1. Introduction
Cancer, an illness arising from the unchecked and abnormal growth of cells, stands as one of the most lethal illnesses throughout the world. In numerous countries, it holds the grim distinction of being the second leading cause of mortality. In 2022 alone, an estimated 1.9 million new cases of cancer are projected to be identified, with approximately 609,360 individuals succumb to the disease in the United States alone[1]. Conventional treatments including chemotherapy, radiotherapy present significant side effects and toxicity[2]. In contrast to conventional treatments, immunotherapy presents clear advantages, including the establishment of long-term memory, prevention of tumor recurrence, and control of metastasis. This approach could be particularly advantageous for patients lacking adequate levels of pre-existing anti-tumor T cells and/or immune checkpoint molecules, thereby benefiting a broader range of individuals[3]. Nevertheless, numerous obstacles have hindered the widespread clinical adoption of cancer immunotherapies[4]. A notable challenge is the limited clinical response rate, with fewer than one-fourth of patients exhibit a positive response to immune checkpoint inhibitors (ICIs). While combining multiple ICIs may enhance clinical efficacy, it also gives rise to severe adverse events, including dermatitis and liver damage[3].
Cancer vaccines hold the potential of activating immune system through the stimulation of T cells via antigens, fostering a specific and targeted immune response. Tumor antigens recognized by T lymphocytes are central to the efficacy of cancer vaccines[5]. T cells recognize foreign antigens only when they are displayed on the surfaces of the body's own cells, which may derive from pathogens like viruses or intracellular bacteria replicating within cells or from pathogens or their components internalized through endocytosis from the extracellular fluid[6]. Antigen presentation refers to the procedure wherein antigens are showcased on the surface of antigen-presenting cells (APCs). This presentation occurs in conjunction with MHC class II molecules during interactions with CD4+ helper T cells or with MHC class I molecules when engaging with CD8+ cytotoxic T cells. Successful presentation relies on the strong binding of peptides to MHC class II molecules; peptides with insufficient binding capacity are not presented, resulting in an ineffective immune response[7]. After maturing in the thymus, T cells circulate throughout the body. When they encounter their specific antigen displayed on the surface of an APC, the T cell receptors on both CD4+ helper T cells and CD8+ cytotoxic T cells bind to the antigen within the Major Histocompatibility Complex (MHC) on the APC surface[7]. The interaction between the presented antigen and MHC class II molecules with the CD4+ helper T cell receptor activates the CD4+ lymphocyte, leading to the release of IL-2 and the expression of IL-2 receptors on the lymphocyte surface[7]. The IL-2 produced by the activated cell not only stimulates its own receptors but also those of mononuclear phagocytes, intensifying their microbicidal activity, prompting B cells to generate antibodies[8]. This interaction initiates the activation of the T cells. Cancer vaccines that target the initial stages of antigen processing could potentially enhance both therapeutic and preventive effectiveness, addressing not only primary tumors but also inoperable metastases or relapses[9].
Though the concept of vaccines as a Cancer treatment has become more appealing, significant challenges remain. Existing drug delivery techniques often face challenges such as imprecise drug distribution, potential toxicity, and environmental repercussions arising from the use of non-degradable materials. These constraints impede the effectiveness of treatments, compromise patient adherence, and contribute to environmental issues. Furthermore, the inadequate induction of immune responses through conventional vaccination approaches has hindered successful treatment and complete tumor eradication[10]. In other words, there has been a high demand for a more effective delivery method[4].
Gold nanoparticles, when used in conjunction with cancer vaccines, offer an optimal approach for enhancing cellular immunity.. Among the various nanoparticles, gold nanoparticles have gained considerable interest in cancer therapy due to their minimal toxicity, strong stability, ease of uptake by cells, impressive optical characteristics, and versatile surface functionalities. Gold nanoparticles can function as adjuvants enhancing vaccine efficacy by stimulating antigen-presenting cells (APCs) and facilitating controlled antigen release. Additionally, they are chemically inert, biocompatible, and easily producible[3]. Drug or treatments can attach to gold nanoparticles through covalent or noncovalent interactions, and the surface of gold nanoparticles can be modified to enhance drug encapsulation and delivery[11]. Specific modifiers like antibodies, aptamers, carbohydrates, and other ligands recognizing tumor-associated markers enable targeted drug delivery. Gold nanoparticles could also serve as a vaccine adjuvant. Their entry into immune cells induces cytokine production, stimulates T cells, activates immune response genes, improves antigen processing, and prompts B cells to secrete antibodies. This makes nanoparticles promising carriers and adjuvants in the formulation of antibodies and vaccines targeting infections[11]. Ovalbumin (OVA), a glycoprotein with a molecular weight of approximately 45 kDa, represents the predominant protein found in chicken egg whites[12]. Its sufficient size and complexity elicit a mild immunogenic response, making OVA a widely utilized model antigen in experimental immunization and vaccination research[13,14].
Building on the provided background, we undertook the modification of the surface of AuNP using PEI and loaded OVA (-) onto both the positive and negative electrodes of PEI, creating a gold nanoparticle vaccine. With the PEI modified AuNP, OVA could easily form bonds with the nanoparticles. Furthermore, the positively charged nature of PEI facilitates easier penetration through cell membranes, thereby reducing the distance between the cells and the nanoparticles. This strategic modification not only addressed the technical challenges of connecting OVA to AuNP but also boosted the enhanced cellular penetration capabilities of PEI, contributing to the overall efficacy of the gold nanoparticle vaccine. Modified AuNP could deliver OVA to the APC that can activate dendritic cell (DC) maturation, leading to antigen specific immune response.
