1. Introduction
The term "circadian rhythm" was originally proposed by Halberg to describe the near 24-hour endogenous oscillations in biological processes related to the Earth's rotation cycle [1]. Disruptions in circadian rhythms can occur for various reasons and affect a wide range of populations, including shift workers, night owls, and those whose endogenous-sunlight oscillations are misaligned due to excessive use of electronic devices [2]. These disruptions also include phase and frequency instability of circadian rhythms in people experiencing jet lag, new mothers, and those with mood fluctuations or affective disorders, as well as the reduced amplitude of circadian rhythms associated with aging and neurodegenerative diseases. Such disruptions can potentially lead to numerous health conditions, including cancer, obesity, arthritis, atherosclerosis, and mental illnesses [3], and may also result in decreased productivity and increased healthcare costs, imposing a social burden.
Current therapeutic approaches for circadian rhythm disruptions primarily include pharmacotherapy, light therapy, electromagnetic therapy, and cognitive behavioral therapy (CBT). CBT is considered safer than pharmacotherapy; however, its effectiveness partially depends on the willingness of participants to accept and implement the therapeutic recommendations. Furthermore, in the treatment of insomnia, only 23-46% of patients achieve complete remission through CBT-I [4]. Electromagnetic therapy demands less participation from patients but is costly and not yet widely practiced. Additionally, some individuals prefer to adhere to "freedom", experiencing a sense of "control" within the chaos and believing that their problems are merely cognitive, hence refusing treatment.
Here, I put forward "Autonomous Cognitive Instant-Engagement"(ACIE) as a new conceptual direction that acknowledges the immediate, tangible impact of personal agency, reflexivity, and cognitive activities on physiological processes. Its specific effects have already been directly or indirectly demonstrated in related studies [5] and CBT outcomes. It requires no medical costs, and according to the Common-Sense Model of Self-Regulation (CSM) [6], when individuals perceive physical discomfort caused by circadian rhythm disruptions, this stimulates the activation of normal self-prototypes and memory structures, generating psychological representations of the illness threat (i.e., beliefs about the identity, cause, control, consequences, and duration/timeline of the illness). Consequently, individuals will seek possible treatments and action plans or self-explanations—the first opportunity for treatment lies in seeking help from oneself.
There are numerous examples of autonomous cognitive instant engagement being integrated into CBT treatment outcomes, where experts supplement CBT with recommendations and education on cognitive correction, light therapy, sleep restriction, diet, exercise, and social contact. The reasons for the efficacy of these interventions may include the financial cost involved, the enhanced belief in change brought about by the therapist's social connection and strategic reinforcement, and the specific effects of zeitgebers such as light [7], temperature [8], food [9], social factors [10], exercise [11], and cognition [5]. This makes it difficult to isolate and separately assess the role of cognition.
In discussions of the effects of autonomous cognitive engagement on insomnia, factors such as rumination , emotional states like anxiety and arousal, and mindfulness have been frequently explored [12,13]. However, these discussions have mostly emphasized horizontal differences and the impact of the past on the present, without addressing the vertical influence from the present to the future (i.e., immediacy). Randomized controlled trials have also investigated the effect of mindfulness meditation alone on improving circadian rhythms [14], but these studies predominantly rely on subjective survey questionnaires derived from scales, with limited simultaneous exploration of mechanisms and effects. Moreover, there may be other cognitive activities beyond meditation that are effective, but the lack of discussion on cognition as a zeitgeber has limited the imagination in this area.
This review aims to explore the following questions: (i) Which aspects of autonomous cognitive activities can be considered to have been physiologically proven to influence circadian rhythms? (ii) What are the immediate and sustained effects corresponding to different intensities, durations, and times of application? (iii) To what extent have the mechanisms underlying these effects been demonstrated? (iv) The potential mechanisms by which autonomous instant cognitive activities influence circadian rhythms. (v) Other possible autonomous instant cognitive activities that may be effective. (vi) The causal relationship between cognitive activities and circadian rhythms. (vii) Future experimental directions.
Box 1 Abbreviations | |||
ACSF | artificial cerebro-spinal fluid | MWM | Morris water maze |
AUC | area under the curve of cortisol | SAT | sustained attention training |
BOA | total bins of activity | SL | selectively deafferent basal forebrain cholinergic projections to the SCN |
DD | dark-dark | SCN | suprachiasmatic nucleus |
dSAT | distracted SAT | SRTT | simple reaction time task |
EL | electrolytic lesion of the SCN | SV | short-version |
LD | light-dark | PMR | progressive muscle relaxation |
LV | long-version | ZT4 | the fourth hour after turning on |
MSDR | male Sprague-Dawley rats | ZT16 | the sixteenth hour after turning on |
2. Evidences
2.1. Sustained attention
Reaching a certain intensity, regardless of the duration of each session, SAT (Sustained Attention Task) administered at any time of day can significantly entrain the circadian rhythm of mice. This is achieved through the obstruction of SCN (suprachiasmatic nucleus) rhythm by cholinergic signals projected from the basal forebrain, which also allows the entrainment of non-SCN clocks, as demonstrated by Gritton's experiments [5,17,18].
