1. Introduction
Bone injuries and defects remain a significant challenge in today’s medical field, often requiring complex surgical interventions and lengthy recovery periods. To deal with these problems, the fabrication and innovation of effective bone repair scaffolds have been focused by researchers for a long time in clinical industry. Simultaneously, the exploration of various elemental applications in bone repair scaffolds has also seen considerable advancements in recent years, greatly improving the regenerative performance of bone repair scaffolds.
Bone repair scaffolds are three-dimensional structures designed to provide a supportive and stable environment for the reformation and repair of damaged or lost bone tissue. These scaffolds typically can be fabricated from multiple types of biomaterials, including some polymers (natural and synthetic), ceramics, and composites. They are engineered to simulate the extracellular matrix of the original bone [1]. The structure, porosity, and mechanical properties of the scaffolds play an essential role in promoting cell attachment, differentiation, and proliferation, as well as the scaffold’s overall integration with the surrounding environment.
A key recent advancement of bone repair scaffolds is that they are engineered to have the ability to serve as a delivery platform for small molecules that promote the development of new bone, such as growth factors and stem cells, which can further enhance the regenerative process [2]. Alongside the development of bone repair scaffolds, the incorporation of various metallic elements into scaffolds has received significant attention in recent years. Several metallic materials, such as titanium, cobalt and magnesium etc. have been successfully used in other clinical industries. Recent studies have showed that those incorporated specific metallic elements can significantly improve various characteristics of the scaffolds, including material strength and porosity. Apart from these, added metallic ions can greatly induce various genes associated with cell proliferation, cell differentiation, osteogenesis and angiogenesis etc. In this review three types of metallic elements (strontium+silicon, magnesium and titanium) added bone repair scaffolds will be introduced.
2. Sr5(PO4)2SiO4 bioceramic scaffold
Bioceramic often occupy an important position in bone regeneration. Recently, strontium (Sr) and Silicon (Si) have received attention in clinical industry because of their distinguished functions in bioceramics [3,4]. Si, which is one of the essential trace elements in the body, has been shown its imperative role for keeping connective tissues such as bone and cartilage articular healthy [5]. It is immediately involved in the course of biomineralization of newly developed bone and the production of secreted type 1 collagen [6]. Besides, it also acts as a key role in the synthetic process of cartilage extracellular matrix (ECM), osteoblast differentiation and growth of bone [7]. Due to these special characteristics in bone and cartilage regeneration, Si has been broadly adopted in fabrication of bioceramics and bioactive glass, improving their biological performance [8,9]. Sr is a vital trace element that has an activity nearly the same as calcium in bone [10]. Sr is significantly important for maintaining the functions of human tissue, increasing the osteoconductive ability of calcium phosphate and enhancing the mechanical strength of bone tissue [11]. While promoting the differentiation of osteoblast and suppressing that of osteoclast, Sr exhibits the activity to reduce degeneration of cartilage and apoptosis of chondrocyte in osteoarthritis therapy [12,13]. Considering of these beneficial traits, Sr-containing biomaterials have been developed for many years.
Hence, it is deduced that the combination of Sr and Si used in bioceramics may have a better clinical performance in bone tissue regeneration. Sr5(PO4)2SiO4(SPS) is a type of mineral with a single-phase apatite structure, containing Sr, P and Si [14]. It belongs to the family of apatite structure with general formula of M5(XO4)3Z, where M is divalent cations, XO4 as trivalent or tetravalent anions and Z is a monovalent anion, respectively [15]. In this apatite structure, the charge was imbalanced due to substitution of tetravalent anion of SiO44- at monovalent anion (X-) and this excessive charge was balanced by minor loss of trivalent anions (PO43-) to retain the structural stability of SPS [16]. SPS bioceramic scaffold can be fabricated through 3D plotting technique and sol-gel method. In the study of Zhu, H et al., 2018, they successfully fabricated 3D plotted SPS bioceramic scaffold. It can be apparently observed that the scaffold had uniform pores in designed shapes (100 μm) and only the characteristic peaks of SPS phase could be detected in the XRD pattern (Fig. 1).