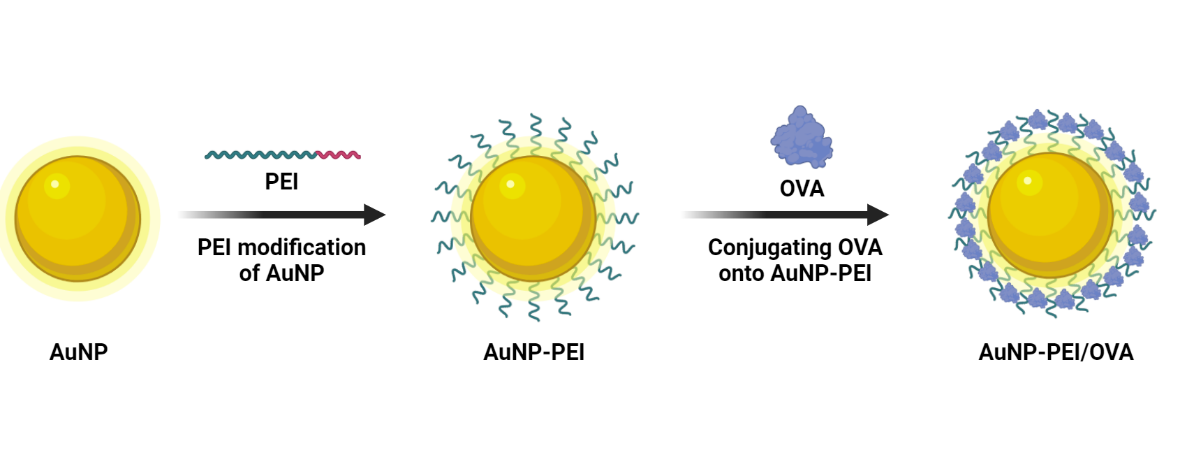
Scheme 1. Scheme for an overview of the modifying AuNP into AuNP-PEI/OVA.
2. Materials and methods
2.1. Cell culture
Prepare cultural dishes: new Petri dishes, centrifuge tubes and add appropriate culture media to them. Culture the cells to a confluency of 80%-90%, collect the cells, and wash them 1-2 times with cell culture medium to remove cell debris and waste. Appropriate trypsin enzyme was used to to digest cell adhesion at 37°C. Then culture medium containing 10% fetal bovine serum was added to stop digestion, and blow the supernatant evenly into the centrifuge tube. Centrifuge the cell suspension to collect cell pellets. An appropriate amount of culture medium was used to suspend the cells. Transplanted the prepared cell suspension into a new culture dish and distribute it evenly. Place the culture dish in an appropriate environment, control factors such as temperature, humidity, CO2 concentration, etc., replace the culture medium regularly, and observe the cell growth. All of the operating procedures should be strictly followed to avoid any possible contamination. RAW 264.7 cells are cultured in DMEM, complemented with 10% Fetal Bovine Serum (FBS) and 1% Penicillin Streptomycin (Pen/Strep). DC 2.4 cells are cultured with RPMI, complemented with 10% FBS and 1% Pen/Strep.
2.2. Synthesis of AuNP-PEI/OVA
As shown in Figure 1, AuNPs were synthesized with high monodispersity by slightly modifying the Turkevich method. Trisodium citrate dehydrate solution (3%, 1 mL) (Sigma-Aldrich, TX, USA) was added to hydrogen tetrachloroaurate (III) trihydrate (HAuCl4·3H2O) solution (0.25 mM, 100 mL) (SigmaAldrich) and stirred for 10 min under reflux system. After that, the solution was cooled to room temperature, and the synthesized nanoparticles were used in the following step.
Polyethylenimine (PEI) (MW 2000 by LS, SigmaAldrich) was used to modify the surface of AuNPs. A stock solution of PEI (2%, v/v) was prepared and then added to a stirring solution of AuNPs in 0.005% (v/v) concentration. After stirring for 1 h with the PEI solution, the synthesized AuNPs were coated with PEI. AuNPs were characterized in terms of shape, size, LSPR peak and zeta potential. In the final step, PEI-coated AuNPs were purified by centrifuging at 7000× g for 30 min and dispersing in 18.2 MΩ deionized water.
To determine the optimal OVA loading, different AuNP-PEI: OVA weight ratios (0.5, 1 and 1.5) were prepared. By adding the calculated quantity of OVA to the nanoparticle solution and pipetting up and down at least ten-times and stirring for 30 min, the antigen could load onto the AuNP. After centrifuging in 1000 rpm for 20 min, extract the supernatant without discarding it. Using the Bicinchoninic acid assay (BCA) method, the quantity of the remaining OVA within the supernatant could be calculated. The loading efficiency of the different OVA ratios were calculated by subtracting the OVA left over in the supernatant from the OVA loaded onto the cells. Using TEM and DLS to determine the AuNP-PEI/OVA diameter.