Table 1. Overview of autonomous cognitive instant-engagement: task characteristics and outcome measures
Study | Condition | Sample groups | Cognitive adjustment | Treatment Duration | Circadian modification results | Free-run |
Field-based studies | ||||||
Thaddeus et al., 2022 [15] USA | Breath, voice, reminding | 35 career firefighters | Meditation APP | 10 consecutive days | Salivary Cortisol After 2 days free-running, there was a significant difference in cortisol concentration and cortisol AUC upon waking before and after the intervention. | Only experimentalize two days |
Mark et al., 1985 [16] USA | Music by Debussy, PMR | 12 female nurses | Guided imagery with PMR then music | One month, 5 times a week | Urinary Cortisol The mean amplitude variation decreased significantly. Oral temperature The normal and cortisol phase was 180°, and the phase displacement increased significantly after training. | × |
Laboratory-based studies | ||||||
Gritton et al., 2009 [17] USA | LD 12:12 LD 6:12:6 after the phase stabilization, ZT4 to ZT10, ZT4 to ZT10 | 8 MSDR | H2O | 14 consecutive days | Locomoter activity No significant difference in the daytime/dark day activity ratio between the H2O group and the H20-SRTT group compared with the absence of water deprivation and SRTT. In the H2O-SAT group, exercise was significantly concentrated on SAT time, and the greater the exercise range, the better the performance on SAT and dSAT. ZT4 | √ |
4 MSDR | H2O+SRTT | 20 consecutive days | √ | |||
11 MSDR | H20+SRTT-SAT-dSAT in turn, after each stage to achieve the next | Until right | √ | |||
to ZT10 showed a close correlation between daytime and black-day motion ratio, indicating that SAT had the same effect on circadian rhythm before and after the light phase shift. Different rats in DD behaved differently, but most of the rats still did not return to normal circadian rhythms after resuming light exposure. | ||||||
Gritton et al., 2012 | LD 12:12 | 12 MSDR | SAT-ZT4 | Until right | Locomoter activity All SAT groups showed entrainment of attention training | √ |
12 MSDR | SAT-ZT10 | Until right | √ | |||
12 MSDR | SAT-ZT16 | Until right | √ | |||
[18] USA | 12 MSDR | SAT/dSAT ZT4-ZT16 | right-2-34-right-2 | activities, and all MWM groups showed little difference compared with SAT groups and no significant differences between ZT4 and ZT16. SAT-ZT4 has two phenotypes, one with delayed activity at night and one with intermittent daytime activity until several hours after the lights are turned off. SAT8min-ZT4 found no significant difference in activity ratio, total activity and DD stage performance between 40min and 40min. | √ | |
12 MSDR | SAT/dSAT ZT16-ZT4 | right-2-34-right-2 | √ | |||
12 MSDR | SAT40min/SAT8min-ZT4 | 115-35 | √ | |||
12 MSDR | MWM-ZT4 | 28 consecutive days | √ | |||
12 MSDR | MWM- ZT16 | 28 consecutive days | √ | |||
Gritton et al., 2013 [5] USA | LD 12:12 | 5 MSDR- ACSF | SAT-ZT4 | 25 consecutive days | Locomotor activity Little difference between ACSF and SL pre-training and normal LD cycles. Each group's exercise was significantly focused on SAT time. The activity of ZT4 in ACSF was the most significant, with the expected activity | 8.3±1.4 days |
6 MSDR- ACSF | SAT-ZT16 | 25 consecutive days | 13.7±0.2 days | |||
5 MSDR-SL | SAT-ZT4 | 25 consecutive days | 1.8±0.3 days | |||
6 MSDR-SL | SAT-ZT16 | 25 consecutive days | 13.3±0.3 days | |||
2 MSDR-EL | SAT-ZT4 | 17 consecutive days | occurring earlier, and the amplitude and duration of ZT4 activity in SL were relatively reduced. The expected motion associated with SAT was significantly greater in ZT4 ACSF than in ZT4 SL, with EL being the strongest. The activity of EL was completely disordered at the beginning, significantly synchronized after SAT, and the LD activity ratio was the highest. Body temperature ZT4 showed a bimodal temperature rhythm, and ZT16 showed a single peak centered on the dark phase. The increase of ACSF ZT4 in body temperature relative to light was significantly earlier than that in SL, and two ZT16 were significantly earlier than two ZT4. ZT4's body temperature rose significantly earlier than SAT's expected body temperature. Relationships ZT4 was more out of sync between animal movement and temperature rhythms, especially in the ACSF group. | — | ||
6 MSDR-EL | SAT-ZT4 | 150 consecutive days | 2.9±0.8 days | |||
In the 2009 experiment [17], Gritton tested a method of training rats' attention by inducing moderate water deprivation. In this scenario, the rats were guided to complete attention tasks with water rewards and rehydration provided at appropriate times each day. The SAT was divided into three stages: the first stage involved simple task training, where two retractable levers alternated after every five presses to designate the "correct" lever. Pressing the correct lever triggered a reward. The second stage involved a signal light, and the rats had to press the correct lever within a limited time after the light appeared; failing to do so resulted in a miss or false alarm. Rats that met the accuracy criteria for three consecutive days proceeded to the next stage. The third stage added ambient lighting, increasing the demand for attention, and shortened the signal light duration, requiring rats to achieve a 70% correct response rate in a 500ms signal trial, with limited misses allowed. Completion of all three stages constituted the successful completion of SAT training. All animals completed the training within three months. Subsequently, the room light was flashed at 0.5 Hz, and the experimental rats were tested with the dSAT task. In the SRTT (Simple Reaction Time Task), the rats were rewarded the same regardless of which lever they pressed. Initially, the light cycle was set at a 12:12 LD ratio; once the rats showed stable performance in SAT training, the cycle was changed to 6:12:6, and once stability was achieved again, the cycle was switched to DD mode without SAT training to observe the lasting impact of SAT training on the rats.
