]. Chen, H., Nie, Q., Hu, J., et al. (2019). Hypoglycemic and Hypolipidemic Effects of Glucomannan Extracted from Konjac on Type 2 Diabetic Rats. Journal of Agricultural and Food Chemistry, 67(18), 5278-5288.
[25]. Vuksan, V., Sievenpiper, J. L., Owen, R., et al. (2000). Beneficial effects of viscous dietary fiber from Konjac-mannan in subjects with the insulin resistance syndrome results of a controlled metabolic trial. Diabetes Care, 23(1), 9-14.
[26]. Yeh, S. L., Lin, M. S., & Chen, H. L. (2010). Partial hydrolysis enhances the inhibitory effects of konjac glucomannan from Amorphophallus konjac C. Koch on DNA damage induced by fecal water in Caco-2 cells Food Chemistry, 119(2), 614-618.