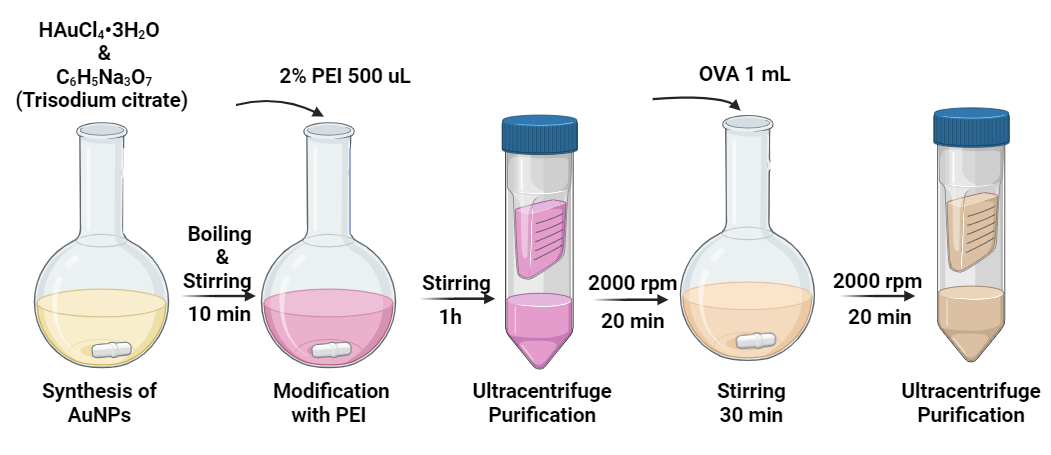
Figure 1. The protocol of synthesis of AuNP-PEI/OVA nanovaccine.
2.3. Cell-counting kits-8 assay
RAW264.7 and DC2.4 cells were used to assess the cytotoxicity of AuNP-PEI/OVA and AuNP-OVA. Cells were collected by centrifuging in 1200 rpm for three minutes. The cells were counted, then diluted to 5×104 /mL confluency, and incubated 100 uL per well in a 96-well plate for 24 hours to ensure adherence. Subsequently, varying concentrations (0, 10, 20, 50, 100, 200, and 300 µg/mL) of AuNP-PEI/OVA were added to each well. After 24 hours, 10 µL of CCK-8 solution were added to each well, followed by a 2-hour incubation in a cell incubator. The absorbance was measured at a wavelength of 450 nm using a microplate reader to calculate cytotoxicity.
2.4. The conjugation of Cy5 with OVA
By using flow cytometer and laser confocal scanning microscope, cellular uptake of AuNP-PEI/OVA could be determined. Cy5 powder was dissolved in DMSO 10ug/mL. Cy5 and OVA were mixed in 10:1 molar ratio. They were then reacted in room temperature for two hours. The reacted Cy5 and OVA mixture was purified by ultracentrifuge. First, the reacted mixture was added into ultracentrifuge tube, centrifuged at 2000 rpm for 20 minutes. The liquid in the outer tube was discarded. 500 uL of PBS was added into the inner tube. The process was repeated three times. Cy5-labeled OVA was mixed with AuNP-PEI to form fluorescence-labeled nanoparticles, which was co-cultured with RAW264.7 or DC2.4 for 24 h. Then one patch of cells was collected and detected for Cy5 signal by flow cytometer. The other patch of cells was stained with DAPI after co-culture for 30 min then proceeding confocal imaging to check if Cy5 signal is in cells.
2.5. Flow cytometer
By using flow cytometer, cellular uptake of AuNP-PEI/OVA could be determined. Cy5-labeled OVA was mixed with AuNP-PEI to form fluorescence-labeled nanoparticles, which was co-cultured with RAW264.7 or DC2.4 for 24 h. Then one patch of cells was collected and detected for Cy5 signal by flow cytometer. For detecting DC cells maturation, flow cytometer was applied to determine if AuNP-PEI/OVA could stimulate maturation. Cells were then stained with anti-CD11c-Cy5, anti-CD86-PE, anti-CD80-APC for 1h at room temperature. After 3-times of washing with PBS complemented 1% Fetal Bovine Serum (FBS), cells were detected for fluorescence signals with flow cytometer to compare percentage of mature BMDCs between different groups.
2.6. Laser scanning confocal microscopy
Cy5-labeled OVA was mixed with AuNP-PEI to form fluorescence-labeled nanoparticles, then AuNP-PEI/OVA-Cy5 was co-cultured with RAW264.7 or DC2.4 for 4 h at concentration of 10 μg/mL (OVA). Afterwards, the cells were washed 3 times with PBS and stained with DAPI dye for 30 min. After aspirating the supernatant, the cells were washed for 3 times with PBS then imaging under laser scanning confocal microscope.
2.7. BMDCs maturation
The BMDC cells were a gift from PhD Wu laboratory. Flow cytometer was applied to determine if AuNP-PEI/OVA could stimulate maturation. BMDCs were co-cultured with 2 µg/mL lipopolysaccharide (LPS), 10 µg/mL OVA, 10 µg/mL AuNP-OVA, 10 µg/mL AuNP-PEI/OVA for 24 h in CO2 incubator. Cells were then stained with anti-CD11c-Cy5, anti-CD86-PE, anti-CD80-APC for 1h at room temperature. After 3-times PBS with 1% FBS washes, cells were detected for fluorescence signals with flow cytometer to compare percentage of mature BMDCs between different groups.
2.8. ELISA
The supernatant of the BMDC cells were used to detect for the cytokines presented. BMDC cell were centrifuged and collected without discarding the supernatant. A day prior to the experiment, the capture antibody was coated on the 96 well plate in 1 ug/mL and cultured in 4 ℃ overnight. The well was taken out and the supernatant of the captured antibody was removed. PBST was added 200 uL into each well to clean the wells by removing it after three minutes. This process was repeated three times. Each well was added with BMDC cells’ supernatant for 50 uL each well. At room temperature, the supernatant was cultured for 2 hours. After two hours, the supernatant was discarded completely. Using 200 uL of PBST, the cells were cleaned again like the cleaning process above (cultured for three minutes, repeat for three times). Detection antibody was diluted by PBS in 1:200 ratio. Each well was added in 200 uL of the diluted detection antibody. The well was cultured again in 37℃ for an hour. After the culturing process, the supernatant is discarded, and the well was cleaned again by PBST like the cleaning process above. HRP conjugated secondary antibody was diluted with PBS in 1:200 ratio and 100 uL was added into the wells for color reaction. The wells were cultured in 37℃ for an hour. After removing the supernatant, the well was again cleaned by PBST for three times like the process above. Each well was added with 100 uL of TMB and reacted within 37℃ environment for 15 minutes. 1M of H2SO4 was added for 50 uL in each well to terminate the reaction. Microplate reader was used to analyze the resulting color reaction of the experiment. Excel was used to create a linear graph which was used to calculate the concentration of the cytokines within the supernatant.