The results showed that, before water deprivation, these rats were normally active only at night. After water deprivation, the control group that received water and the simple SRTT group showed no significant change in activity patterns, while the SAT group exhibited significant changes, with activity beginning before the SAT task commenced. Moreover, when the light cycle was changed to 6:12:6, the activity pattern did not shift in accordance with the light! After the DD phase, all rats took at least a few days to return to nocturnal activity patterns, and some rats never fully reverted.
In the 2012 experiment [18], Gritton examined the effects of SAT training administered at different times of the day, both during the day and night, and compared these with MWM (Morris Water Maze) training—a task that induces stress, relies on hippocampus-dependent spatial memory, and requires visual and proprioceptive feedback—to compare the differences in cognitive activities. The results showed that the MWM group did not exhibit cognitive entrainment, whereas all SAT groups, regardless of day or night training, showed significant entrainment. Moreover, there was no significant difference between 8-minute SAT training and 40-minute daily SAT training in terms of day-night activity ratio, total activity level, or performance during free running in the DD phase.
In the 2013 experiment [5], Gritton explored the mechanisms of SAT entrainment by setting up groups in which cholinergic projections from the basal forebrain to the SCN were removed using 192 IgG-saporin (SL group), groups that received only ACSF (artificial cerebrospinal fluid) (ACSF group), and groups with electrolytic lesions of the SCN (EL group). The results showed that the activity patterns of the ACSF and SL groups did not show significant circadian rhythm changes under normal lighting, while the EL group’s patterns were completely disrupted. After adding SAT training, all three groups exhibited a significant concentration of activity during the SAT training times, with the EL group showing the highest day-night activity ratio, earlier onset time, and longer daytime activity duration compared to the ACSF group, which in turn showed significantly higher levels than the SL group. After the DD phase, none of the groups immediately resumed nocturnal activity, with the SL group returning to normal faster than the ACSF group. In this experiment, Gritton also measured body temperature, finding that ZT16 showed a single peak centered on the dark phase, while ZT4 exhibited a biphasic pattern with peaks in both the dark and light phases. Interestingly, ZT4, particularly in the ACSF group, showed greater desynchronization between activity and temperature rhythms, which might simulate the experience of night shift workers. In explaining the related mechanisms, Gritton proposed a model distinguishing between peripheral oscillations and core oscillations of the SCN. The impact of SAT largely acts on peripheral oscillations, while cholinergic projections to the SCN inhibit the SCN’s intrinsic oscillations. The resulting oscillations are a combination of both effects, explaining why SAT has the greatest impact in the absence of SCN oscillations and the least impact when cholinergic projections to the SCN are reduced.
These three sets of experiments all included controls, distinguished entrainment from "masking" under DD conditions, and continuously recorded activity patterns over 24 hours. They considered different training tasks, varying degrees of training, different training durations, and certain mechanisms, making the findings comprehensive and convincing. Unfortunately, these experiments did not further assess the rats' physical health or measure clock proteins in their tissues.
2.2. Meditation
Thaddeus's study[15] employed a 1-group pretest-posttest design to evaluate the effectiveness of a new mobile meditation app designed for firefighters. The app consisted of 10 individual 10-minute sessions, one session per day for 10 consecutive days, covering key themes such as awareness, connection, insight, and purpose. On the first day, the practice focused on breathing in the present moment; on the second day, it focused on bodily sensations; the third day on sounds; the fourth day on thoughts, emotions, and breathing; the fifth day on shifting awareness from the body to one’ s uniqueness and capabilities; the sixth day on gratitude; the seventh day on empathy; the eighth day on examining unconscious beliefs; the ninth day on using personal values to make judgments; and the tenth day on finding meaning in the challenges faced by oneself and others.
Saliva samples were collected six times over two days before the intervention—upon waking, 30 minutes after waking, and at bedtime. The same six samples were collected two days after the intervention. The results showed a statistically significant reduction in both the cortisol concentration in the saliva upon waking and the area under the curve (AUC) of cortisol levels. This suggests that when firefighters take time for self-reflection, self-compassion, empathy for others, and connection with others, they become more focused and engaged in their work.
However, with a sample of 35 professional firefighters from a city in the southwestern United States, there was considerable variability between individuals, and the sample size was relatively small. Additionally, the final measurements lacked continuity, and there were reminders from the researchers via text messages or phone calls, which may have introduced some social zeitgeber interference.
2.3. Guided imagery
In Mark's study [16], a sample of 12 full-time female nurses was used, with 6 nurses regularly rotating shifts every 10-14 days between morning (700-1500) and evening (1500-2300) shifts, and 6 nurses working night shifts long-term. The music tape played Debussy's Prelude to the Afternoon and Clouds, as well as McLaughlin's Peace of Mind. Previous studies have shown that these pieces of music have a relaxing effect. Based on Debussy's Prelude, progressive muscle relaxation was narrated over the music, guiding the participants to sequentially tense and relax eight muscle groups. The guided imagery training involved participants finding a "place of rest" in their minds and experiencing this place vividly with their senses during the muscle group exercises. During Clouds, listeners were asked to imagine themselves (1) becoming a cloud and rising to a place of relaxation, (2) focusing on an area of concern in their life, such as work or relationships, and (3) from their elevated position as a cloud, visualizing the problem area and attempting to see aspects that had previously been unclear.
Over the course of a month, the participants listened to the tape every 4-5 days. On those days, urine samples were collected at waking, 4-5 hours after waking, 4-5 hours after listening to the tape, and before bed. Oral temperature was recorded simultaneously with urine collection. The study measured average urine cortisol levels, circadian rhythm amplitude, and the interaction between circadian rhythm and body temperature. The results showed a significant reduction in circadian rhythm amplitude and a greater phase difference between urinary cortisol and temperature rhythms.