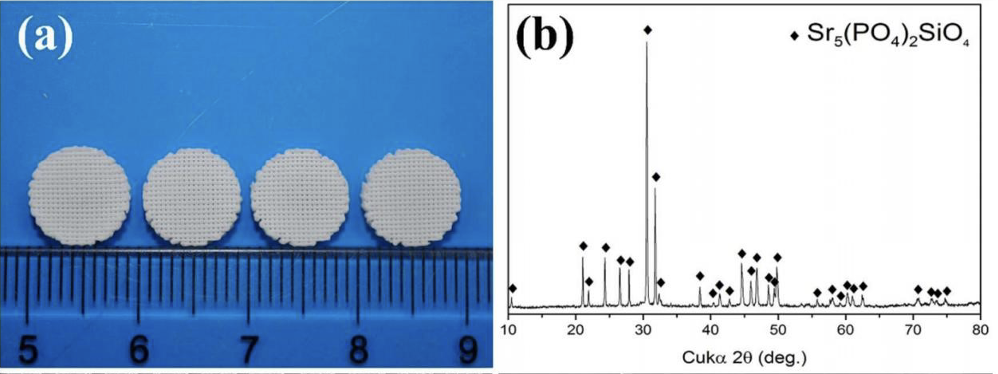
Figure 1. (a) the actual picture of plotted SPS bioceramic scaffold. (b). result of XRD [5]
The compressive strength and in vitro weight lost of SPS scaffolds were much higher than that of β-TCP scaffolds with similar porosity [3]. Apart from these physical advancements, SPS bioceramic scaffolds had stimulatory effects on osteogenesis and angiogenesis in vitro. MTT assay showed that the proliferation of rabbit bone marrow-derived mesenchymal stem cells (rBMSCs) seeded in SPS ceramic scaffolds increased significantly. Those proliferated rBMSCs can then differentiate into various types of cells, forming bone tissues. Simultaneously, a gradual increase in the alkaline phosphatase (ALP) activity of rBMSCs can be seen. RT-qPCR analysis demonstrated that the expression of osteogenic genes of rBMSCs were enhanced significantly by SPS extracts in the experimental groups [3]. Notably, a study of mBMSCs conducted by A. Udduttula et al showed similar results that the expression of genes of ALP, Runx2 and OCN were induced by SPS extracts. Moreover, it was found that the expression of genes of VEGF, KDR, eNOS and HIF-1ɑ was up-regulated drastically, which suggested that the angiogenesis was induced by SPS extracts. Besides, it is showed that rBMSC and HUVECs cultured in SPS scaffolds presented well-defined actin, stress fibre and cytoskeleton, which are beneficial for cell migration [16].
To conclude, SPS bioceramic scaffold is a novel strategy with distinguished physical characteristics for bone tissue regeneration. Importantly, Sr and Si ions in it have remarkable effects on up-regulating various genes related to cell proliferation, osteogenesis and angiogenesis. Therefore, these results suggested that 3D-plotted SPS bioceramic scaffold could be an effective strategy for bone tissue regeneration.
3. Porous magnesium scaffold
Magnesium (Mg) is seen as a promising metallic element used for bone repair scaffold because of its several distinguished properties. One of them is biodegradability, which is the main advantage of Mg over currently used metals [17]. While playing a key role in physiology systems, Mg ions have been shown the ability to stimulate bone formation [18,19]. Moreover, some features of Mg, e.g. high specific strength and elastic modulus, are proved to be closer to those of natural human bone in comparison with the other traditional metal implants [20,21]. However, Mg has a key shortcoming: the emission of hydrogen gas when rapid degradation [22]. To solve this problem, a variety of methods have been adopted. For example, many methods like microarc oxidation, ion implantation, plasma anodization, electro-deposition, etc. have been substantiated to improve the corrosion resistance of Mg and greatly reduce the volume of released hydrogen gas [23].
