1. Introduction
Originating from the technological advancements of the 1980s, Deep Brain Stimulation (DBS) has since evolved into a pivotal modality for addressing neurological disorders. Recent innovations in DBS have introduced closed-loop control mechanisms, significantly enhancing the operational efficiency of Implantable Pulse Generators (IPGs), shrinking their physical footprint, and mitigating the risks associated with implantation procedures. More importantly, these advances facilitate a more precise monitoring of patient status, enabling automated adjustments of stimulation parameters in real-time-a cornerstone of Adaptive DBS (aDBS).
At the heart of aDBS lies the principle of continuous brain activity surveillance, leveraging sophisticated algorithms to decipher neural signatures and tailor the stimulation regimen accordingly. For Parkinson’s disease (PD), investigators have honed in on specific neural signals correlated with tremor and rigidity. Epilepsy research, conversely, focuses on detecting aberrant electrical patterns that precede seizure events. In the realm of major depressive disorder (MDD), scientists are delving into the functional dynamics of brain regions implicated in mood regulation and cognition. By critically evaluating the convergences and divergences across these disorders, this paper elucidates the optimal feedback signals for each condition, paving the way for the design of more refined closed-loop control systems.
In recent years, many scholars have carried out detailed research according to different diseases, but the industry lacks holistic thinking. This paper analyzes the similarities and differences of PD, epilepsy, and MDD and the corresponding aDBS feedback signals, to provide some evidence and development possibilities for the classified development of DBS technology.
2. Neuropsychiatric health
2.1. PD
Delving into the intricacies of PD, a complex neurodegenerative disorder, uncovers a series of nuanced physiological alterations. Central to this process is the progressive degeneration of dopaminergic neurons within the compact part of the substantia nigra (Substantia Nigra Pars Compacta, SNC). These unique neuronal cells serve as the primary source for dopamine synthesis and release in the brain, playing an indispensable role in modulating motor coordination, emotional regulation, and cognitive functions. As PD ensues stealthily, the population of dopaminergic neurons dwindles significantly, precipitating a marked decline in dopaminergic levels within the basal ganglia. This neurotransmitter depletion directly triggers the hallmark motor impairments associated with PD, encompassing resting tremor, rigidity, bradykinesia, and disturbances in gait and posture control.
2.1.1. β wideband signal The continuous production of synchronized STNβ wideband signal is an obvious symptom of Parkinson's disease [1,2]. STNβ broadband signal generally refers to the brain wave activity signal generated in the subthalamic nucleus within the range of 13 ~ 30Hz, which is rhythmical, periodic, relatively small amplitude, and affected by voluntary mobiles.
Dopamine plays a key role in the production of beta broadband signals. In Parkinson's disease, due to the loss of dopamine neurons, dopamine levels in the basal ganglia decrease significantly. Since dopamine is an important neurotransmitter that transmits information about motor control, the regulation of certain inhibitory and excitatory signals in some basal ganglia is unbalanced, and STN neurons show abnormal synchronization and excessive beta rhythm activity. At the same time, the patient showed tremor and other typical symptoms of Parkinson's disease. This may also explain why scientists can use the beta broadband signal to monitor Parkinson's disease in patients and use it as a control signal for DBS.
2.1.2. γ narrow-band signal STN γ narrow-band signal generally refers to the brain wave activity signal in the range of 13 ~ 30Hz generated in the subthalamic nucleus. Like the β wideband signal, γ narrow-band signal also has rhythm and periodicity. The difference is that its amplitude is smaller than that of the β-wideband signal, and it is minimally affected by autonomous motion [3,4]. Therefore, although the γ narrow-band signal is characterized by its high frequency, which can represent cognitive diseases, the γ narrow-band signal is still a more stable signal and is being studied whether it can be applied in adaptive DBS as a feedback signal for motor control diseases.
2.2. Epilepsy
Epilepsy is a common chronic neurological disorder characterized by spontaneous recurrent seizures and affects around 60 million patients worldwide [5]. Epilepsy can be caused by a variety of factors, but the most critical cause is an imbalance of excitatory and inhibitory neurotransmitters in the brain. In general, patients with epilepsy have an increase in excitatory neurotransmitters (such as glutamate) and a decrease in inhibitory neurotransmitters (such as GABA), resulting in overexcitation of neurons, resulting in abnormal firing of brain neurons resulting in motor control disorders. Epilepsy patients may suffer from epilepsy because of a variety of factors, such as genetics, glial cell function abnormalities, etc., but they are usually accompanied by neurotransmitter disorders, and relieving neurotransmitter disorders can effectively promote the treatment of epilepsy.