However, the sample size was small, other life events may have caused interference, and there was potential interference from the music via the auditory pathway.
3. Discussion
3.1. Main findings
"Autonomous Instant Cognitive-Engagement" is a method in which one's proactive and immediate cognitive engagement influences physiological processes to regulate circadian rhythms. Existing research has demonstrated the effects of immediate engagement in meditation, guided imagery, and sustained attention on altering circadian rhythms, with measured factors including cortisol levels, motor activity, and temperature rhythms. Sustained attention has been shown to require a certain intensity, is not linearly related to the duration of each session, can occur at any time of the day, and has lasting effects. This insight encourages people to engage in activities requiring focused attention during the day. Although the other two types of cognitive engagement still face issues such as insufficient data continuity, small sample sizes, and many uncontrollable factors, they also suggest that practicing these techniques before bed could lower cortisol levels and facilitate quicker sleep onset. The overview of these experiments can be seen in Table 1.
3.2. Possible mechanisms
The alteration of circadian rhythms can ultimately be traced back to the transcription-translation feedback loop (TTFL) of clock genes, a topic that has been systematically reviewed in numerous studies. In simple terms, a BMAL1 and CLOCK dimer acts as a transcriptional activator by binding to the E-box (CACGTG) response element in the promoter region of clock genes, leading to the transcription of clock genes such as Per and Cry. The proteins encoded by these genes form complexes under the action of casein kinase, which then enter the nucleus to further inhibit the transcription of Bmal1 and Clock. The transcriptional activator SIRT1 forms a protein that inhibits the expression of PER2 through deacetylation. Activated RORs and Rev-Erbs either bind to or inhibit the RRE element (AGGTCA) on the Bmal1 gene. Other clock genes are also involved in this process. These interactions of mutual promotion and inhibition result in the expression cycles of these proteins, each lasting approximately 24 hours.
Hormones secreted by the human body regulate TTFL. Glucocorticoids, after binding with cytoplasmic glucocorticoid receptors, enter the nucleus together and bind to GREs elements in the promoter region of clock genes, stimulating the transcriptional oscillation of multiple clock genes, including Per1, 2, 3, Cry1, 2, Rev-Erb, and Bmal1. Notably, one GRE in Per2 remains continuously occupied during rhythmic expression [19]. Norepinephrine (NA) can induce transient expression of Per1 mRNA by activating β2-adrenergic receptors in C6 glioma cells, involving the PKA-CREB and Src-GSK-3beta pathways [20]. Melatonin, via the MT1 receptor, affects the expression of clock genes Per1, Clock, Bmal1, and NPAS2 in primary neurons prepared from the striatum [21]. In melatonin-proficient mice (C3H), the adrenal cortex and medulla show circadian variations in PER1, CRY2, and BMAL1 protein levels, whereas in melatonin-deficient mice (C57BL), clock proteins do not exhibit such variations in the adrenal cortex [22].
Meditation can be deconstructed into two core components: focused attention and open monitoring. Focused attention involves concentrating attention on a specific object, while open monitoring involves non-reactive monitoring of the content of experience, aimed at maintaining a calm and continuous focus on thoughts [23]. Anatomically, long-term meditators have been observed to have thicker lateral and medial prefrontal cortices (PFC) and temporal cortices, and thinner lateral and medial parietal regions; there is increased gyrification in the insula and increased thickness of the corpus callosum [24]. In the nervous system, meditation has been shown to activate the prefrontal cortex (PFC), hippocampus, amygdala, and anterior cingulate cortex, and to enhance the neuroplasticity of white matter in the anterior cingulate cortex [25]. EEG studies have shown significantly increased alpha and theta wave activity in the frontal cortex, with widespread changes in gamma frequency [26]. Stimulation of the PFC triggers excitation of the hypothalamus-ventromedial nucleus via the excitatory neurotransmitter glutamate, which in turn stimulates the peripheral parasympathetic nervous system [27]. The dominance of the parasympathetic nervous system leads to decreased heart rate, blood pressure, and oxygen metabolism, alleviates oxidative stress, and promotes neuronal growth. Biochemically, serotonin metabolism increases in the urine, GABA levels increase in the serum, and preliminary evidence suggests increased levels of BDNF, IL-6, TNF-α, and IFN-γ, with a decrease in IL-12 in the plasma [28,29].
Although there is no direct evidence of meditation affecting the circadian clock, three aspects of fluid regulation might play a role when considering the aforementioned mechanisms. First, melatonin levels in the plasma have been found to increase sharply during meditation [30]. Second, due to decreased heart and respiratory rates, the nucleus of the solitary tract reduces the influence of the locus coeruleus, leading to decreased norepinephrine production and distribution by the locus coeruleus. This results in reduced stimulation of the hypothalamic paraventricular nucleus, lowering the release of corticotropin-releasing hormone (CRH) and, consequently, cortisol levels [31]. Third, meditation may influence the control of hypothalamic peptide release by neurotransmitters, thereby altering the secretion of pituitary hormones by either increasing inhibitory factors from the hypothalamus or decreasing stimulatory factors such as thyrotropin-releasing hormone (TRH) or growth hormone-releasing factor [32].
Sustained attention can be defined as the readiness to detect the presence of a rarely occurring signal over an extended period and the ability to accurately distinguish this signal from non-signal events or "noise". There is evidence that adding the cholinergic agonist carbachol to SCN slices induces a phase shift in PER1 protein rhythms, dependent on muscarinic receptors [33].
Guided imagery is a cognitive activity based on cues such as images and sounds to create an imaginative scenario beyond everyday life, with the core goal of generating vivid mental imagery. It may further affect the hypothalamic-pituitary-adrenal (HPA) axis by influencing the fronto-limbic system.