Magnesium scaffolds can be fabricated via many ways including 3D gel-printing(3DGP), titanium wire space holder method (TWSH) and Solid free-form fabrication (SFF) method. Lin, T. et al. successfully produced porous magnesium scaffolds by 3DGP method in 2021. In their study, the optical density (OD450), namely a specific index regarding to the density of cell, of MC3T3-E1 cells on oxide-coated Mg scaffolds was higher than the blank group at 24 h. Conversly, the viability is lower than that of the blank group at 48 h. Specifically, on the oxide-coated Mg scaffolds, it was observed that the cells were flatter, spreading over almost the whole surfaces of the samples. Simultaneously, oxide-coated Mg scaffolds can promote osteogenesis and the growth of bone tissue compared to TCP scaffolds, which are the control groups [24]. Earlier, Liu, Y. J., et al. studied the osteogenic and biodegradative characteristics of Mg scaffolds in 2014. Firstly, they modified the surface of the Mg porous scaffolds using microarc oxidation method and then disinfected them with ethylene oxide. Mg scaffolds and hydroxyapatite (HA) scaffolds were implanted into New Zealand white rabbits. The micro-CT scans showed that Mg porous scaffolds had a high bioabsorption (7.80 ± 0.50mm3) and could induce more bone formation (9.29 ± 1.27 mm3) compared to HA scaffolds, which induced 1.40 ± 0.49 mm3 new bone formation and had 0 mm3 bioabsorption. This experiment is a preliminary attempt for the performance of magnesium scaffolds in osteogenesis and biodegradation, further studies should be conducted on larger animals with porous Mg scaffolds. Overall, porous Mg scaffold showed good biocompatibility, physical properties, biodegradability and the ability for osteogenesis, suggesting that it could be a promising material in bone regeneration industry.
4. Porous Ti scaffold
Titanium (Ti) is one of the metal materials that being used in clinical application due to its outstanding biocompatibility, specific strength, stiffness, and corrosive resistance etc. Conventional Ti implants’ Young’s modulus (110GPa) are much higher than that of bone tissue (4-30GPa). This imbalance may cause load redistribution, implant loosening or autogenous bone fracture. The consequence of this imbalance is known as stress shielding. Stress shielding effect is the principal factor responsible for instability of the interface between the scaffolds and the tissues [25]. One of the solutions to this issue is using porous structure instead of cast material [26]. Dabrowski, B. et al. manufactured Ti porous scaffolds by powder metallurgy (PM) technique in 2019. The Ti porous scaffolds showed good porosity with open pores in a 3-dimensional network under SEM investigation. Additionally, they found that the Young’s modulus of the Ti porous scaffolds (8 GPa for total porosity of 45% and decrease to 1 GPa for porosity of 75%) is much closer to that of the natural bone in comparison with that of the conventional Ti scaffolds. Because of the samples with 75% porosity had some similar traits with cancellous bone, they were then examined by XRD and electrochemical test. In XRD test, no residual phases induced by space holder materials were observed. This meant that paraformaldehyde, a space holder, does not affect the phase composition of the Ti scaffolds. Furthermore, it apparently improved the porosity, pore size, also homogeneity of the pore distribution.
In electrochemical test, porous Ti scaffolds were observed to have 100 times-smaller values of polarization resistance, compared to those of cast Ti scaffolds in the same conditions. After that, EIS test was conducted to investigate the corrosive potential. It is showed that the corrosive potential for porous ones on 80 mV is smaller than for solid ones in 0.9% NaCl electrolyte solution. Therefore, these results proved that the porous titanium scaffolds had lower corrosive resistance 26. Similarly, Chen, Y. et al. successfully produced porous TI scaffolds with powder metallurgy approach in 2017. In this study, they used spherical magnesium powder, which facilitated cell migration, as the space holder as per a review by Loh et al. [27]. Firstly, the density of the samples was examined to be 2.25 g/cm3, which is close to the average bone mineral density (BMD) of human (approximately 3.88 g/cm2 for males and 2.90 g/cm2 for females respectively). This characteristic helps patients to be more comfortable and keeps the failure rate of the implant surgery low. In addition, they also demonstrated that the porous structures had an excellent interconnected pore network (above 95% pores are interconnected). Moreover, according to the Micro-CT analysis, the porous Ti scaffolds exhibited a great consistency between the designed porosity and the obtained one, with uniformly distributed pores in round and complete shapes. Then, the Young’s modulus (ability for solid materials to resist deformation) and yield strength of scaffolds with different porosity were determined. Figure. 2 shows the compression stress-strain of CP (commercially pure) scaffolds and those Ti scaffolds with different porosity.

