1. Introduction
Diabetes is one of the most common diseases in the world. People in various countries suffer from this disease, especially type 2 diabetes(T2DM). It affects nearly 10.5% of the global population, with a significant increase in the incidence among teenagers. The population with T2DM is projected to be 643 million by 2030 and 783 million by 2045 [1]. It is mainly caused by the body’s resistance to insulin and inefficient insulin secretion. Its symptoms include hunger, fatigue, frequent urination, and difficulty of healing injury. Current treatment for T2DM such as thiazolidinediones, sulfonylureas and meglitinides. But they have the same side effect, which is weight gain. Thiazolidinediones may increases the risk of cardiovascular disease, weight gain, water retention, and edema. Sulfonylureas have a greater risk of failure as monotherapy than metformin or rosiglitazone (a thiazolidinedione) [2]. Obesity is another global health issue, 650 millions of adults in the world are affected by it [3]. In Europe, the prevalence of obesity is five times higher than it was after World War III [4]. It also causes a series of other disease, such as high blood lipids, fatty liver, coronary heart disease. Most obese people also have diabetes. To treat these diseases, the cost and burden on medical services are increased. The medical cost for obesity treatment in China are expected to reach 418 billion RNB by 2030 [5]. To solve the problem of obesity and reduce the cost of social services, this article is going to introduce the one of drug used to treat obesity, which is glucagon-like-peptide-1(GLP-1), also called semaglutide. In 1986 Habener and Mojsov found that GLP-1 in the intestine is a cleavage product of glucagon precursors. Glucagon is secreted by pancreatic alpha cells when blood glucose is low and acts on the liver to stimulate glycog-enolysis, gluconeogenesis, and hepatic glucose output, thereby raising blood glucose and preventing hypoglycemic sequelae. Subsequent studies using radioimmunoassay showed that glucagon-like peptides in the gut are released in response to glucose, raising the possibility that the glucagon gene product acts as incretin [6]. At the same time, it also causes weight loss by reduce appetite and food intake. On January 16, 2024, National Medical Products Administration approved the application for the marketing in China of GLP-1 produced by Novo Nordisk [7]. Through the network, GLP-1 becomes a popular drug to treat obesity and T2DM currently. Sales of semaglutide injection Wegovy and Ozempic manufactured by Novo Nordisk, are expected to increase significantly in 2024 [8]. The estimated sales ranking is shown in Figure 1.
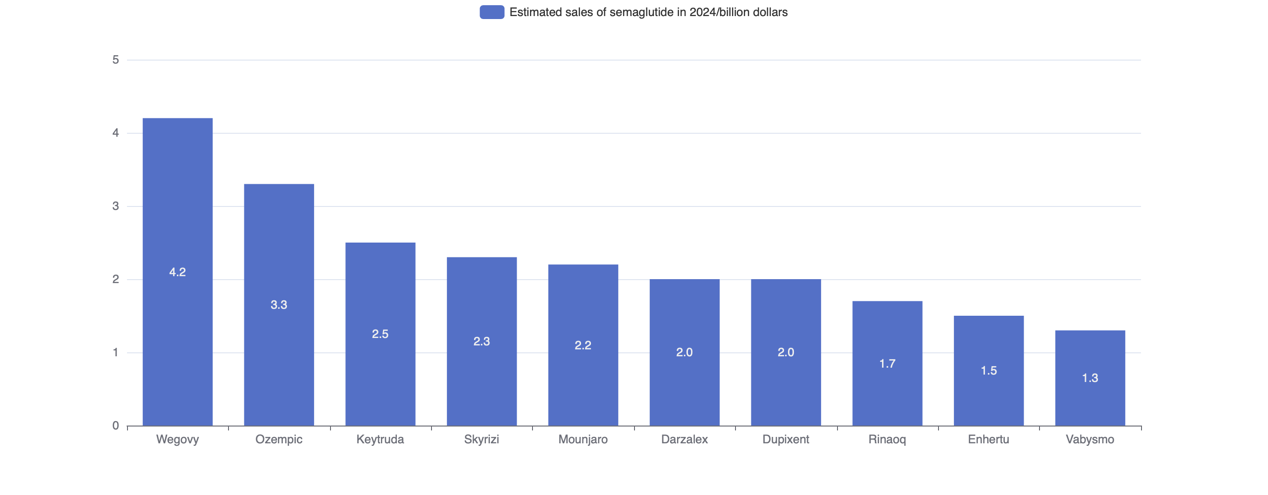
Figure 1. Estimated sales of semaglutide of different brands in 2024.