3.3. Other kinds of “autonomous cognitive instant-engagement”
Self-suggestion. Self-suggestion differs from guided imagery in that the cognitive content involves attitudinal evaluations about one's future moments. In a randomized controlled trial with elderly patients, participants were divided into a self-suggestion group and a non-suggestion group. The self-suggestion group followed specific phrasing techniques to write positive affirmations about their lives and recorded themselves reading these affirmations aloud for 15 minutes. They then listened to these recordings an average of four times a day for 30 days as a form of self-affirmation. After accounting for baseline conditions, it was found that the elderly in the self-suggestion group rated their quality of life higher subjectively compared to the non-suggestion group. Additionally, their IL-6 levels significantly decreased, cortisol levels in the serum moved closer to the normal range, and there was a trend towards increased neuroplasticity in the prefrontal cortex [34].
3.4. Causal relationship between cognitive activity and circadian rhythm
In the causal relationship between cognitive activities and circadian rhythms, most existing studies have focused on the impact of circadian rhythms on cognitive activities. There have been studies examining the circadian rhythms of cognitive function and emotions, as well as the immediate and delayed predictions of cognitive performance by circadian rhythms [35]. However, when it comes to the influence of cognitive activities on circadian rhythms, most research compares different groups of individuals, and the effects of certain mental conditions, such as Alzheimer's disease, Parkinson's disease, Huntington's disease, ADHD, autism, major depression, bipolar disorder, and stress on circadian rhythms, have already been discussed. Additionally, treatment methods like magnetic stimulation are based on this premise.
In Gritton's experiments [5,18], besides examining the entrainment of circadian activity by cognitive tasks, the impact of training time and circadian activity on training outcomes was also investigated. The results showed that when the training tasks were conducted at night—aligning with the original circadian activity patterns—the mice performed better in both the SAT and dSAT tasks, and their learning progression was more effective. Interestingly, during ZT4, better performance was associated with higher daytime activity, while during ZT16, better performance was linked to lower nighttime activity. In the water maze experiment, mice trained at ZT4 exhibited greater memory loss two weeks after training cessation compared to those trained at ZT16.
This experimental result inspires us to propose a causal model where cognition and circadian rhythms iteratively influence each other over time, effectively forming a mutually causal relationship. Within this relationship, neither aspect is entirely deterministic. Other zeitgebers can introduce additional factors into the interaction, and cognition itself can introduce new factors, thereby further influencing the shape of circadian rhythms affected by cognition at the next moment. It can be described in figure 1.
Clarifying the causal relationship between the two, especially the part of cognitive activities that is not determined by circadian rhythms, is crucial for self-understanding and for developing therapeutic approaches for patients with circadian rhythm disorders.
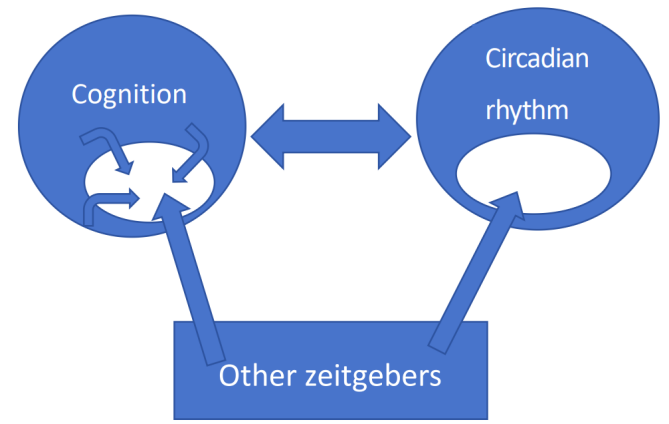
Figure 1. A causal model between cognitive activity and circadian rhythm
3.5. Future research
There are still many unresolved issues. The precise quantification and evaluation of the effects of sustained attention, meditation, and imagery on the human body need further investigation. Additionally, there is a need for direct evidence and mechanistic explanations regarding the impact of other cognitive processes, such as self-suggestion and brainstorming, on circadian rhythm alterations. Glucocorticoids and the HPA axis also exhibit ultradian rhythms regulated by clock genes such as Per2 and Cry1, but the causal relationships between these elements remain unclear.
Research methods should also be expanded. Concurrent studies on both humans and animals should be conducted, leveraging the advantage of more controllable environmental variables in animal experiments. In terms of measurement methods, detailed assessments of biomarkers that are representative of circadian rhythms should be carried out, along with enhanced measurements at the transcriptional level of clock genes.
4. Conclusion
Although the literature is limited, current research findings suggest that sustained attention, meditation, and imagery in autonomous instant cognitive activities can have tangible effects on the body's endocrine system, thereby adjusting circadian rhythms when applied to a certain extent. Additionally, other cognitive activities, such as self-suggestion and brainstorming, may also be effective. More comprehensive social and laboratory research is needed in the future.