Figure 2. samples after compression test [28]
The results showed that the elastic modulus for samples with 30% porosity is 44.2 GPa, samples with 40% porosity is 24.7 GPa, and samples with 50% porosity is 15.4 GPa [28]. Importantly, it has been shown that human bone’s elastic modulus ranged from 4 GPa to 30 GPa, which meant that the values of elastic modulus for all these samples are similar with those of natural bone [29]. Furthermore, the yield strength analysis proved that the samples with 50% porosity had even larger values than that of the human bones, and the ones with 40% porosity had the closest values of yield strength and stiffness to the natural bone. At last, the scaffolds were seeded with hMSCs to test interaction with cells. They observed that hMSCs appeared to gather in the pores of the scaffolds, distribution of cells was heterogeneous, cells exhibited a partially spread morphology and the viability of the cells were the same as the conventional tissue culture plats. Taking together, these properties of the porous Ti scaffolds are proved to be a promising biomaterial for bone repair scaffolds [27].
5. Conclusion
The addition of metallic elements in bone repair scaffolds has been widely regarded as a promising strategy for bone tissue regeneration. This review has explored the potential of three key elements: strontium (Sr), silicon (Si), and magnesium (Mg), highlighting their individual contributions to scaffold properties and their synergistic effects when combined.
Sr5(PO4)2SiO4 (SPS) bioceramic scaffolds, enriched with Sr and Si, demonstrate superior mechanical strength, and exhibit induced osteogenesis and angiogenesis in vitro. The presence of these elements upregulates genes associated with cell proliferation, differentiation, and vascularization, making SPS scaffolds a promising candidate for bone tissue regeneration. Porous magnesium (Mg) scaffolds, with their biodegradability and ability to stimulate bone formation, offer an alternative to traditional metal implants. The use of Mg in scaffolds addresses the limitations of conventional materials, promoting bone regeneration and minimizing the risk of implant-related complications. Porous titanium (Ti) scaffolds, manufactured through powder metallurgy, address the issue of stress shielding associated with conventional Ti implants. The interconnected pore network and controlled porosity of these scaffolds mimic the properties of natural bone, leading to improved biocompatibility, cell interaction, and enhanced bone regeneration. The research presented in this review suggest that the integration of metallic elements into bone repair scaffolds holds great promise for innovative and effective strategies for bone tissue repair. Further study is needed to optimize the design, as well as the fabrication of these scaffolds, ensuring their efficacy and safety in clinical applications in the future.
References
[1]. Amini, A. R., Laurencin, C. T., & Nukavarapu, S. P. (2012). Bone tissue engineering: recent advances and challenges. Critical Reviews in Biomedical Engineering, 40(5), 363-408.
[2]. Lichte, P., Pape, H. C., Pufe, T., Kobbe, P., & Fischer, H. (2011). Scaffolds for bone healing: concepts, materials and evidence. Injury, 42(6), 569-573.
[3]. N. Neves, D. Linhares, G. Costa, C.C. Ribeiro, M.A. Barbosa, In vivo and clinical application of strontium-enriched biomaterials for bone regeneration: a systematic review, Bone. Joint. Res. 6 (6) (2017) 366–375.
[4]. C. Wu, Z. Chen, D. Yi, J. Chang, Y. Xiao, Multidirectional effects of Sr-, Mg-, and Sicontaining bioceramic coatings with high bonding strength on inflammation, osteoclastogenesis and osteogenesis, ACS Appl. Mater. Interfaces. 6(6)(2014)4264–4276.