Besides, injection of GLP-1 can effectively reduce the glycosylated hemoglobin content in T2DM patients. It also has a dose-dependent benefit, which can reduce blood sugar without the increasing the incidence of hypoglycemia and shows a good clinical effect [9]. This article will discuss the clinical application of GLP-1 and its prospects.
2. Mechanism of action
GLP-1 has shown significant effect in the treatment of obesity. Its main mechanism is that it regulates neuronal activity related to appetite in the hypothalamus and posterior brain regions, stimulate POMC and CART (food inhibitory neuropeptides) and inhibit AgRP and NPY (food promoting neuropeptides), then achieve weight loss [9]. Another method is stimulating the secretion of insulin. Activation of GLP-1R leads to multiple signaling pathways that increase intracellular calcium, ultimately leading to pancreatic insulin secretion (Figure 2). By mimicking the endogenous effects of GLP-1, GLP-1 RAs activates GLP-1R, a G protein-coupled receptor (GPCR). Once GLP-1 RAs binds, the GTP-bound Gαs subunit of the GPCR complex activates adenylyl cyclase, which converts ATP to cyclic adenosine monophosphate (cAMP). This activates protein kinase A (PKA), which phosphorylates the sulfonylurea receptor-1 (SUR1) subunit of the K+/ atpase channel, leading to premature closure of this channel and amplification of physiological Ca2+ influx through voltage-gated calcium channels. Increased insulin secretion can also occur through the Gq/ phospholipase C (PLC) signaling pathway, in which inositol triphosphate 3 (IP3) binds to its receptor (IP3R) and