References
[1]. Halberg, F. (1959) Physiologic 24-hour periodicity; general and procedural considerations with reference to the adrenal cycle. Internationale Zeitschrift fur Vitaminforschung. Beiheft 10, 225-296
[2]. Merikanto, I. et al. (2013) Associations of Chronotype and Sleep With Cardiovascular Diseases and Type 2 Diabetes. Chronobiol. Int. 30, 470-477. 10.3109/07420528.2012.741171
[3]. Stevens, R.G. et al. (2011) Considerations of circadian impact for defining 'shift work' in cancer studies: IARC Working Group Report. Occupational and Environmental Medicine, 68, 154-162. 10.1136/oem.2009.053512
[4]. Ong, J.C. et al. (2014) A randomized controlled trial of mindfulness meditation for chronic insomnia. Sleep, 37, 1553-1563. 10.5665/sleep.4010
[5]. Gritton, H.J. et al. (2013) Cognitive performance as a zeitgeber: cognitive oscillators and cholinergic modulation of the SCN entrain circadian rhythms. PLoS One, 8, e56206. 10.1371/journal.pone.0056206
[6]. Leventhal, H. et al. (2016) The Common-Sense Model of Self-Regulation (CSM): a dynamic ramework for understanding illness self-management. Journal of Behavioral Medicine, 39, 935-946. 10.1007/s10865-016-9782-2
[7]. Wright, K.P. et al. (2013) Entrainment of the Human Circadian Clock to the Natural Light-Dark Cycle. Current Biology, 23, 1554-1558. 10.1016/j.cub.2013.06.039
[8]. Rensing, L. and Ruoff, P. (2002) Temperature effect on entrainment, phase shifting, and amplitude of circadian clocks and its molecular bases. Chronobiol. Int. 19, 807-864. 10.1081/cbi-120014569
[9]. Hepsomali, P. et al. (2023) An Examination of the Associations between Nutritional Composition, Social Jet Lag and Temporal Sleep Variability in Young Adults. Nutrients, 15. 10.3390/nu15153425
[10]. Chang, C.S. et al. (2024) Associations between social loneliness trajectories and chronotype among adolescents. Eur Child Adolesc Psychiatry, 33, 179-191. 10.1007/s00787-023-02160-5
[11]. Weinert, D. and Gubin, D. (2022) The Impact of Physical Activity on the Circadian System: Benefits for Health, Performance and Wellbeing. Applied Sciences-Basel, 12. 10.3390/app12189220
[12]. Watkins, E.R. and Roberts, H. (2020) Reflecting on rumination: Consequences, causes, mechanisms and treatment of rumination. Behaviour Research and Therapy, 127. 10.1016/j.brat.2020.103573
[13]. Fabbri, M. (2023) Mindfulness, Subjective Cognitive Functioning, Sleep Timing and Time Expansion during COVID-19 Lockdown: A Longitudinal Study in Italy. Clocks & Sleep, 5, 313-332. 10.3390/clockssleep5020024
[14]. Kwok, J.Y.Y. et al. (2023) A randomized controlled trial on the effects and acceptability of individual mindfulness techniques - meditation and yoga - on anxiety and depression in people with Parkinson's disease: a study protocol. BMC Complement Med Ther, 23, 241. 10.1186/s12906-023-04049-x
[15]. Pace, T.W.W. et al. (2022) Feasibility, Acceptability, and Preliminary Efficacy of an App-Based Meditation Intervention to Decrease Firefighter Psychological Distress and Burnout: A One-Group Pilot Study. JMIR Form Res, 6, e34951. 10.2196/34951
[16]. Rider, M.S. et al. (1985) The effect of music, therapy, and relaxation on adrenal corticosteroids and the re-entrainment of circadian rhythms. J Music Ther, 22, 46-58. 10.1093/jmt/22.1.46
[17]. Gritton, H.J. et al. (2009) Interactions between cognition and circadian rhythms: attentional demands modify circadian entrainment. Behav Neurosci, 123, 937-948. 10.1037/a0017128
[18]. Gritton, H.J. et al. (2012) Bidirectional interactions between circadian entrainment and cognitive performance. Learn Mem, 19, 126-141. 10.1101/lm.023499.111
[19]. So, A.Y.L. et al. (2009) Glucocorticoid regulation of the circadian clock modulates glucose homeostasis. Proceedings of the National Academy of Sciences of the United States of America, 106, 17582-17587. 10.1073/pnas.0909733106
[20]. Morioka, N. et al. (2010) Noradrenaline induces clock gene Per1 mRNA expression in C6 glioma cells through beta(2)-adrenergic receptor coupled with protein kinase A - cAMP response element binding protein (PKA-CREB) and Src-tyrosine kinase - glycogen synthase kinase-3beta (Src-GSK-3beta). J Pharmacol Sci, 113, 234-245. 10.1254/jphs.10031fp
[21]. Imbesi, M. et al. (2009) The melatonin receptor MT1 is required for the differential regulatory actions of melatonin on neuronal 'clock' gene expression in striatal neurons in vitro. Journal of Pineal Research, 46, 87-94. 10.1111/j.1600-079X.2008.00634.x
[22]. Torres-Farfan, C. et al. (2006) Immunocytochemical demonstration of day/night changes of clock gene protein levels in the murine adrenal gland:: differences between melatonin-proficient (C3H) and melatonin-deficient (C57BL) mice. Journal of Pineal Research, 40, 64-70. 10.1111/j.1600-079X.2005.00279.x
[23]. Jindal, V. et al. (2013) Molecular Mechanisms of Meditation. Molecular Neurobiology, 48, 808-811. 10.1007/s12035-013-8468-9
[24]. Kang, D.H. et al. (2013) The effect of meditation on brain structure: cortical thickness mapping and diffusion tensor imaging. Soc Cogn Affect Neurosci, 8, 27-33. 10.1093/scan/nss056
[25]. Brefczynski-Lewis, J.A. et al. (2007) Neural correlates of attentional expertise in long-term meditation practitioners. Proc Natl Acad Sci U S A, 104, 11483-11488. 10.1073/pnas.0606552104
