1. Introduction
In recent years, this therapy achieves targeted killing of tumors by transforming the T-cells into cells with specific tumor antigen-recognition capabilities [1]. Since the first FDA approval of a CAR-T product for blood cancers’ treat in 2017, the therapy has been widely used for addressing a broad spectrum of refractory and relapsed hematological malignancies, including lymphoma and multiple myeloma. Nevertheless, the widespread use of CAR-T therapy still faces many challenges, such as treatment-related toxicity, long-term effects of treatment, and treatment cost. Existing studies have shown that it has demonstrated high complete remission rates in the treatment of hematological malignancies, but the durability of efficacy remains unclear. Particularly in patients with multiple myeloma, despite significant initial efficacy, subsequent recurrence rates are high, suggesting the need to further optimize the design and manufacturing process of CAR-T cells [2]. In addition, treatment-associated cytokine release syndrome (CRS) and neurotoxicity (ICANS) are significant factors limiting the application of this therapies, and how to effectively predict and manage these adverse effects is the focus of current research. This study will pay attention on the current status of CAR-T cell therapy in hematological malignancies, specifically including evaluation of clinical effectiveness and safety of acute lymphoblastic leukemia, lymphoma and multiple myeloma.Also, this study will explore the differences in specific antigen recognition and killing effects of different CAR-T cell products and how these differences affect the efficacy and incidence of side effects.Through a systematic review of relevant literature, the study in order to to summarize the current advantages and shortcomings of this therapy, and to provide guidance for future clinical applications and research.The significance of this study is that it will not only help to deepen the comprehension of how it works and clinical application of it in hematological malignancies, but will also provide theoretical support for optimizing the existing therapies, reducing the side-effects, and improving the therapeutic efficacy. Meanwhile, this study will provide a key recommendation for future direction of new CAR-T cell products and therapeutic strategies, thus promoting the further development of this field [3].
2. Overview and Mechanism of CAR-T Cell Therapy
2.1. Overview of CAR-T-cell therapy
This treatment represents a groundbreaking method for cancer immunotherapy, mainly utilized for hematological cancers. It functions use genetically modifying the patient’s T-cells, enabling them to generate chimeric antigen receptors (CARs) that can identify and eliminate targeted tumor cells. Developers of this therapy initially faced several challenges, for instance, the inhibition of therapeutic effects by the tumour microenvironment. But now CAR-T therapy has gradually overcome these challenges and has demonstrated significant efficacy [4].
2.2. Mechanism of action
The core of this therapy is genetic modification. The process begins by collecting patients' T cells and introducing the gene encoding the chimeric antigen receptor (CAR) into the T cell genome via a viral vector or non-viral vector. Chimeric antigen receptors usually consist of three functional domains: an extracellular antigen recognition region (usually a single-chain antibody fragment), a transmembrane region, and an intracellular signal transduction region. The structure of CAR-T cells and their mechanism of action are illustrated in Figure 1. CAR recognizes specific antigens on the surface of cancer cells, such as the CD19 antigen (commonly found in B-cell hematological tumours), and activates the killing function of T-cells through intracellular signal transduction regions. Activated T cells release cytokines that initiate a cytotoxic response, thereby destroying the target tumor cells [5].In addition, CAR-T cells can proliferate in vivo to form "active drugs" that continue to seek out and kill remaining tumor cells for a certain period of time.This property gives therapy a significant long-term tumor-inhibiting potential. Mechanism of action of CAR-T cells is shown as Figure.1.