diacylglycerol (DAG) binds to the ryanodine receptor to stimulate calcium-induced calcium release. In another pathway, exchange proteins directly activated by cAMP (Epac2) activate ras-proxime-1 (Rap1), thereby activating PLC and increasing downstream IP3/DAG levels, similarly inducing calcium release. Epac2 further promotes insulin exocytosis because Epac2/Rim2/Piccolo complex binds to the Rab3-insulin interface to excrete insulin particles. The calcium-permeable transient receptor potential melastatin 2 (TRPM2) channel is also involved in glp-1 related insulin secretion through cAMP and PKA signaling. Desensitization of GLP-1R is prevented by regular internalization of intracellular trafficking and recycling mediated by Gaq and β-arrestin pathways [10].
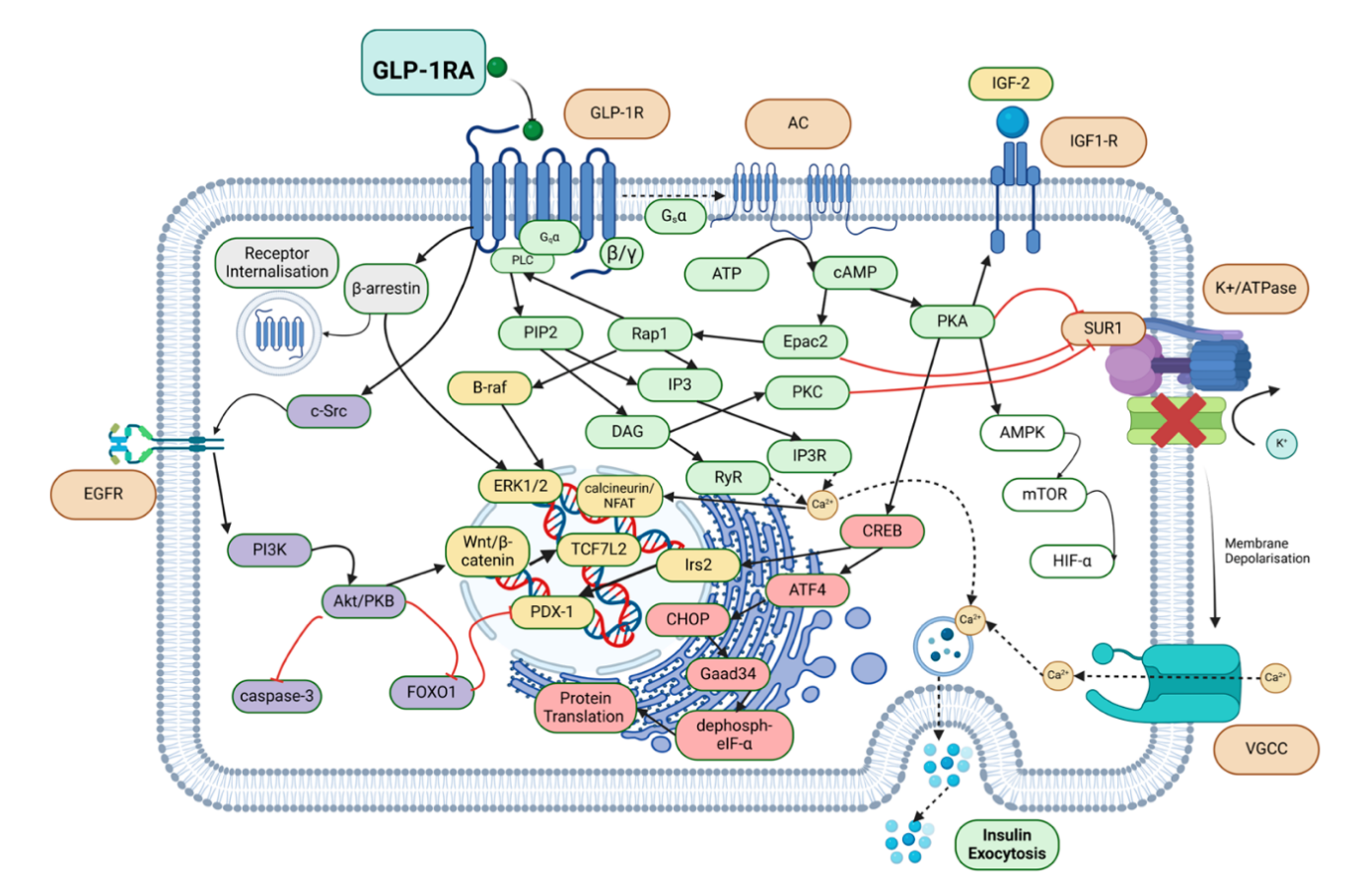
Figure 2. Intracellular signaling pathways induced by glp-1ra in combination with GLP-1R These results alleviate ER stress, inhibit apoptosis, increase insulin exocytosis and β-cell proliferation, and improve glucose metabolism and homeostasis [10]
In the treatment of T2DM, preliminary studies in rodent models have shown that GLP-1 is highly effective as an insulin-stimulating agent in non-diabetic animals but has significantly reduced bioactivity with a previously identified glucose-dependent insulin-stimulating polypeptide in diabetic animals. However, when GLP-1 was administered to patients with hyperglycemia, the insulin-promoting activity of both GLP-1 (7-36 amide) and GLP-1(7-36) was maintained, accompanied by a short-term reduction in plasma glucose within the normal fasting range [11].