[5]. Zhu, H., Zhai, D., Lin, C., Zhang, Y., Huan, Z., Chang, J., & Wu, C. (2016). 3D plotting of highly uniform Sr5(PO4)2SiO4 bioceramic scaffolds for bone tissue engineering. Journal of Materials Chemistry B, 4(37), 6200-6212.
[6]. R. Jugdaohsingh, K.L. Tucker, N. Qiao, L.A. Cupples, D.P. Kiel, J.J. Powell, Dietary silicon intake is positively associated with bone mineral density in men and premenopausal women of the Framingham Offspring cohort, J. Bone. Miner. Res. 19 (2) (2004) 297–307.
[7]. D.M. Reffitt, N. Ogston, R. Jugdaohsingh, H.F.J. Cheung, B.A.J. Evans, R.P.H. Thompson, J.J. Powell, G.N. Hampson, Orthosilicic acid stimulates collagen type 1 synthesis and osteoblastic differentiation in human osteoblast-like cells in vitro, Bone.32 (2) (2003) 127–135.
[8]. Bohner, M. (2009). Silicon-substituted calcium phosphates–a critical view. Biomaterials, 30(32), 6403-6406.
[9]. Hing, K. A., Revell, P. A., Smith, N., & Buckland, T. (2006). Effect of silicon level on rate, quality and progression of bone healing within silicate-substituted porous hydroxyapatite scaffolds. Biomaterials, 27(29), 5014-5026.
[10]. Udduttula, A., Teng, B., Chandrashekar, B. N., Li, J., Yu, X. F., Liu, C., ... & Ren, P. G. (2020). Novel Sr5 (PO4) 2SiO4-graphene nanocomposites for applications in bone regeneration in vitro. Applied Surface Science, 507, 145176.
[11]. K. Maciejewska, Z. Drzazga, M. Kaszuba, Role of trace elements (Zn, Sr, Fe) in bone development: Energy dispersive X-ray fluorescence study of rat bone and tooth tissue, BioFactors. 40 (4) (2014) 425–435.
[12]. V. Mourino, J.P. Cattalini, A.R. Boccaccini, Metallic ions as therapeutic agents in tissue engineering scaffolds: an overview of their biological applications and strategies for new developments, J. Royal Soc. Interface. 9 (68) (2011) 401–419.
[13]. D.G. Yu, H.F. Ding, Y.Q. Mao, M. Liu, B. Yu, X. Zhao, X.Q. Wang, Y. Li, G.W. Liu, S.B. Nie, S. Liu, Strontium ranelate reduces cartilage degeneration and subchondral bone remodeling in rat osteoarthritis model, Acta. Pharmacol. Sin. 34 (3) (2013)393–402.
[14]. Huang, Y., Gan, J., & Seo, H. J. (2011). Luminescence investigation of Eu-activated Sr5 (PO4) 2SiO4 phosphor by combustion synthesis. Journal of the American Ceramic Society, 94(4), 1143-1148.
[15]. Y. Zheng, G. Dong, C. Deng, Effect of silicon content on the surface morphology of silicon-substituted hydroxyapatite bio-ceramics treated by a hydrothermal vapor method, Ceram. Int. 40 (2014) 14661–14667.
[16]. Udduttula, A., Li, J., Zhao, P. Y., Wang, G. C., Zhang, J. V., & Ren, P. G. (2019). Sol-gel derived nanosized Sr5 (PO4) 2SiO4 powder with enhanced in vitro osteogenesis and angiogenesis for bone regeneration applications. Ceramics International, 45(3), 3148-3158.
[17]. F. Witte, The history of biodegradable magnesium implants: a review, Acta Bio-mater. 6 (2010) 1680–1692.
[18]. C. Palacios, The role of nutrients in bone health, from A to Z, Crit. Rev. Food Sci. Nutr. 46 (2006) 621–628.