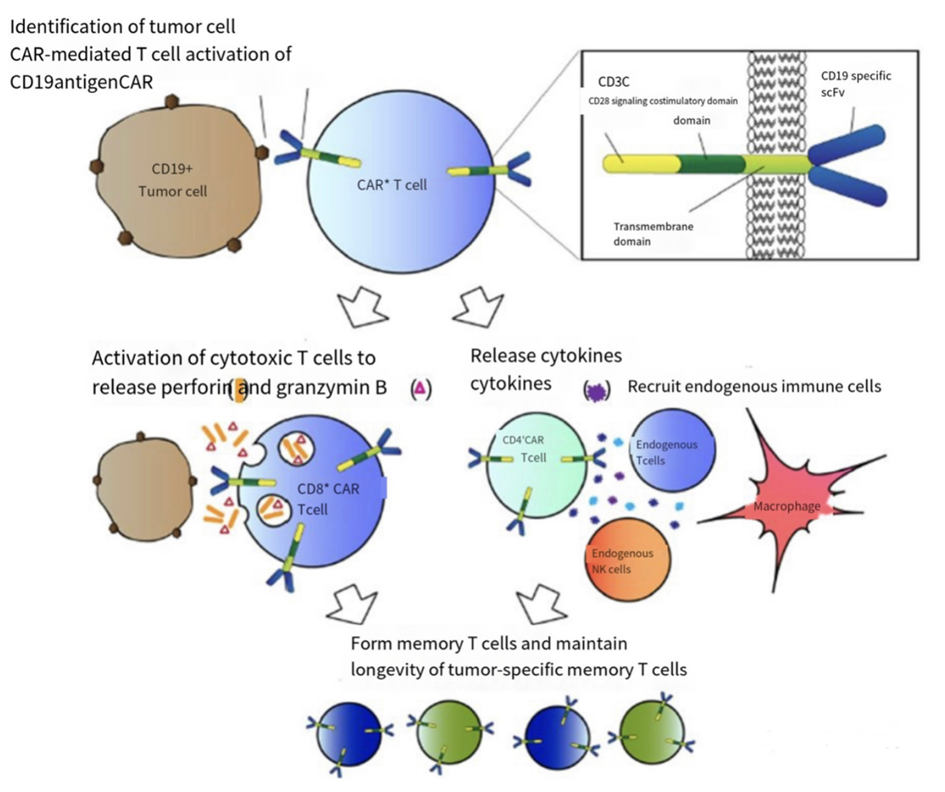
Figure 1. Mechanism of action of CAR-T cells[6]
3. Clinical Application of CAR-T Cell Therapy in Hematologic Malignancies
3.1. Leukemia
For managing Acute Lymphoblastic Leukemia, ALL in children, this therapy has achieved significant success. ALL is for which conventional treatment options are often ineffective in relapsed or refractory cases. However, CAR-T therapies offer new treatment options for these patients. Kymriah (tisagenlecleucel) was the first CAR-T cell therapy granted FDA approval, specifically for treating patients with ALL. In clinical trials, tisagenlecleucel has demonstrated impressive remission rates. According to Maude et al, 52 out of 68 patients with relapsed or refractory B-cell ALL achieved complete remission after treatment with tisagenlecleucel, and the majority of patients maintained disease-free survival for the following 12 months [7]. Despite the remarkable efficacy of this therapy in leukemia patients, there are still some challenges in the treatment process, especially the occurrence of cytokine release syndrome (CRS), a serious side effect that usually occurs in the early stages of treatment and is characterised by an overactive immune response, which may lead to multi-organ failure. In most cases, the use of tolizumab, an IL-6 receptor antagonist, is effective in alleviating the symptoms of CRS [8].
3.2. Lymphoma
It has also demonstrated significant efficacy in the treatment of B-cell lymphoma, particularly in the treatment of large B-cell lymphoma (DLBCL). Yescarta is the first FDA-approved CAR-T cell therapy for treating this kind of disease. In a ZUMA-1 trial, 82% of 101 patients with relapsed to Yescarta, with 54% achieving complete remission [9]. Extended monitoring data show that some patients remain in complete remission more than two years after treatment. Although this therapy has shown notable results in lymphoma patients, patients may experience relapse after treatment. This may be related to the lack of persistence of CAR-T cells in the body. In order to solve issue, experimenters are exploring ways to enhance the persistence of CAR-T cells, such as extending T-cell lifespan through gene editing or developing bispecific CAR-T cells [10].