3. Clinical application and function
3.1. Application
GLP-1 is applicable to three main mechanisms: glucose dependent insulin stimulating action, inhibit glucagon hypersecretion except during hypoglycemia episodes and gastric emptying slows down [11]. Semaglutide is a long acting GLP-1 analogue, it is primarily used to control type 2 diabetes and is also suitable for promoting weight loss in people with mild obesity. The treatment for obesity is a subcutaneous injection once a week. For both diabetic and non-diabetic patients, semaglutide can lead to weight loss. Since 2007, Novo Nordisk has conducted global multi-center clinical studies of semaglutide in its weekly SUSTAIN and oral PIONEER series, to evaluate the safety, glycemic control, cardiovascular and renal protection. The SUSTAIN series of trials showed that whether used semaglutide alone or in combination, it could reduce the HBA1C value significantly better than placebo, the oral hypoglycemic agents sitagliptin, canagliflozin, insulin glargine. And it performs better than other type of GLP-1RA drugs, such as dulaglutide and liraglutide. The PIONEER series shows the same results as SUSTAIN series. In 2021, the “Diabetes Diagnosis and Treatment Standards” issued by American Diabetes Association (ADA) proposed that to use GLP-1RA as injection hypoglycemic drug for patients with T2DM, instead of insulin. In 2022, the ADA guidelines raised the recommendation for hypoglycemic efficacy of GLP-1RA to “high”, putting it par with metformin, insulin thiazolidine and sulfonylureas. The STEP series is a clinical trial of the efficacy of semaglutide in weight loss of overweight and obese adults. The results of studies showed that in obese subjects with or without T2DM, once-weekly administration of semaglutide leads to weight loss, and the effect is better than placebo and other type of GLP-1RA drugs. Use weekly semaglutide for up to 68 weeks is associated with sustained weight loss. With exercise and diet control, the effect of weight loss can be up to 104 weeks. The OASIS series of semaglutide tablets has shown good safety and tolerance, and the common side effects are mild or moderate gastrointestinal discomfort [12].
3.2. Similar drugs
Liraglutide stimulates the GLP-1 receptor and is approved for glycemic control in children with type 2 diabetes (FDA approval 2019). The drug under the trade name Victoza was approved for this indication. In 2014, the FDA approved an alternative brand for liraglutide, “Saxenda”. It is used in the long-term management of weight in adults, together with a caloric-restricted diet and increased physical activity. Liraglutide is an acylated synthetic human GLP-1 receptor agonist with an amino acid sequence that shares 97% with natural human GLP-1. Its function is similar to that of endogenous GLP-1. Liraglutide binds to the GLP-1 receptor present on the cell surface and promotes its activation. Despite its similarity to native GLP-1, liraglutide has the unique property that it is not degraded by dipeptidyl peptidase 4 (DPP-4) and neutral endopeptidase (NEP). 52 Common adverse effects that have been reported in 5% or more of cases with liraglutide include vomiting, nausea, hypoglycemia, diarrhea, constipation, headache, dizziness, decreased appetite, dyspepsia, abdominal pain, fatigue, and lipase elevations. In addition, the liraglutide label contains warnings and precautions related to several potential adverse effects, such as thyroid C-cell neoplasms, acute pancreatitis, acute gallbladder disease, marked hypoglycemia with insulin secretion agonists such as sulfonylureas, increased heart rate, renal dysfunction, and suicidal thoughts and behavior [13].
4. Research status and future development trend
4.1. Status quo
Diabetes and obesity are important causes of Major Adverse Cardiovascular Events. Semaglutide can benefit the cardiovascular system by reducing inflammation, improving metabolism, and providing vascular protection and other mechanisms. In the field of metabolic associated Fatty liver disease, semaglutide has been clinically applied and has become a hot drug that is expected to solve this global medical problem. In the field of PD and AD, GLP-1 analogs have been shown to improve damaged nerves, and semaglutide has also been preliminarily explored in the treatment of neurodegenerative disease. Recent studies have shown that semaglutide has anticancer cell potential by improving NK cell function and increasing IFN-γand granzyme B. Studies on the long-term efficacy of semaglutide are relatively inadequate. Although short-term results have shown excellent performance in improving core hypoglycemic markers and body mass loss, whether this effect can be sustained in long-term use has not been fully verified. This will be the focus of future research, and long-term studies will provide insight into the lasting effects of semaglutide in the treatment of chronic disease and the potential long-term adverse effects.