3. Results and discussions
3.1. Synthesis of AuNPs and characterization
As demonstrated by TEM analysis, AuNP-PEI/OVA’s size is around 50 nm and highly monodisperse (Figure 2a). AuNP-PEI in demonstrates a hydrodynamic diameter of 146.3 nm and with polydispersity index (PDI) of 0.252 (Figure 2b). AuNP-PEI/OVA has a hydrodynamic diameter of 151.9 nm and PDI of 0.193 (Figure 2c). Additionally, AuNP-PEI has a zeta potential of 40 (units while when conjugated with OVA exist a negative charge, demonstrating the success of conjugating OVA onto the nanoparticles (Figure 2d). The gold nanoparticles are then verified with UV-vis spectrophotometer at 560 nm localized surface plasmon resonance (LSPR) peak (Figure 2e).
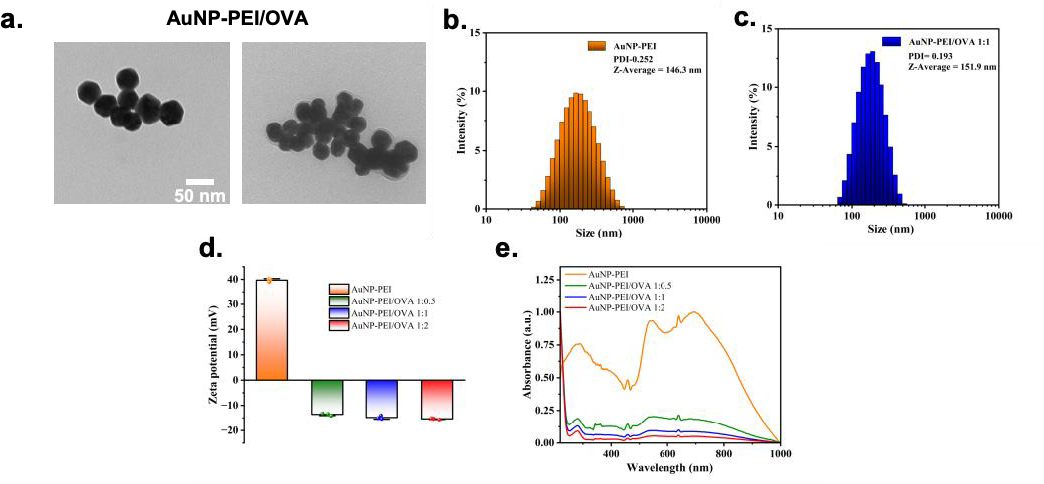
Figure 2. Characterization of AuNP-PEI/OVA nanoparticle.
3.2. Low cytotoxicity of AuNP-PEI/OVA nanovaccine
Next, the effect of AuNP-PEI/OVA on viability of two antigen-presenting cells was analyzed. In short, RAW264.7 and DC2.4 cells were cultured with varying concentrations of AuNP-PEI/OVA or AuNP-OVA in a 96-well plate. CCK-8 solution was added to each well to enhance the color reaction, and the absorbance at 450 nm wavelength was measured using a microplate reader to determine cytotoxicity. Figure 3a illustrates that AuNP-PEI/OVA nanoparticles led to a slight reduction in the viability of DC2.4 cells to approximately 75% after 24 hours of co-culture at a concentration of 150 μg/mL. Similar outcomes were observed in the RAW264.7 group treated with AuNP-PEI/OVA (Figure 3b). Meanwhile, AuNP-PEI/OVA only decreased cell viability to around 90% at concentration of 10 μg/mL for DC2.4 cells (Figure 3a). Additionally, RAW264.7 cells treated with AuNP-PEI/OVA reflect negligible cytotoxicity at 10 μg/mL (Figure 3b). Overall, these results indicate that AuNP-PEI/OVA demonstrates good biocompatibility and could be applied as nanovaccine in delivering tumor antigen.
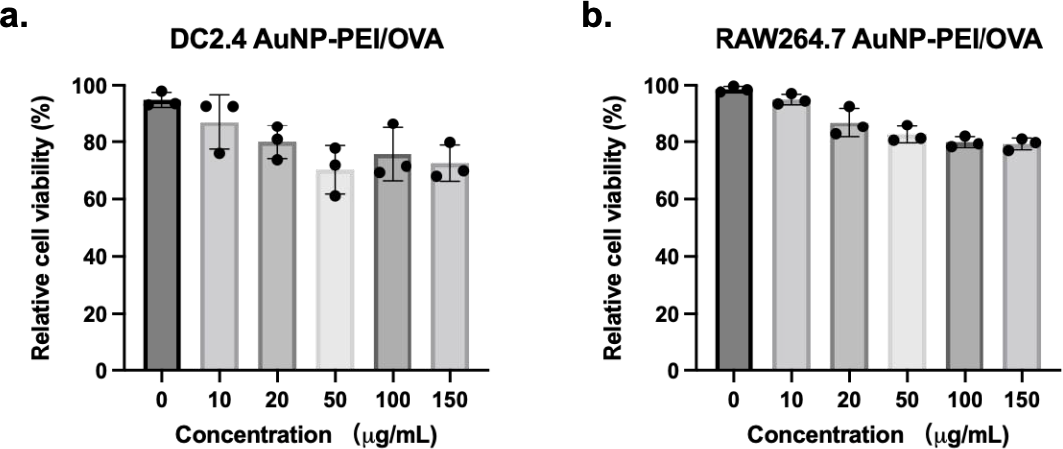
Figure 3. Low cytotoxicity of AuNP-PEI/OVA nanovaccine.