[19]. Y. Zhang, J. Xu, Y.C. Ruan, M.K. Yu, M. O’laughlin, H. Wise, D. Chen, L. Tian, D. Shi, J. Wang, S. Chen, J.Q. Feng, D.H. Chow, X. Xie, L. Zheng, L. Huang, S.Huang, K. Leung, N. Lu, L. Zhao, H. Li, D. Zhao, X. Guo, K. Chan, F. Witte, H.C. Chan, Y. Zheng, L. Qin, Implant-derived magnesium induces local neuronal production of CGRP to improve bone-fracture healing in rats, Nat. Med. 22 (2016) 1160–1169.
[20]. H. Hornberger, S. Virtanen, A. Boccaccini, Biomedical coatings on magnesium alloys–a review, Acta Biomater. 8 (2012) 2442–2455.
[21]. M. Razavi, M. Fathi, O. Savabi, D. Vashaee, L. Tayebi, Biodegradation, bioactivity and in vivo biocompatibility analysis of plasma electrolytic oxidized (PEO) biodegradable Mg implants, Phys. Sci. Int. J. 4 (2014) 708–722.
[22]. Gray JE, Luan B. Protective coatings on magnesium and its alloys - a critical review. J Alloys Compd 2002; 336: 88-113, doi: 10.1016/S0925-8388(01)01899-0.
[23]. Liu, Y. J., Yang, Z. Y., Tan, L. L., Li, H., & Zhang, Y. Z. (2014). An animal experimental study of porous magnesium scaffold degradation and osteogenesis. Brazilian Journal of Medical and Biological Research, 47(8), 715-720.
[24]. Lin, T., Wang, X., Jin, L., Li, W., Zhang, Y., Wang, A., ... & Shao, H. (2021). Manufacturing of porous magnesium scaffolds for bone tissue engineering by 3D gel-printing. Materials & Design, 209, 109948.
[25]. Yu, G., Li, Z., Li, S., Zhang, Q., Hua, Y., Liu, H., ... & Wang, X. (2020). The select of internal architecture for porous Ti alloy scaffold: A compromise between mechanical properties and permeability. Materials & Design, 192, 108754.
[26]. Dabrowski, B., Swieszkowski, W., Godlinski, D., & Kurzydlowski, K. J. (2010). Highly porous titanium scaffolds for orthopaedic applications. Journal of Biomedical Materials Research Part B: Applied Biomaterials, 95(1), 53-61.
[27]. Loh Q L and Choong C 2013 Three-dimensional scaffolds for tissue engineering applications: role of porosity and pore size. Tissue Eng. Part B. Rev. 19 485–502
[28]. Chen, Y., Frith, J. E., Dehghan-Manshadi, A., Attar, H., Kent, D., Soro, N. D. M., ... & Dargusch, M. S. (2017). Mechanical properties and biocompatibility of porous titanium scaffolds for bone tissue engineering. Journal of the mechanical behavior of biomedical materials, 75, 169-174.
[29]. Elias, C. N., Lima, J. H. C., Valiev, R., & Meyers, M. A. (2008). Biomedical applications of titanium and its alloys. Jom, 60, 46-49.
Cite this article
Sun,Z. (2024). The Use of Metallic Elements in Bone Repair Scaffolds. Theoretical and Natural Science,63,38-44.
Data availability
The datasets used and/or analyzed during the current study will be available from the authors upon reasonable request.
Disclaimer/Publisher's Note
The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of EWA Publishing and/or the editor(s). EWA Publishing and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
About volume
Volume title: Proceedings of the 4th International Conference on Biological Engineering and Medical Science
© 2024 by the author(s). Licensee EWA Publishing, Oxford, UK. This article is an open access article distributed under the terms and
conditions of the Creative Commons Attribution (CC BY) license. Authors who
publish this series agree to the following terms:
1. Authors retain copyright and grant the series right of first publication with the work simultaneously licensed under a Creative Commons
Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this
series.
2. Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the series's published
version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial
publication in this series.
3. Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and
during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See
Open access policy for details).