Several GLP-1 agonists are currently in various stages of clinical trials: retatrutide, pemvidutide, cotadutide, danuglipron, and orforglipron. Although all these agents provide patients with the ability to achieve clinically meaningful weight loss, retatrutide shows promise. Retatrutide is an investigational GLP-1/GIP/ glucagon triple agonist currently undergoing phase 3 trials for the treatment of obesity. This peptide shows significant promise in medical weight optimization by combining the anorexigenic effects of GLP-1 and GIP and the enhanced energy expenditure of glucagon. At 48 weeks, participants receiving the highest dose of retatrutide had significantly reduced body weight by 24.2%. Orforglipron is a novel, non-peptide, small-molecule GLP-1 receptor agonist that has shown significant weight loss in phase 2 trials with a safety profile like other incretin-based therapies. Preliminary study results at 36 weeks showed a 14.7% reduction in total body weight at the highest dose, marking a promising future for incretin-based oral therapy in medical weight management. In addition, the non-peptide structure of this drug may provide a more cost-effective alternative to the more expensive peptide drugs currently on the market. [14]
4.2. Risks and limitation
The safety of semaglutide in clinical use mainly relates to intestinal reaction, gallbladder and biliary tract risk, pancreatitis risk, tumor risk, and the possible risk of delayed gastric emptie. Semaglutide may increase the risk of acute gallbladder disease and should be carefully considered when increasing dosage. The risk of pancreatitis is controversial, and a patient’s triglyceride levels, and epigastric pain symptoms need to be closely monitored. The current study lacks a detailed analysis of different subgroups. The patient’s physiological condition, age, gender and other factors may have an impact on the efficacy and safety of semaglutide, but current studies have not fully considered these factors. Future research will focus more on individualized treatment strategies to achieve precision treatment by in-depth analysis of differences response among different subgroups [15]. The most common adverse effects of GLP-1 receptor agonists at the beginning of treatment are gastrointestinal complaints, including nausea, vomiting, diarrhea, and abdominal discomfort. These may decrease over time and are related to the serum levels of the drug. Adverse effects such as hypoglycemia, injection site reactions, pancreatitis, neoplasms, and gallbladder disease were rare. Some studies have suggested that patients using GLP-1 receptor agonists may be at increased risk for developing thyroid C-cell neoplasms. Patients should be screened for contraindications such as pancreatitis, diabetic retinopathy, and medullary thyroid carcinoma before initiating treatment. Caution should also be exercised in users of inhibitors of the renin-angiotensin system because of the increasing risk of acute kidney injury [16].
5. Conclusion
As a representative drug of GLP-1 RA, semaglutide has shown broad prospects in the treatment of diabetes, obesity and related field. In the treatment of diabetes, compared with traditional hypoglycemic drugs, semaglutide not only performed well in the core hypoglycemic indicators, but also improved the medication compliance of patients with chronic diseases. It also shows advantages in pharmacoeconomic. In the treatment of obesity, semaglutide has established its important position in this class of drugs with fewer adverse effects and significant weight loss advantages. The potential application of semaglutide in neurodegenerative disease is an area of interest for future research. GLP-1 receptor is widely distributed in the nervous system, and its activation is related to the neuroprotective mechanism, makes GLP-1 RA become a promising therapy and new development direction.
Unanswered questions remain regarding the effect of semaglutide on outcomes, and ongoing trials will further clarify these issues. Subcutaneous semaglutide is being evaluated for diabetic nephropathy and retinopathy, and it will be determined whether oral it has a positive CV benefit [17].
References
[1]. Ruze, R., Liu, T., Zou, X., Song, J., Chen, Y., Xu, R., ... & Xu, Q. (2023). Obesity and type 2 diabetes mellitus: connections in epidemiology, pathogenesis, and treatments. Frontiers in endocrinology, 14, 1161521.
[2]. Susilawati, E., Levita, J., Susilawati, Y., & Sumiwi, S. A. (2023). Review of the case reports on metformin, sulfonylurea, and thiazolidinedione therapies in type 2 diabetes mellitus patients. Medical Sciences, 11(3), 50.
[3]. Chao AM, Tronieri JS, Amaro A, & Wadden TA. (2023). Semaglutide for the treatment of obesity. Trends in cardiovascular medicine, 159-66.
[4]. Wang J.Y., Wang Q.W., Yang X.Y., Yang W., Li D.R., Zhang H.C., & Zhang X.F.. (2023). GLP− 1 receptor agonists for the treatment of obesity: Role as a promising approach. Frontiers in endocrinology, 14.