3.3. Multiple myeloma
These therapies have demonstrated increasing medical achievement have attracted widespread attention. Multiple myeloma is a refractory hematological malignancy in which patients often show resistance to conventional therapies and have a poor prognosis. Abecma (Idecabtagene Vicleucel) is the first FDA-approved BCMA-targeted CAR-T cell therapy for treating this case. In a pivotal testing, 128 patients with multiple myeloma who had failed multiple treatment regimens were treated with Abecma, of which 73% produced an objective response and 33% achieved complete remission [11]. Despite the promising this therapy in multiple myeloma, the persistence of therapeutic effects remains a challenge. Researchers are trying to overcome the problems of target loss, immune escape, and the inability of cells to remain in patients for extended durations.
4. Current Status of Research and Future Development Trend
4.1. Progress and challenges in CAR-T Cell Therapy
In recent years, great progress has been made in this therapy, but as the clinical application of the therapy has been gradually promoted, scientists are aware of its limitations and potential problems, especially its limited application in the treatment of solid tumours. In addition, serious pains remain key barriers to further dissemination of the therapy.
4.2. Innovations and future directions
In view of this, researchers are exploring a variety of new CAR design strategies to augment their safety, performance, and practical application. Current research is focused on optimizing the design of CAR-T cell therapies, improving their efficacy in solid tumours, developing 'existing' allogeneic CAR-T cells, and combining CAR-T therapies with other therapies. With these strategies, researchers hope to overcome the limitations of existing these therapies and expand their application to more cancer types. Firstly, the structural design of this therapy is continuously being optimized to overcome problems such as immune escape and tumour antigen loss. Current research has seen the emergence of bispecific CAR-T cells, which are capable of recognizing two different tumour antigens at the same time, thereby improving their target specificity and reducing the risk of recurrence associated with antigen loss.In addition, researchers have introduced a "suicide switch" into the CAR structure, which allows CAR-T cells to be rapidly inactivated by an external control mechanism in the event of complication, thus enhancing security of the therapy [12]. Secondly, this will also face many more difficult challenges, such as hands-on practice in solid masses. Unlike hematological malignancies, solid masses have a more complex microenvironment that contains a large number of inhibitory cytokines and immunosuppressive cells, which greatly impede the penetration and activation of CAR-T cells.In addition, solid tumours typically exhibit antigenic heterogeneity and low antigen density, which further diminish the efficacy of cells.To address these challenges, experimenters are developing CAR-T cells that can resist the inhibitory tumour microenvironment, such as gene editing to desensitise CAR-T cells to inhibitory signals, or designing CAR structures that can reverse inhibitory signals [13].Another research direction that has attracted much attention is the development of allogeneic CAR-T cells.Most current therapies rely on the patient's own T cells, which results in a cumbersome, costly and lengthy treatment process. To address these issues, researchers are exploring the use of T cells from healthy donors to produce "off-the-shelf" CAR-T cells by removing molecular markers that may trigger graft-versus-host disease (GVHD) through gene editing. This method is expected to significantly lower the cost of treatment, increase accessibility of this cell therapy.
4.3. Combinations therapies and expanded applications
The development of combination therapies is an important future direction for this therapy. Many scientists have experimented with it and found that when used alongside other therapies such as PD-1 inhibitors and radiotherapy, may further improve treatment efficacy and reduce tumour resistance to single therapies. For example, PD-1 inhibitors can lift the immunosuppression within the mass's environment, thus enhancing the killing activity of CAR-T cells, whereas chemotherapy or radiotherapy can create better conditions for them to do their job by reducing the tumour volume or destroying the immune escape mechanism of tumour cells [14]. Overall, CAR-T cell therapy research is promising, but also challenging. Due to the continuous improvement of biotechnology and gene editing technology, CAR-T therapies will be more diverse and personalized in future clinical applications. In the future, researchers will continue to explore ways to increase the efficacy of the it, reduce patients' paints and expand its use in more cancer types.