References
[1]. Amini, A. R., Laurencin, C. T., & Nukavarapu, S. P. (2012). Bone tissue engineering: recent advances and challenges. Critical Reviews in Biomedical Engineering, 40(5), 363-408.
[2]. Lichte, P., Pape, H. C., Pufe, T., Kobbe, P., & Fischer, H. (2011). Scaffolds for bone healing: concepts, materials and evidence. Injury, 42(6), 569-573.
[3]. N. Neves, D. Linhares, G. Costa, C.C. Ribeiro, M.A. Barbosa, In vivo and clinical application of strontium-enriched biomaterials for bone regeneration: a systematic review, Bone. Joint. Res. 6 (6) (2017) 366–375.
[4]. C. Wu, Z. Chen, D. Yi, J. Chang, Y. Xiao, Multidirectional effects of Sr-, Mg-, and Sicontaining bioceramic coatings with high bonding strength on inflammation, osteoclastogenesis and osteogenesis, ACS Appl. Mater. Interfaces. 6(6)(2014)4264–4276.
[5]. Zhu, H., Zhai, D., Lin, C., Zhang, Y., Huan, Z., Chang, J., & Wu, C. (2016). 3D plotting of highly uniform Sr5(PO4)2SiO4 bioceramic scaffolds for bone tissue engineering. Journal of Materials Chemistry B, 4(37), 6200-6212.
[6]. R. Jugdaohsingh, K.L. Tucker, N. Qiao, L.A. Cupples, D.P. Kiel, J.J. Powell, Dietary silicon intake is positively associated with bone mineral density in men and premenopausal women of the Framingham Offspring cohort, J. Bone. Miner. Res. 19 (2) (2004) 297–307.
[7]. D.M. Reffitt, N. Ogston, R. Jugdaohsingh, H.F.J. Cheung, B.A.J. Evans, R.P.H. Thompson, J.J. Powell, G.N. Hampson, Orthosilicic acid stimulates collagen type 1 synthesis and osteoblastic differentiation in human osteoblast-like cells in vitro, Bone.32 (2) (2003) 127–135.
[8]. Bohner, M. (2009). Silicon-substituted calcium phosphates–a critical view. Biomaterials, 30(32), 6403-6406.
[9]. Hing, K. A., Revell, P. A., Smith, N., & Buckland, T. (2006). Effect of silicon level on rate, quality and progression of bone healing within silicate-substituted porous hydroxyapatite scaffolds. Biomaterials, 27(29), 5014-5026.
[10]. Udduttula, A., Teng, B., Chandrashekar, B. N., Li, J., Yu, X. F., Liu, C., ... & Ren, P. G. (2020). Novel Sr5 (PO4) 2SiO4-graphene nanocomposites for applications in bone regeneration in vitro. Applied Surface Science, 507, 145176.
[11]. K. Maciejewska, Z. Drzazga, M. Kaszuba, Role of trace elements (Zn, Sr, Fe) in bone development: Energy dispersive X-ray fluorescence study of rat bone and tooth tissue, BioFactors. 40 (4) (2014) 425–435.
[12]. V. Mourino, J.P. Cattalini, A.R. Boccaccini, Metallic ions as therapeutic agents in tissue engineering scaffolds: an overview of their biological applications and strategies for new developments, J. Royal Soc. Interface. 9 (68) (2011) 401–419.
[13]. D.G. Yu, H.F. Ding, Y.Q. Mao, M. Liu, B. Yu, X. Zhao, X.Q. Wang, Y. Li, G.W. Liu, S.B. Nie, S. Liu, Strontium ranelate reduces cartilage degeneration and subchondral bone remodeling in rat osteoarthritis model, Acta. Pharmacol. Sin. 34 (3) (2013)393–402.
[14]. Huang, Y., Gan, J., & Seo, H. J. (2011). Luminescence investigation of Eu-activated Sr5 (PO4) 2SiO4 phosphor by combustion synthesis. Journal of the American Ceramic Society, 94(4), 1143-1148.