[5]. Li J., Dai J., Chen G., Liu C., Jiang Z.. (2023). Rapid health technology assessment for semaglutide in adults with overweight and obesity. Clinical Medication Journal, 5.
[6]. Friedman, J. M. (2024). The discovery and development of GLP-1 based drugs that have revolutionized the treatment of obesity. Proceedings of the National Academy of Sciences, 121(39)
[7]. Lin Y.. (2024). The world's first oral semaglutide was approved in China, and the GLP-1 drug Market or Change Bureau. 21st Century Business Herald, 12.
[8]. Yang S. (2024). Global development of GLP-1 weight-loss drugs. Competitive Intelligence, 55-63.
[9]. Kong S., Wang Chang, & Wang Bingmei. (2024). Overview of the clinical application and mechanism of the weight-loss drug semaglutide. Biology Teaching, 2-5.
[10]. Le, Richard & Nguyen, Mau & Allahwala, Momina & Psaltis, James & Marathe, Chinmay & Marathe, Jessica & Psaltis, Peter. (2024). Cardiovascular Protective Properties of GLP-1 Receptor Agonists: More than Just Diabetic and Weight Loss Drugs. Journal of Clinical Medicine. 13. 4674.
[11]. Nauck M.A., Quast DR, Wefers J & Meier JJ. (2021). GLP-1 receptor agonists in the treatment of type 2 diabetes–state-of-the-art. Molecular metabolism, 46.
[12]. Hong Y.. (2024). GLP-1RA Progress in clinical research of semaglutide. Chinese and foreign medicine Research, 159-164.
[13]. Alorfi, Nasser & Alshehri, Fahad. (2023). Usage of Glucagon-Like Peptide-1 for Obesity in Children; Updated Review of Clinicaltrials.gov. Journal of Multidisciplinary Healthcare. 16. 2179-2187.
[14]. Heckmann, Nathanael & Palmer, Ryan & Mayfield, Cory & Gucev, Gligor & Lieberman, Jay & Hong, Kurt. (2024). Glucagon-Like Peptide Receptor-1 Agonists Used for Medically-Supervised Weight Loss in Patients With Hip and Knee Osteoarthritis: Critical Considerations for the Arthroplasty Surgeon. Arthroplasty Today. 27. 101327.
[15]. Li Z.g, Bai Q.. (2024). Research progress on clinical and safety of semaglutide. Drug Evaluation Studies, 657-664.
[16]. Jaroń, Aleksandra & Wiklińska, Agata & Jastrzębska, Katarzyna & Witkowska, Maria & Skotnicka, Joanna & Błaszczak, Karolina & Turek, Monika & Wojciechowska, Klara & Borkowski, Adrian & Sawicki, Mateusz. (2024). Use of GLP-1 Receptor Agonists in the Treatment of Obesity in Women with PCOS. Quality in Sport. 20. 53831.
[17]. Gallwitz B, Giorgino F.. (2021). Clinical perspectives on the use of subcutaneous and oral formulations of semaglutide. Frontiers in Endocrinology, 12.
Cite this article
Shu,Z. (2024). The clinical application and research progression of glucagon-like-peptide-1. Theoretical and Natural Science,65,113-118.
Data availability
The datasets used and/or analyzed during the current study will be available from the authors upon reasonable request.
Disclaimer/Publisher's Note
The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of EWA Publishing and/or the editor(s). EWA Publishing and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
About volume
Volume title: Proceedings of ICBioMed 2024 Workshop: Computational Proteomics in Drug Discovery and Development from Medicinal Plants
© 2024 by the author(s). Licensee EWA Publishing, Oxford, UK. This article is an open access article distributed under the terms and
conditions of the Creative Commons Attribution (CC BY) license. Authors who
publish this series agree to the following terms:
1. Authors retain copyright and grant the series right of first publication with the work simultaneously licensed under a Creative Commons
Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this
series.
2. Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the series's published
version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial
publication in this series.
3. Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and
during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See
Open access policy for details).