Three kinds of signals and their mixed electrical activity are generated at the lesion, namely spike, sharp wave and slow waves. A Spike is a sharp, high-amplitude electrical wave, usually less than 70 milliseconds in duration. Sharp waves are like spikes, but last slightly longer, usually between 70 and 200 milliseconds. Epileptic lesions sometimes exhibit abnormal slow-wave activity, which usually ranges in frequency from 0.5 to 4 Hz. They combine with each other to form signals such as spik-and-wave Complexes and polyspikes.
2.3. MDD
One of the causes of depression (MDD) is the dysfunction of the corticostriothalamic circuit (CSTC circuit). This circuit involves key brain regions such as the prefrontal cortex, striatum, pallidum and thalamus. The prefrontal cortex plays an important role in cognitive control and emotional regulation, the striatum integrates signals from the cortex, and the pallidum and thalamus are responsible for information transmission and feedback. When activity in these areas is abnormal, such as reduced activity in the prefrontal cortex, reduced signaling efficiency in the striatum, or an imbalance in neurotransmitters, it can lead to dysregulation of emotional regulation. This dysfunction can manifest as persistent low mood, lack of motivation, and cognitive dysfunction, triggering symptoms of depression.
The VCVS (Ventral Capsule/Ventral Striatum) target is one of the important targets of DBS in the treatment of psychiatric disorders such as depression and obsessive-compulsive disorder and is composed of Ventral Capsule and Ventral Striatum. Ventral Capsule, located in the ventral part of the internal capsule, is a bundle of nerve pathways made up of white matter fibers that carry signals from the cortex. Nerve fibers in the abdominal sac connect the prefrontal cortex to the striatum and regulate emotional and cognitive function. Stimulating this area can enhance emotional processing and cognitive control, which can alleviate symptoms of depression and obsessive-compulsive disorder. Ventral Striatum includes the Nucleus Accumbens and adjacent striatum regions, which are strongly associated with reward, motivation, and mood regulation, and stimulation of this region can improve mood and motivation in people with depression.
3. Comprehensive analysis for PD
Birbaumer, N. et al. [6] and Pfurtscheller, G. [7] et al., when monitoring the neural oscillatory activity of LFPs in the dorsolateral region of STN, found that patients receiving levodopamine treatment with a dose sufficient to reduce the symptoms of slow movement and stiffness had increased gamma narrow band signals. event-related synchronization (ERS) is shown. Similarly, when the patient's Parkinson's symptoms are relatively reduced, beta band signals gradually replace gamma band signals, and these beta band signals decrease with exercise, showing event-related desynchronization (ERD).
This experiment suggests that both gamma and beta signals may indicate the mildness and severity of Parkinson's symptoms. Gamma signals increase with symptom reduction, while beta signals replace gamma signals after a certain stage of treatment and decrease with exercise. The whole process starts with ERS and then ERD. From the perspective of feedback signal, the increase of beta signal and the decrease of gamma signal mean the intensification of disease degree, while the enhancement of gamma signal and the decrease of beta signal mean the reduction of disease degree. Beta signals and gamma signals can be used as biomarkers to monitor the degree of PD, but it is determined by the detection range of the equipment and whether to consider the influence of autonomous movement.
4. Comprehensive analysis for motor control disorders
The brain signals of epilepsy are characterized by diversity and complexity. It is closely related to the location of the lesion, the degree of occurrence and the cause of the disease [8]. Young et al. completed the monitoring of epileptic signals in the rat brain. They first inhibited spontaneous SWDs in the brain with an 800-Hz, 40% duty cycle, and 30-40-μa stimulation pulse train for 0.5 s. The rats were then injected with proconvulsant PTZ (20 mg/kg, i.p.) to induce convulsive SWDs. EEG images of the rats in wakefulness, spike-wave discharge, slow-wave sleep, and movement-artifact were eventually obtained (Figure 1) [9].