[26]. Baijal, S. and Srinivasan, N. (2010) Theta activity and meditative states: spectral changes during concentrative meditation. Cognitive Processing, 11, 31-38. 10.1007/s10339-009-0272-0
[27]. Devinsky, O. (1997) Neurological aspects of the conscious and unconscious mind. Annals of the New York Academy of Sciences, 835, 321-329. 10.1111/j.1749-6632.1997.tb48639.x
[28]. Esch, T. et al. (2002) The role of stress in neurodegenerative diseases and mental disorders. Neuro Endocrinol Lett, 23, 199-208
[29]. Jones, B.M. (2001) Changes in cytokine production in healthy subjects practicing Guolin Qigong : a pilot study. BMC Complement Altern Med, 1, 8. 10.1186/1472-6882-1-8
[30]. Tooley, G.A. et al. (2000) Acute increases in night-time plasma melatonin levels following a period of meditation. Biol Psychol, 53, 69-78. 10.1016/s0301-0511(00)00035-1
[31]. Jevning, R. et al. (1992) The physiology of meditation: a review. A wakeful hypometabolic integrated response. Neuroscience and biobehavioral reviews, 16, 415-424. 10.1016/s0149-7634(05)80210-6
[32]. Bevan, A.J.W. (1980) ENDOCRINE CHANGES IN TRANSCENDENTAL MEDITATION. Clinical and Experimental Pharmacology and Physiology, 7, 75-76
[33]. Dojo, K. et al. (2017) Carbachol Induces Phase-dependent Phase Shifts of Per1 Transcription Rhythms in Cultured Suprachiasmatic Nucleus Slices. J Biol Rhythms, 32, 101-108. 10.1177/0748730417691205
[34]. Sari, N.K. et al. (2017) The role of autosuggestion in geriatric patients' quality of life: a study on psycho-neuro-endocrine-immunology pathway. Social Neuroscience, 12, 551-559. 10.1080/17470919.2016.1196243
[35]. Roybal, K. et al. (2007) Mania-like behavior induced by disruption of CLOCK. Proceedings of the National Academy of Sciences of the United States of America, 104, 6406-6411. 10.1073/pnas.0609625104
Cite this article
Liu,Y. (2024). Ask yourself for help: a review of objective evidence on “Autonomous Cognitive Instant-Engagement” regulating the circadian rhythm. Theoretical and Natural Science,64,57-67.
Data availability
The datasets used and/or analyzed during the current study will be available from the authors upon reasonable request.
Disclaimer/Publisher's Note
The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of EWA Publishing and/or the editor(s). EWA Publishing and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
About volume
Volume title: Proceedings of the 4th International Conference on Biological Engineering and Medical Science
© 2024 by the author(s). Licensee EWA Publishing, Oxford, UK. This article is an open access article distributed under the terms and
conditions of the Creative Commons Attribution (CC BY) license. Authors who
publish this series agree to the following terms:
1. Authors retain copyright and grant the series right of first publication with the work simultaneously licensed under a Creative Commons
Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this
series.
2. Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the series's published
version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial
publication in this series.
3. Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and
during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See
Open access policy for details).
References
[1]. Halberg, F. (1959) Physiologic 24-hour periodicity; general and procedural considerations with reference to the adrenal cycle. Internationale Zeitschrift fur Vitaminforschung. Beiheft 10, 225-296
[2]. Merikanto, I. et al. (2013) Associations of Chronotype and Sleep With Cardiovascular Diseases and Type 2 Diabetes. Chronobiol. Int. 30, 470-477. 10.3109/07420528.2012.741171
[3]. Stevens, R.G. et al. (2011) Considerations of circadian impact for defining 'shift work' in cancer studies: IARC Working Group Report. Occupational and Environmental Medicine, 68, 154-162. 10.1136/oem.2009.053512
[4]. Ong, J.C. et al. (2014) A randomized controlled trial of mindfulness meditation for chronic insomnia. Sleep, 37, 1553-1563. 10.5665/sleep.4010
[5]. Gritton, H.J. et al. (2013) Cognitive performance as a zeitgeber: cognitive oscillators and cholinergic modulation of the SCN entrain circadian rhythms. PLoS One, 8, e56206. 10.1371/journal.pone.0056206
[6]. Leventhal, H. et al. (2016) The Common-Sense Model of Self-Regulation (CSM): a dynamic ramework for understanding illness self-management. Journal of Behavioral Medicine, 39, 935-946. 10.1007/s10865-016-9782-2
[7]. Wright, K.P. et al. (2013) Entrainment of the Human Circadian Clock to the Natural Light-Dark Cycle. Current Biology, 23, 1554-1558. 10.1016/j.cub.2013.06.039
[8]. Rensing, L. and Ruoff, P. (2002) Temperature effect on entrainment, phase shifting, and amplitude of circadian clocks and its molecular bases. Chronobiol. Int. 19, 807-864. 10.1081/cbi-120014569
[9]. Hepsomali, P. et al. (2023) An Examination of the Associations between Nutritional Composition, Social Jet Lag and Temporal Sleep Variability in Young Adults. Nutrients, 15. 10.3390/nu15153425
[10]. Chang, C.S. et al. (2024) Associations between social loneliness trajectories and chronotype among adolescents. Eur Child Adolesc Psychiatry, 33, 179-191. 10.1007/s00787-023-02160-5