3.3. AuNP-PEI/OVA nanovaccine cell permeability
In order to confirm if AuNP-PEI/OVA could effectively enter antigen-presenting cells, Cy5-labeled OVA was mixed with AuNP-PEI to form fluorescence-labeled nanoparticles. Then AuNP-PEI/OVA-Cy5 was co-cultured with RAW264.7 or DC2.4 for 12 h. After co-culture, the Cy5 signal in the cells was detected using FACS. The data of FACS assay was analyzed by using Flowjo. The Figure 4a shows the gating strategy that was used to separate the living and dead cells. Subsequently, Cy5 signals in single living cells were analyzed. As illustrated in the histogram, the peak of cells treated with OVA-Cy5 only shows slight shift when compared to untreated cells (Figure 4b). In contrast, The peak of cells treated with AuNP-PEI/OVA-Cy5 demonstrates significant shift compared to cells treated with AuNP-OVA-Cy5, indicating that the modification of PEI on AuNPs significantly enhanced the cellular uptake of antigen. The similar results were observed in the DC2.4 group (Figure 4b). The statistic results illustrate that DC2.4 cells treated with AuNP-PEI/OVA-Cy5 pose about 90% positive events: about 20% positive events higher than cells treated with AuNP-OVA-Cy5 (Figure 4c). These data indicate that AuNP-PEI could deliver antigen into antigen-presenting cells efficiently.
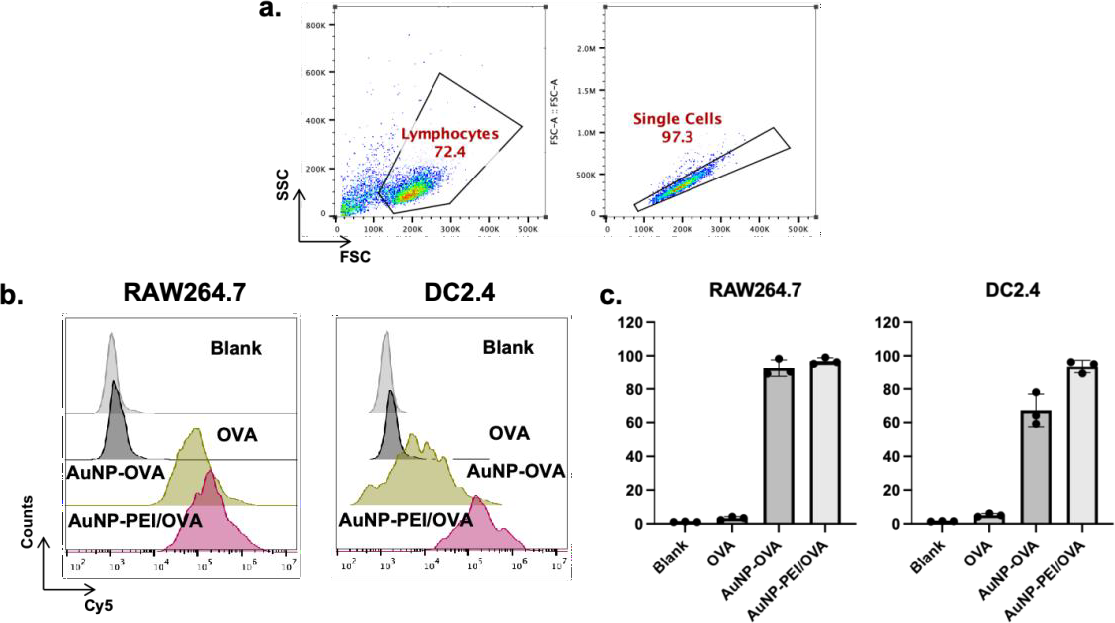
Figure 4. AuNP-PEI/OVA nanovaccine cell permeability.
Using a confocal fluorescent microscope to determine the cellular uptake of gold nanoparticles in cells can confirm the successful entry of AuNP-PEI/OVA into antigen-presenting cells (APCs). Firstly, AuNP-PEI/OVA-Cy5 is co-cultured with RAW264.7 and DC2.4. After co-culture, The cells are then washed with PBS after culture. The cells are then examined by confocal fluorescent microscope to quantify the level of cellular uptake represented in the charts. Red glow created by the Cy5 represents OVA protein , while the blue fluorescence created by DAPI, which binds to DNA in the cell nuclei, highlights the cells in the dark field (Figure 5). By combining the two channels, it is possible to determine whether the nanoparticles have entered the cells (Figure 5). Represented by the chart, AuNP/OVA and AuNP-PEI/OVA efficiently delivered antigens along with the fluorescence inside the cells (Figure 5). In conclusion, the charts generated by FACS and fluorescence imaging indicate AuNP-PEI/OVA’s efficiency in entering the cells.