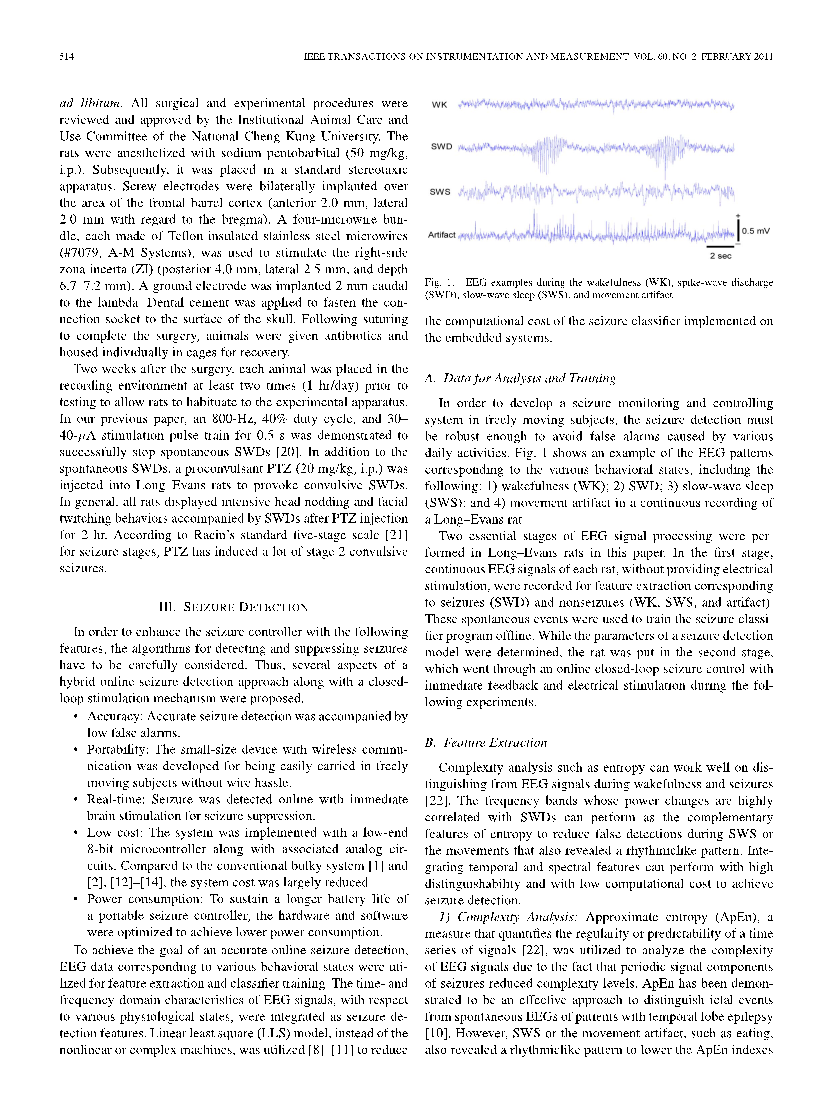
Figure 1. EEG images of rats in four states [9]
Epileptic rats showed high rhythmic and high amplitude electrical abnormalities in the SWDs images. This shows that epileptic focal discharge also has the characteristics of high rhythm. This is also a significant feature of PD. It can be inferred that the brain locations of motor control diseases have high rhythmic characteristics. In fact, according to the characteristics of symptoms shared by PD and epilepsy, such as quiescence tremor, the rhythm of EEG electrical signals is consistent with the rhythm of co-occurring tremor symptoms, indicating that one of the common characteristics of the biomarkers of motor control disease is rhythmicity.
The EEG signals of epilepsy have a variety, most of which are mainly spike-wave and have the characteristics of high amplitude. However, one of the main characteristics of PD signals, whether beta broadband or gamma narrow band, is low amplitude. This indicates that PD and epilepsy still have significant differences in etiology and symptoms. Considering that the biomarkers of PD are concentrated in STN, while epilepsy is concentrated in a variety of lesions, which may be STN or ANT, and the pathology of the two is inconsistent, it can explain the reason why the signal amplitude of epilepsy lesions is high, and the PD signal is low. From the point of view of symptoms, PD is persistent, mostly static tremor, and the EEG amplitude is low. Epilepsy is often more rapid, the symptoms are more intense, and the electrical amplitude of the brain is higher. This is a key difference between epilepsy and PD when it comes to acquiring feedback signals
5. Comprehensive analysis for two kinds of disorders
MDD, recognized as a prototypical cognitive ailment, has revealed the distinctive value of its feedback signals in recent research endeavors. Scangos et al.'s study [10], through the implantation of ten stereoelectroencephalography (SEEG) electrodes bilaterally in the orbitofrontal cortex (OFC), amygdala, hippocampus, ventral capsule/ventral striatum (VC/VS), and subgenual cingulate cortex (SGC), has engaged in stimulation-response mapping targeting emotional circuits. Their findings illuminate the roles of various brain regions in emotion regulation, indicating that the amygdala and hippocampus integrate signals across numerous brain areas with the highest weighted indices, underscoring their paramount importance in emotional and mnemonic processes. Moreover, the study highlights the VC/VS's influence on distal brain regions, reinforcing its centrality within the emotional regulation network. The research further demonstrates that the gamma signal intensity from the bilateral amygdala alone is sufficient to effectively monitor the severity of MDD symptoms, emerging as a crucial feedback signal for adaptive DBS therapy. This revelation accentuates the amygdala's pivotal role in MDD treatment, as fluctuations in gamma signals can precisely reflect the patient's emotional state, thus providing a basis for real-time modulation.