[11]. Weinert, D. and Gubin, D. (2022) The Impact of Physical Activity on the Circadian System: Benefits for Health, Performance and Wellbeing. Applied Sciences-Basel, 12. 10.3390/app12189220
[12]. Watkins, E.R. and Roberts, H. (2020) Reflecting on rumination: Consequences, causes, mechanisms and treatment of rumination. Behaviour Research and Therapy, 127. 10.1016/j.brat.2020.103573
[13]. Fabbri, M. (2023) Mindfulness, Subjective Cognitive Functioning, Sleep Timing and Time Expansion during COVID-19 Lockdown: A Longitudinal Study in Italy. Clocks & Sleep, 5, 313-332. 10.3390/clockssleep5020024
[14]. Kwok, J.Y.Y. et al. (2023) A randomized controlled trial on the effects and acceptability of individual mindfulness techniques - meditation and yoga - on anxiety and depression in people with Parkinson's disease: a study protocol. BMC Complement Med Ther, 23, 241. 10.1186/s12906-023-04049-x
[15]. Pace, T.W.W. et al. (2022) Feasibility, Acceptability, and Preliminary Efficacy of an App-Based Meditation Intervention to Decrease Firefighter Psychological Distress and Burnout: A One-Group Pilot Study. JMIR Form Res, 6, e34951. 10.2196/34951
[16]. Rider, M.S. et al. (1985) The effect of music, therapy, and relaxation on adrenal corticosteroids and the re-entrainment of circadian rhythms. J Music Ther, 22, 46-58. 10.1093/jmt/22.1.46
[17]. Gritton, H.J. et al. (2009) Interactions between cognition and circadian rhythms: attentional demands modify circadian entrainment. Behav Neurosci, 123, 937-948. 10.1037/a0017128
[18]. Gritton, H.J. et al. (2012) Bidirectional interactions between circadian entrainment and cognitive performance. Learn Mem, 19, 126-141. 10.1101/lm.023499.111
[19]. So, A.Y.L. et al. (2009) Glucocorticoid regulation of the circadian clock modulates glucose homeostasis. Proceedings of the National Academy of Sciences of the United States of America, 106, 17582-17587. 10.1073/pnas.0909733106
[20]. Morioka, N. et al. (2010) Noradrenaline induces clock gene Per1 mRNA expression in C6 glioma cells through beta(2)-adrenergic receptor coupled with protein kinase A - cAMP response element binding protein (PKA-CREB) and Src-tyrosine kinase - glycogen synthase kinase-3beta (Src-GSK-3beta). J Pharmacol Sci, 113, 234-245. 10.1254/jphs.10031fp
[21]. Imbesi, M. et al. (2009) The melatonin receptor MT1 is required for the differential regulatory actions of melatonin on neuronal 'clock' gene expression in striatal neurons in vitro. Journal of Pineal Research, 46, 87-94. 10.1111/j.1600-079X.2008.00634.x
[22]. Torres-Farfan, C. et al. (2006) Immunocytochemical demonstration of day/night changes of clock gene protein levels in the murine adrenal gland:: differences between melatonin-proficient (C3H) and melatonin-deficient (C57BL) mice. Journal of Pineal Research, 40, 64-70. 10.1111/j.1600-079X.2005.00279.x
[23]. Jindal, V. et al. (2013) Molecular Mechanisms of Meditation. Molecular Neurobiology, 48, 808-811. 10.1007/s12035-013-8468-9
[24]. Kang, D.H. et al. (2013) The effect of meditation on brain structure: cortical thickness mapping and diffusion tensor imaging. Soc Cogn Affect Neurosci, 8, 27-33. 10.1093/scan/nss056
[25]. Brefczynski-Lewis, J.A. et al. (2007) Neural correlates of attentional expertise in long-term meditation practitioners. Proc Natl Acad Sci U S A, 104, 11483-11488. 10.1073/pnas.0606552104
[26]. Baijal, S. and Srinivasan, N. (2010) Theta activity and meditative states: spectral changes during concentrative meditation. Cognitive Processing, 11, 31-38. 10.1007/s10339-009-0272-0
[27]. Devinsky, O. (1997) Neurological aspects of the conscious and unconscious mind. Annals of the New York Academy of Sciences, 835, 321-329. 10.1111/j.1749-6632.1997.tb48639.x
[28]. Esch, T. et al. (2002) The role of stress in neurodegenerative diseases and mental disorders. Neuro Endocrinol Lett, 23, 199-208
[29]. Jones, B.M. (2001) Changes in cytokine production in healthy subjects practicing Guolin Qigong : a pilot study. BMC Complement Altern Med, 1, 8. 10.1186/1472-6882-1-8
[30]. Tooley, G.A. et al. (2000) Acute increases in night-time plasma melatonin levels following a period of meditation. Biol Psychol, 53, 69-78. 10.1016/s0301-0511(00)00035-1
[31]. Jevning, R. et al. (1992) The physiology of meditation: a review. A wakeful hypometabolic integrated response. Neuroscience and biobehavioral reviews, 16, 415-424. 10.1016/s0149-7634(05)80210-6
[32]. Bevan, A.J.W. (1980) ENDOCRINE CHANGES IN TRANSCENDENTAL MEDITATION. Clinical and Experimental Pharmacology and Physiology, 7, 75-76
[33]. Dojo, K. et al. (2017) Carbachol Induces Phase-dependent Phase Shifts of Per1 Transcription Rhythms in Cultured Suprachiasmatic Nucleus Slices. J Biol Rhythms, 32, 101-108. 10.1177/0748730417691205
[34]. Sari, N.K. et al. (2017) The role of autosuggestion in geriatric patients' quality of life: a study on psycho-neuro-endocrine-immunology pathway. Social Neuroscience, 12, 551-559. 10.1080/17470919.2016.1196243
[35]. Roybal, K. et al. (2007) Mania-like behavior induced by disruption of CLOCK. Proceedings of the National Academy of Sciences of the United States of America, 104, 6406-6411. 10.1073/pnas.0609625104