[15]. Y. Zheng, G. Dong, C. Deng, Effect of silicon content on the surface morphology of silicon-substituted hydroxyapatite bio-ceramics treated by a hydrothermal vapor method, Ceram. Int. 40 (2014) 14661–14667.
[16]. Udduttula, A., Li, J., Zhao, P. Y., Wang, G. C., Zhang, J. V., & Ren, P. G. (2019). Sol-gel derived nanosized Sr5 (PO4) 2SiO4 powder with enhanced in vitro osteogenesis and angiogenesis for bone regeneration applications. Ceramics International, 45(3), 3148-3158.
[17]. F. Witte, The history of biodegradable magnesium implants: a review, Acta Bio-mater. 6 (2010) 1680–1692.
[18]. C. Palacios, The role of nutrients in bone health, from A to Z, Crit. Rev. Food Sci. Nutr. 46 (2006) 621–628.
[19]. Y. Zhang, J. Xu, Y.C. Ruan, M.K. Yu, M. O’laughlin, H. Wise, D. Chen, L. Tian, D. Shi, J. Wang, S. Chen, J.Q. Feng, D.H. Chow, X. Xie, L. Zheng, L. Huang, S.Huang, K. Leung, N. Lu, L. Zhao, H. Li, D. Zhao, X. Guo, K. Chan, F. Witte, H.C. Chan, Y. Zheng, L. Qin, Implant-derived magnesium induces local neuronal production of CGRP to improve bone-fracture healing in rats, Nat. Med. 22 (2016) 1160–1169.
[20]. H. Hornberger, S. Virtanen, A. Boccaccini, Biomedical coatings on magnesium alloys–a review, Acta Biomater. 8 (2012) 2442–2455.
[21]. M. Razavi, M. Fathi, O. Savabi, D. Vashaee, L. Tayebi, Biodegradation, bioactivity and in vivo biocompatibility analysis of plasma electrolytic oxidized (PEO) biodegradable Mg implants, Phys. Sci. Int. J. 4 (2014) 708–722.
[22]. Gray JE, Luan B. Protective coatings on magnesium and its alloys - a critical review. J Alloys Compd 2002; 336: 88-113, doi: 10.1016/S0925-8388(01)01899-0.
[23]. Liu, Y. J., Yang, Z. Y., Tan, L. L., Li, H., & Zhang, Y. Z. (2014). An animal experimental study of porous magnesium scaffold degradation and osteogenesis. Brazilian Journal of Medical and Biological Research, 47(8), 715-720.
[24]. Lin, T., Wang, X., Jin, L., Li, W., Zhang, Y., Wang, A., ... & Shao, H. (2021). Manufacturing of porous magnesium scaffolds for bone tissue engineering by 3D gel-printing. Materials & Design, 209, 109948.
[25]. Yu, G., Li, Z., Li, S., Zhang, Q., Hua, Y., Liu, H., ... & Wang, X. (2020). The select of internal architecture for porous Ti alloy scaffold: A compromise between mechanical properties and permeability. Materials & Design, 192, 108754.
[26]. Dabrowski, B., Swieszkowski, W., Godlinski, D., & Kurzydlowski, K. J. (2010). Highly porous titanium scaffolds for orthopaedic applications. Journal of Biomedical Materials Research Part B: Applied Biomaterials, 95(1), 53-61.
[27]. Loh Q L and Choong C 2013 Three-dimensional scaffolds for tissue engineering applications: role of porosity and pore size. Tissue Eng. Part B. Rev. 19 485–502
[28]. Chen, Y., Frith, J. E., Dehghan-Manshadi, A., Attar, H., Kent, D., Soro, N. D. M., ... & Dargusch, M. S. (2017). Mechanical properties and biocompatibility of porous titanium scaffolds for bone tissue engineering. Journal of the mechanical behavior of biomedical materials, 75, 169-174.
[29]. Elias, C. N., Lima, J. H. C., Valiev, R., & Meyers, M. A. (2008). Biomedical applications of titanium and its alloys. Jom, 60, 46-49.