3.4. Efficiency of AuNP-PEI/OVA nanovaccine in Dendritic cell maturation
Flow cytometry was used to determine the effectiveness of AuNP-PEI/OVA and AuNP/OVA on the maturation of dendritic cells (DCs).. The x axis indicates the maturation of CD86 and the y axis indicates the maturation of CD 80. AuNP-PEI/OVA along with other materials, were co-cultured with BMDC for 24 hours. The cells were then labled with flourecence antibody, and the cells were analyzed for their expression on CD 86 and CD 80. Figure 6a demonstrates cells that were untreated, with LPS, with OVA, with AuNP-OVA, and with AuNP-PEI/OVA. These cells maturation results were in 2.61%, 74.2%, 13.0%, 42.1%, 57.0% respectfully. The results indicate that cells with AuNP-PEI treatment proves significant increase in DC cells maturation in comparison to cells with OVA or cells with AuNP.
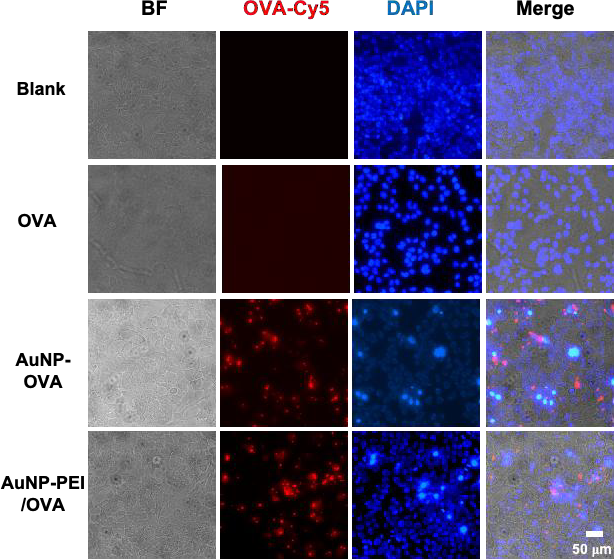
Figure 5. AuNP-PEI/OVA nanovaccine cell permeability.
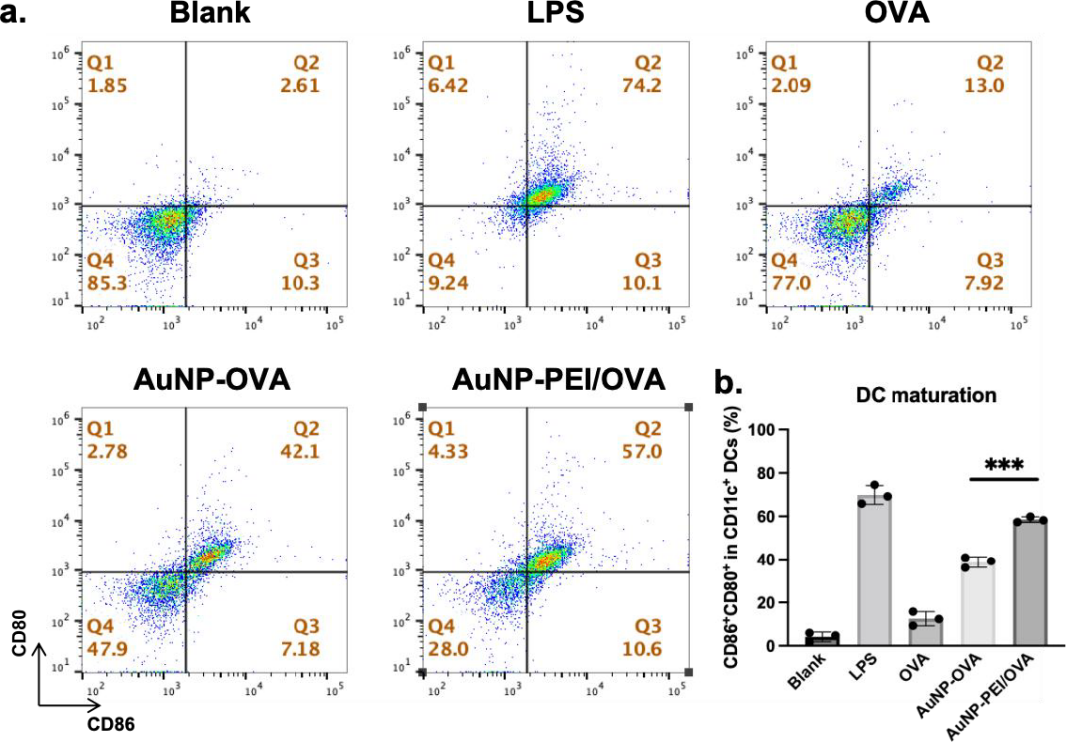
Figure 6. Efficiency of AuNP-PEI/OVA nanovaccine in dendritic cell maturation.
3.5. ELISA to determine the level of secreting cytokines within supernatant
Using the experiment involves coating a 96-well plate with a capture antibody, blocking with 3% BSA PBS, and adding the BMDC supernatant. After incubation and washing, detection antibodies and HRP-labeled secondary antibodies are added. A color reaction with TMB and HRP is initiated, then stopped with 1M H2SO4. Results are measured with a microplate reader and analyzed in Excel. As shown in figure 7a, blank reached a concentration at 50 pg/mL, OVA reached at around 100 pg/mL, AuNP-OVA reached at around 140 pg/mL. AuNP-PEI/OVA reached at close to 170 pg/mL. The concentration of cytokine IL-12 is significantly less in the OVA sample in comparison to the AuNP-PEI/OVA. Similarly in figure 7b, the OVA and the AuNP-PEI/OVA samples showed strong contrast. With the help of nanoparticles, the AuNP-PEI/OVA sample demonstrated significant present of the IL-2 cytokines. In contrast to the 130 pg/mL concentration of OVA sample, AuNP-PEI/OVA obtained close to 250 pg/mL, increased in detection of cytokines to almost double the amount. The results indicate that the nanoparticles delivery system with PEI increased the secretion of IL-2 and IL-12 by the BMDC cells that indicate an increase in the maturation of the cells.