References
[1]. Ruze, R., Liu, T., Zou, X., Song, J., Chen, Y., Xu, R., ... & Xu, Q. (2023). Obesity and type 2 diabetes mellitus: connections in epidemiology, pathogenesis, and treatments. Frontiers in endocrinology, 14, 1161521.
[2]. Susilawati, E., Levita, J., Susilawati, Y., & Sumiwi, S. A. (2023). Review of the case reports on metformin, sulfonylurea, and thiazolidinedione therapies in type 2 diabetes mellitus patients. Medical Sciences, 11(3), 50.
[3]. Chao AM, Tronieri JS, Amaro A, & Wadden TA. (2023). Semaglutide for the treatment of obesity. Trends in cardiovascular medicine, 159-66.
[4]. Wang J.Y., Wang Q.W., Yang X.Y., Yang W., Li D.R., Zhang H.C., & Zhang X.F.. (2023). GLP− 1 receptor agonists for the treatment of obesity: Role as a promising approach. Frontiers in endocrinology, 14.
[5]. Li J., Dai J., Chen G., Liu C., Jiang Z.. (2023). Rapid health technology assessment for semaglutide in adults with overweight and obesity. Clinical Medication Journal, 5.
[6]. Friedman, J. M. (2024). The discovery and development of GLP-1 based drugs that have revolutionized the treatment of obesity. Proceedings of the National Academy of Sciences, 121(39)
[7]. Lin Y.. (2024). The world's first oral semaglutide was approved in China, and the GLP-1 drug Market or Change Bureau. 21st Century Business Herald, 12.
[8]. Yang S. (2024). Global development of GLP-1 weight-loss drugs. Competitive Intelligence, 55-63.
[9]. Kong S., Wang Chang, & Wang Bingmei. (2024). Overview of the clinical application and mechanism of the weight-loss drug semaglutide. Biology Teaching, 2-5.
[10]. Le, Richard & Nguyen, Mau & Allahwala, Momina & Psaltis, James & Marathe, Chinmay & Marathe, Jessica & Psaltis, Peter. (2024). Cardiovascular Protective Properties of GLP-1 Receptor Agonists: More than Just Diabetic and Weight Loss Drugs. Journal of Clinical Medicine. 13. 4674.
[11]. Nauck M.A., Quast DR, Wefers J & Meier JJ. (2021). GLP-1 receptor agonists in the treatment of type 2 diabetes–state-of-the-art. Molecular metabolism, 46.
[12]. Hong Y.. (2024). GLP-1RA Progress in clinical research of semaglutide. Chinese and foreign medicine Research, 159-164.
[13]. Alorfi, Nasser & Alshehri, Fahad. (2023). Usage of Glucagon-Like Peptide-1 for Obesity in Children; Updated Review of Clinicaltrials.gov. Journal of Multidisciplinary Healthcare. 16. 2179-2187.
[14]. Heckmann, Nathanael & Palmer, Ryan & Mayfield, Cory & Gucev, Gligor & Lieberman, Jay & Hong, Kurt. (2024). Glucagon-Like Peptide Receptor-1 Agonists Used for Medically-Supervised Weight Loss in Patients With Hip and Knee Osteoarthritis: Critical Considerations for the Arthroplasty Surgeon. Arthroplasty Today. 27. 101327.
[15]. Li Z.g, Bai Q.. (2024). Research progress on clinical and safety of semaglutide. Drug Evaluation Studies, 657-664.
[16]. Jaroń, Aleksandra & Wiklińska, Agata & Jastrzębska, Katarzyna & Witkowska, Maria & Skotnicka, Joanna & Błaszczak, Karolina & Turek, Monika & Wojciechowska, Klara & Borkowski, Adrian & Sawicki, Mateusz. (2024). Use of GLP-1 Receptor Agonists in the Treatment of Obesity in Women with PCOS. Quality in Sport. 20. 53831.
[17]. Gallwitz B, Giorgino F.. (2021). Clinical perspectives on the use of subcutaneous and oral formulations of semaglutide. Frontiers in Endocrinology, 12.