Contrary to the feedback signals in most motor control disorders, such as Parkinson's disease, which predominantly manifest in lower frequency bands, MDD feedback signals predominantly exhibit in the form of gamma signals, characterized by higher frequencies. This distinction aligns with the window theory, suggesting a significantly higher degree of precision in feedback mechanisms at the level of cognitive control compared to motor control. Thus, this disparity becomes a critical watershed in differentiating therapeutic approaches for motor control diseases and cognitive disorders. In clinical practice, it is impermissible to categorize feedback signals merely based on frequency. Instead, a comprehensive evaluation incorporating the patient's etiology, specific lesion location, and the anatomical positioning of feedback signals within the brain is imperative for more accurate discernment.
Besides, although gamma narrowband signals also play a significant role as feedback signals in Parkinson's disease, the fundamental differences in neurophysiological mechanisms between MDD and Parkinson's disease underscore the necessity for a more nuanced analysis and understanding of these signals' functional attributes when devising therapeutic strategies for distinct disorders. This approach not only facilitates the development of more personalized treatment plans but also propels the field forward in unraveling the intricate mechanisms underlying various neuropsychiatric conditions.
6. Future development
The analysis reveals that neurological disorders can broadly be categorized into two major types: movement control disorders and cognitive disorders. The symptomatic features, underlying causes, and characteristics of feedback signals relevant to adaptive DBS in clinical practice show notable correlations. Specifically, feedback signals in movement control disorders exhibit a high rhythmicity, typically characterized by a lower frequency, and are primarily localized in structures such as the thalamus or STN. These signals are closely tied to the regulation of motor functions. In contrast, feedback signals in cognitive disorders display a higher frequency, mainly found within the CSTC loops or associated cortical regions, often relating to cognitive and emotional processing.
This classification provides an essential reference for future closed-loop DBS therapies. In the face of complex, poorly understood, or urgent neurological conditions, initial symptom classification can guide us in identifying potentially effective feedback signal locations. Additionally, abnormal EEG signals can aid in inferring the type of disorder, thus offering valuable insights for the application of DBS and the selection of pharmacological treatments.
In addition, the commonalities in these feedback signal characteristics open new possibilities for the advancement of DBS technology. In forthcoming clinical applications, it will be advantageous to utilize distinct mathematical models and tailor algorithms according to different types of feedback signals. This strategic refinement will enhance the capacity to address both high-frequency and low-frequency signals, facilitating the professional growth of aDBS. Such developments promise not only to improve the precision of closed-loop DBS control but also to augment the overall efficacy of treatments.
7. Conclusion
This paper mainly compares the similarities and differences of feedback signals in clinical practice of aDBS in three diseases: PD, epilepsy and MDD. This paper first analyzes the etiology and symptoms of these three diseases, and summarizes the types of feedback signals commonly used in clinical practice and their characteristics, such as frequency, amplitude, rhythm and location. Then this paper mainly describes the ways of obtaining partial feedback signals and its features in previous studies, and the relationship between them and symptoms through three experiments. Later, the comparison between PD and epilepsy showed the similarities and differences of feedback signals of motor control diseases and the correlation between the signals and disease symptoms as well as etiology. Then, the differences between motor control diseases and cognitive diseases are demonstrated by comparing two motor control diseases and MDD. The conclusions are as follows: Most of the feedback signals of motor control disorders are low frequency signals, characterized by high rhythm; Cognitive disorders have a higher frequency of feedback signals, which is related to the precision of the brain's cognitive and motor control. This paper presents a new idea for the selection of aDBS feedback signals and a new possibility for the development of aDBS specialization.
References
[1]. Piña F. D, Beudel M, Little S, et al. Adaptive deep brain stimulation as advanced Parkinson's disease treatment (ADAPT study): protocol for a pseudo-randomised clinical study. BMJ Open 2019; 9(6): e029652.
[2]. Rosa M, Arlotti M, Ardolino G, et al. Adaptive deep brain stimulation in a freely moving parkinsonian patient. Mov Disord 2015; 30(7).