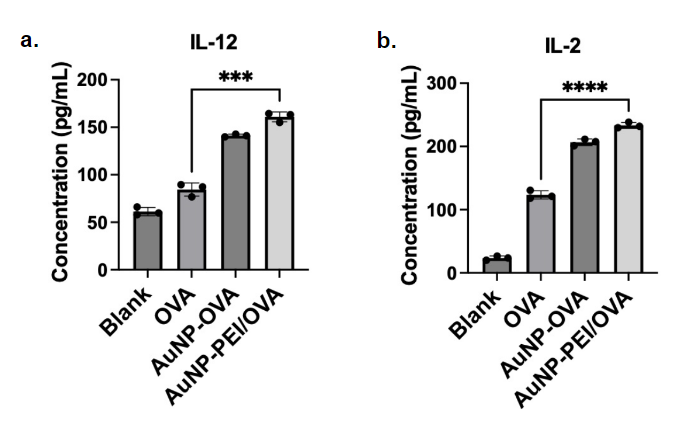
Figure 7. Cytokines IL-2 and IL-12 presented within the BMDC supernatant.
4. Conclusions
This study designed a high-efficient strategy to deliver cancer antigen as described above. First, the AuNP-PEI/OVA nanovaccine prepared size is around 50 nm and highly monodisperse and AuNP-PEI/OVA possesed a hydrodynamic diameter of 146.3 nm and with polydispersity index (PDI) of 0.252. In the cytotoxicity test, we confirmed that the prepared AuNP-PEI/OVA excellent safety towards RAW264.7 and DC2.4 cells, which facilitate the further applications in cancer vaccine. Further, the experiment results generated by FACS and fluorescence imaging together indicate AuNP-PEI/OVA’s efficiency in entering the cells. In addition, AuNP-PEI treatment proves significant increase in DC cells maturation in comparison to cells with OVA or cells with AuNP, which means AuNP-PEI antigen-delivering system could elicit robust antigen-specific immune response to fight cancer.
Acknowledgement
I extend my gratitude to all those who have contributed to this research article. First and foremost, I am profoundly thankful to my mentor, Phd. Wu, for his invaluable guidance, unwavering support, and constructive feedback throughout the entire research process. His expertise and mentorship have been instrumental in shaping the methodology and content of this article. I would also like to express my appreciation to my Cancer Immunology program teacher Dr. Aguilar and fellow classmate / teammate Davis Novak, who have generously shared their insights and expertise in inspiring me to research about Gold Nanoparticles. The collaborative spirit within our research community has significantly enriched the depth of this work. Special thanks are also due to the University of Science and Technology of China, whose laboratory played a crucial role in facilitating the execution of this research. I am grateful to my family for their unwavering encouragement and understanding during the phases of this project. Their support has been my source of strength. Thank you to all participants who contributed their time and insights to this study, your involvement is deeply appreciated. Lastly, I acknowledge the broader academic community, whose collective body of knowledge has been instrumental in shaping the context and framing of this research. Each of you has played a vital role in the completion of this research article, and for that, I extend my sincere thanks.
References
[1]. Sung, H.; Ferlay, J.; Siegel, R. L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F., Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. Ca-a Cancer Journal for Clinicians 2021, 71 (3), 209-249.
[2]. Siegel, R. L.; Miller, K. D.; Fuchs, H. E.; Jemal, A., Cancer statistics, 2022. Ca-a Cancer Journal for Clinicians 2022, 72 (1), 7-33.
[3]. Huang, H. Q.; Liu, R. H.; Yang, J.; Dai, J.; Fan, S. H.; Pi, J.; Wei, Y. B.; Guo, X. R., Gold Nanoparticles: Construction for Drug Delivery and Application in Cancer Immunotherapy. Pharmaceutics 2023, 15 (7).
[4]. Fan, Y.; Moon, J. J., Nanoparticle Drug Delivery Systems Designed to Improve Cancer Vaccines and Immunotherapy. Vaccines 2015, 3 (3), 662-85.
[5]. Liu, J.; Fu, M. Y.; Wang, M. N.; Wan, D. D.; Wei, Y. Q.; Wei, X. W., Cancer vaccines as promising immuno-therapeutics: platforms and current progress. Journal of Hematology & Oncology 2022, 15 (1).
[6]. Akira, S.; Uematsu, S.; Takeuchi, O., Pathogen recognition and innate immunity. Cell 2006, 124 (4), 783-801.
[7]. Banchereau, J.; Steinman, R. M., Dendritic cells and the control of immunity. Nature 1998, 392 (6673), 245-252.
[8]. Sakaguchi, S.; Yamaguchi, T.; Nomura, T.; Ono, M., Regulatory T cells and immune tolerance. Cell 2008, 133 (5), 775-787.
[9]. Banchereau, J.; Briere, F.; Caux, C.; Davoust, J.; Lebecque, S.; Liu, Y. T.; Pulendran, B.; Palucka, K., Immunobiology of dendritic cells. Annual Review of Immunology 2000, 18, 767-+.
[10]. Nath, P. C.; Sharma, R.; Debnath, S.; Nayak, P. K.; Roy, R.; Sharma, M.; Inbaraj, B. S.; Sridhar, K., Recent advances in production of sustainable and biodegradable polymers from agro-food waste: Applications in tissue engineering and regenerative medicines. International journal of biological macromolecules 2024, 129129.