5. Conclusion
The emergence of CAR-T cell therapy marks a major progress in the immunotherapy of hematological malignancies, and its remarkable efficacy has been proven in acute lymphoblastic leukemia, large B-cell lymphoma and multiple myeloma. By genetically modifying T-cells so that they can kill tumor cells, it has brought new light to many individuals with recurrent hematological tumours that have failed to respond to conventional treatment. However, the side effects have become obstacles to its further dissemination. Research in recent years has focus on optimizing CAR design to improve safety and efficacy, in particular by developing bispecific CAR-T cells, introducing suicide switches, and other strategies to counter antigen loss and immune escape. In addition, the application of it in solid tumours faces problems such as microenvironmental inhibition and antigenic heterogeneity, for which researchers are exploring means such as gene editing and signal modulation to enhance its role in complex tumour environments. And improvements in allogeneic CAR-T cells represent progress to increase accessibility while reducing treatment costs. The exploration of combination therapies has also demonstrated the potential for CAR-T therapy to synergise with other immune checkpoint inhibitors, radiotherapy or chemotherapy. In conclusion, although this therapy has made remarkable achievements in hematological malignancies, its future development will depend on further technological innovation and clinical research. Along with increasing advances in biological and gene editing technology, CAR-T therapies are look forwards to achieve more diverse and personalized clinical applications in the coming years, bringing hope for long-term treatment to patients with more cancer types.
References
[1]. Fesnak, A. D., June, C. H., & Levine, B. L.. (2016). Engineered T cells: the promise and challenges of cancer immunotherapy. Nature Reviews Cancer, 16(9), 566-581.
[2]. Neelapu, S. S.. (2017). Axicabtagene ciloleucel CAR T-cell therapy in refractory large B-cell lymphoma. New England Journal of Medicine, 377(26), 2531-2544.
[3]. Hartmann, J.. (2017). Clinical development of CAR T cells—challenges and opportunities in translating innovative treatment concepts. EMBO Molecular Medicine, 9(9), 1183-1197.
[4]. Jackson, H. J., Rafiq, S., & Brentjens, R. J.. (2016). Driving CAR T-cells forward. Nature Reviews Clinical Oncology, 13(6), 370-383.
[5]. June, C. H.. (2018). CAR T cell immunotherapy for human cancer. Science, 359(6382), 1361-1365.
[6]. Maude, S. L.. (2018). Tisagenlecleucel in children and young adults with B-cell lymphoblastic leukemia. New England Journal of Medicine, 378(5), 439-448.
[7]. Neelapu, S. S.. (2017). Axicabtagene ciloleucel CAR T-cell therapy in refractory large B-cell lymphoma. New England Journal of Medicine, 377(26), 2531-2544.
[8]. Wang, M.. (2020). Kymriah (Tisagenlecleucel) CAR T-Cell Therapy for Relapsed/Refractory Diffuse Large B-Cell Lymphoma. Blood Advances, 4(1), 16-26.
[9]. Cohen, A. D.. (2019). BCMA-Targeted CAR T Cells for Multiple Myeloma: Emerging Clinical Data and Future Directions. Blood. Cancer Letters, 134(2), 129-142.
[10]. Munshi, N. C.. (2021). Idecabtagene vicleucel in relapsed and refractory multiple myeloma. New England Journal of Medicine, 384(8), 705-716.
[11]. Garfall, A. L.. (2020). Anti-BCMA CAR T Cells in Multiple Myeloma: Current Data and Future Directions. Leukemia, 34(5), 1351-1362.
[12]. Srivastava, S., & Riddell, S. R. (2015). Engineering CAR-T cells: Design concepts. Trends in Immunology, 36(8), 494-502.
[13]. Rafiq, S., Hackett, C. S., & Brentjens, R. J. (2020). Engineering strategies to overcome the current roadblocks in CAR T cell therapy. Nature Reviews Clinical Oncology, 17(3), 147-167.