[3]. Crone N E, Miglioretti D L, Gordon B, Lesser R P. Functional mapping of human sensorimotor cortex with electrocorticographic spectral analysis. II. Event-related synchronization in the gamma band. Brain 1998; 121: 2301-2315.
[4]. Miller K J, et al. Spectral changes in cortical surface potentials during motor movement. J Neurosci 2007; 27: 2424-2432.
[5]. Engel J J. What can we do for people with drug-resistant epilepsy. Neurology 2016; 87: 2483-2489.
[6]. Birbaumer N, Elbert T, Canavan A G M, Rockstroh B. Slow potentials of the cerebral cortex and behavior. Physiol Rev 1990; 70: 1-41.
[7]. Pfurtscheller G, da Silva F L. Event-related EEG/MEG synchronization and desynchronization: basic principles. Clin Neurophysiol 1999; 110: 1842-1857.
[8]. Wu Y C, Liao Y S, Yeh W H, Liang S F, Shaw F Z. Directions of Deep Brain Stimulation for Epilepsy and Parkinson's Disease. Front Neurosci 2021; 15: 680938.
[9]. Young C P, Liang S F, Chang D W, Liao Y C, Shaw F Z, Hsieh C H. A portable wireless online closed-loop seizure controller in freely moving rats. IEEE Trans Instr Meas 2011; 60: 513-521.
[10]. Scangos K W, Khambhati A N, Daly P M, et al. Closed-loop neuromodulation in an individual with treatment-resistant depression. Nat Med 2021; 27: 1696-1700.
Cite this article
Li,E. (2024). Correlations between neurological disease mechanisms and feedback signals utilized in adaptive DBS: A comparative study. Theoretical and Natural Science,64,27-32.
Data availability
The datasets used and/or analyzed during the current study will be available from the authors upon reasonable request.
Disclaimer/Publisher's Note
The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of EWA Publishing and/or the editor(s). EWA Publishing and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
About volume
Volume title: Proceedings of the 4th International Conference on Biological Engineering and Medical Science
© 2024 by the author(s). Licensee EWA Publishing, Oxford, UK. This article is an open access article distributed under the terms and
conditions of the Creative Commons Attribution (CC BY) license. Authors who
publish this series agree to the following terms:
1. Authors retain copyright and grant the series right of first publication with the work simultaneously licensed under a Creative Commons
Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this
series.
2. Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the series's published
version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial
publication in this series.
3. Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and
during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See
Open access policy for details).
References
[1]. Piña F. D, Beudel M, Little S, et al. Adaptive deep brain stimulation as advanced Parkinson's disease treatment (ADAPT study): protocol for a pseudo-randomised clinical study. BMJ Open 2019; 9(6): e029652.
[2]. Rosa M, Arlotti M, Ardolino G, et al. Adaptive deep brain stimulation in a freely moving parkinsonian patient. Mov Disord 2015; 30(7).
[3]. Crone N E, Miglioretti D L, Gordon B, Lesser R P. Functional mapping of human sensorimotor cortex with electrocorticographic spectral analysis. II. Event-related synchronization in the gamma band. Brain 1998; 121: 2301-2315.
[4]. Miller K J, et al. Spectral changes in cortical surface potentials during motor movement. J Neurosci 2007; 27: 2424-2432.
[5]. Engel J J. What can we do for people with drug-resistant epilepsy. Neurology 2016; 87: 2483-2489.
[6]. Birbaumer N, Elbert T, Canavan A G M, Rockstroh B. Slow potentials of the cerebral cortex and behavior. Physiol Rev 1990; 70: 1-41.
[7]. Pfurtscheller G, da Silva F L. Event-related EEG/MEG synchronization and desynchronization: basic principles. Clin Neurophysiol 1999; 110: 1842-1857.
[8]. Wu Y C, Liao Y S, Yeh W H, Liang S F, Shaw F Z. Directions of Deep Brain Stimulation for Epilepsy and Parkinson's Disease. Front Neurosci 2021; 15: 680938.
[9]. Young C P, Liang S F, Chang D W, Liao Y C, Shaw F Z, Hsieh C H. A portable wireless online closed-loop seizure controller in freely moving rats. IEEE Trans Instr Meas 2011; 60: 513-521.
[10]. Scangos K W, Khambhati A N, Daly P M, et al. Closed-loop neuromodulation in an individual with treatment-resistant depression. Nat Med 2021; 27: 1696-1700.