[11]. Dykman, L. A.; Staroverov, S. A.; Kozlov, S. V.; Fomin, A. S.; Chumakov, D. S.; Gabalov, K. P.; Kozlov, Y. S.; Soldatov, D. A.; Khlebtsov, N. G., Immunization of Mice with Gold Nanoparticles Conjugated to Thermostable Cancer Antigens Prevents the Development of Xenografted Tumors. International Journal of Molecular Sciences 2022, 23 (22).
[12]. Dykman, L. A., Gold nanoparticles for preparation of antibodies and vaccines against infectious diseases. Expert Review of Vaccines 2020, 19 (5), 465-477.
[13]. Sarraf, T. R.; Sen, M., Wnt5A signaling supports antigen processing and CD8 T cell activation. Frontiers in Immunology 2022, 13.
[14]. Nakamura, R.; Ishimaru, M., STUDIES ON OVALBUMIN-S-OVALBUMIN TRANSFORMATION .3. CHANGES IN THE SHAPE AND SURFACE HYDROPHOBICITY OF OVALBUMIN DURING ITS TRANSFORMATION TO S-OVALBUMIN. Agricultural and Biological Chemistry 1981, 45 (12), 2775-2780.
Cite this article
You,X. (2024). Functionalized gold nanoparticles serve as nanovaccine to boost Immune protection for cancer. Theoretical and Natural Science,64,8-17.
Data availability
The datasets used and/or analyzed during the current study will be available from the authors upon reasonable request.
Disclaimer/Publisher's Note
The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of EWA Publishing and/or the editor(s). EWA Publishing and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
About volume
Volume title: Proceedings of the 4th International Conference on Biological Engineering and Medical Science
© 2024 by the author(s). Licensee EWA Publishing, Oxford, UK. This article is an open access article distributed under the terms and
conditions of the Creative Commons Attribution (CC BY) license. Authors who
publish this series agree to the following terms:
1. Authors retain copyright and grant the series right of first publication with the work simultaneously licensed under a Creative Commons
Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this
series.
2. Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the series's published
version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial
publication in this series.
3. Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and
during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See
Open access policy for details).
References
[1]. Sung, H.; Ferlay, J.; Siegel, R. L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F., Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. Ca-a Cancer Journal for Clinicians 2021, 71 (3), 209-249.
[2]. Siegel, R. L.; Miller, K. D.; Fuchs, H. E.; Jemal, A., Cancer statistics, 2022. Ca-a Cancer Journal for Clinicians 2022, 72 (1), 7-33.
[3]. Huang, H. Q.; Liu, R. H.; Yang, J.; Dai, J.; Fan, S. H.; Pi, J.; Wei, Y. B.; Guo, X. R., Gold Nanoparticles: Construction for Drug Delivery and Application in Cancer Immunotherapy. Pharmaceutics 2023, 15 (7).
[4]. Fan, Y.; Moon, J. J., Nanoparticle Drug Delivery Systems Designed to Improve Cancer Vaccines and Immunotherapy. Vaccines 2015, 3 (3), 662-85.
[5]. Liu, J.; Fu, M. Y.; Wang, M. N.; Wan, D. D.; Wei, Y. Q.; Wei, X. W., Cancer vaccines as promising immuno-therapeutics: platforms and current progress. Journal of Hematology & Oncology 2022, 15 (1).
[6]. Akira, S.; Uematsu, S.; Takeuchi, O., Pathogen recognition and innate immunity. Cell 2006, 124 (4), 783-801.
[7]. Banchereau, J.; Steinman, R. M., Dendritic cells and the control of immunity. Nature 1998, 392 (6673), 245-252.
[8]. Sakaguchi, S.; Yamaguchi, T.; Nomura, T.; Ono, M., Regulatory T cells and immune tolerance. Cell 2008, 133 (5), 775-787.
[9]. Banchereau, J.; Briere, F.; Caux, C.; Davoust, J.; Lebecque, S.; Liu, Y. T.; Pulendran, B.; Palucka, K., Immunobiology of dendritic cells. Annual Review of Immunology 2000, 18, 767-+.
[10]. Nath, P. C.; Sharma, R.; Debnath, S.; Nayak, P. K.; Roy, R.; Sharma, M.; Inbaraj, B. S.; Sridhar, K., Recent advances in production of sustainable and biodegradable polymers from agro-food waste: Applications in tissue engineering and regenerative medicines. International journal of biological macromolecules 2024, 129129.
[11]. Dykman, L. A.; Staroverov, S. A.; Kozlov, S. V.; Fomin, A. S.; Chumakov, D. S.; Gabalov, K. P.; Kozlov, Y. S.; Soldatov, D. A.; Khlebtsov, N. G., Immunization of Mice with Gold Nanoparticles Conjugated to Thermostable Cancer Antigens Prevents the Development of Xenografted Tumors. International Journal of Molecular Sciences 2022, 23 (22).
[12]. Dykman, L. A., Gold nanoparticles for preparation of antibodies and vaccines against infectious diseases. Expert Review of Vaccines 2020, 19 (5), 465-477.
[13]. Sarraf, T. R.; Sen, M., Wnt5A signaling supports antigen processing and CD8 T cell activation. Frontiers in Immunology 2022, 13.
[14]. Nakamura, R.; Ishimaru, M., STUDIES ON OVALBUMIN-S-OVALBUMIN TRANSFORMATION .3. CHANGES IN THE SHAPE AND SURFACE HYDROPHOBICITY OF OVALBUMIN DURING ITS TRANSFORMATION TO S-OVALBUMIN. Agricultural and Biological Chemistry 1981, 45 (12), 2775-2780.