[14]. Depil, S.. (2020). ‘Off-the-shelf’ allogeneic CAR T cells: Development and challenges. Nature Reviews Drug Discovery, 19(3), 185-199.
Cite this article
Gao,Y. (2024). Current status and progress of research on CAR-T Cell Therapy for the treatment of hematological malignancies. Theoretical and Natural Science,71,26-31.
Data availability
The datasets used and/or analyzed during the current study will be available from the authors upon reasonable request.
Disclaimer/Publisher's Note
The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of EWA Publishing and/or the editor(s). EWA Publishing and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
About volume
Volume title: Proceedings of ICBioMed 2024 Workshop: Computational Proteomics in Drug Discovery and Development from Medicinal Plants
© 2024 by the author(s). Licensee EWA Publishing, Oxford, UK. This article is an open access article distributed under the terms and
conditions of the Creative Commons Attribution (CC BY) license. Authors who
publish this series agree to the following terms:
1. Authors retain copyright and grant the series right of first publication with the work simultaneously licensed under a Creative Commons
Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this
series.
2. Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the series's published
version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial
publication in this series.
3. Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and
during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See
Open access policy for details).
References
[1]. Fesnak, A. D., June, C. H., & Levine, B. L.. (2016). Engineered T cells: the promise and challenges of cancer immunotherapy. Nature Reviews Cancer, 16(9), 566-581.
[2]. Neelapu, S. S.. (2017). Axicabtagene ciloleucel CAR T-cell therapy in refractory large B-cell lymphoma. New England Journal of Medicine, 377(26), 2531-2544.
[3]. Hartmann, J.. (2017). Clinical development of CAR T cells—challenges and opportunities in translating innovative treatment concepts. EMBO Molecular Medicine, 9(9), 1183-1197.
[4]. Jackson, H. J., Rafiq, S., & Brentjens, R. J.. (2016). Driving CAR T-cells forward. Nature Reviews Clinical Oncology, 13(6), 370-383.
[5]. June, C. H.. (2018). CAR T cell immunotherapy for human cancer. Science, 359(6382), 1361-1365.
[6]. Maude, S. L.. (2018). Tisagenlecleucel in children and young adults with B-cell lymphoblastic leukemia. New England Journal of Medicine, 378(5), 439-448.
[7]. Neelapu, S. S.. (2017). Axicabtagene ciloleucel CAR T-cell therapy in refractory large B-cell lymphoma. New England Journal of Medicine, 377(26), 2531-2544.
[8]. Wang, M.. (2020). Kymriah (Tisagenlecleucel) CAR T-Cell Therapy for Relapsed/Refractory Diffuse Large B-Cell Lymphoma. Blood Advances, 4(1), 16-26.
[9]. Cohen, A. D.. (2019). BCMA-Targeted CAR T Cells for Multiple Myeloma: Emerging Clinical Data and Future Directions. Blood. Cancer Letters, 134(2), 129-142.
[10]. Munshi, N. C.. (2021). Idecabtagene vicleucel in relapsed and refractory multiple myeloma. New England Journal of Medicine, 384(8), 705-716.
[11]. Garfall, A. L.. (2020). Anti-BCMA CAR T Cells in Multiple Myeloma: Current Data and Future Directions. Leukemia, 34(5), 1351-1362.
[12]. Srivastava, S., & Riddell, S. R. (2015). Engineering CAR-T cells: Design concepts. Trends in Immunology, 36(8), 494-502.
[13]. Rafiq, S., Hackett, C. S., & Brentjens, R. J. (2020). Engineering strategies to overcome the current roadblocks in CAR T cell therapy. Nature Reviews Clinical Oncology, 17(3), 147-167.
[14]. Depil, S.. (2020). ‘Off-the-shelf’ allogeneic CAR T cells: Development and challenges. Nature Reviews Drug Discovery, 19(3), 185-199.









