1. Introduction
Chemical small molecule drug refers to chemosynthetic drugs, whose molecular weight is often less than 1000. These drugs have neat spatial dispersion, and their chemical properties lead to good pharmaceutical properties and pharmacokinetic properties. Therefore, recent years, the idea of utilizing small molecules to deal with tumor, including targeting proteins that promote immunosuppressive signals within the tumor microenvironment, has become a hot topic [1]. Based on the scientific research, it is known that small molecule drugs do have brought hope to the treatment of various diseases, such as renal cancer, bladder cancer, leukemia, etc. However, there are still challenges in promoting the use of these drugs: Firstly, the side effects of drugs will enhance under different circumstances, contributing to dissatisfying treatment effect. Second, especially protease inhibitors, some may get drug-resistant mutations, thereby reducing the treatment effect. Gefitinib, as an instance, is a member of epidermal growth factor receptor-tyrosine kinase inhibitor (EGFR-TKI). It was produced by AstraZeneca initially and approved by Food and Drug Administration (FDA) in 2003 to be used in the therapy of non-small cell lung cancers patients (NSCLC).
It was demonstrated that Gefitinib had performed well in patients with epidermal growth factor receptor (EGFR) mutant non-small cell lung cancers patients (NSCLC) in clinical application. However, it has side effects include but not limited to diarrhea, cutireaction, more serious ones such as gastrointestinal perforation, interstitial lung disease, etc. To resolve this problem, companies have developed EGFR-TKI(II), such as afatinib, or combining Gefitinib with other drugs in the therapeutic. The development of Gefitinib not only reflects the progress of medical science but also plays an important role in promoting subsequent drug development. Hence, this research is expected to bring a view on the past progress in context of small molecule drugs, and the need for us to concern so that it could step into new breakthroughs. This research will take three examples regarding lung cancer, liver cancer and leukemia, presented by summarizing papers in the fields related. First, the synthesis methods (mechanism, process, efficacy) of drugs would be discussed, followed by the introduction to treatment effects and clinical statics of the drugs. Ultimately, pros and cons of the drugs would be discussed, followed by their development prospects.
2. Synthesis
2.1. Gefitinib in non-small cell lung cancers (NSCLC)
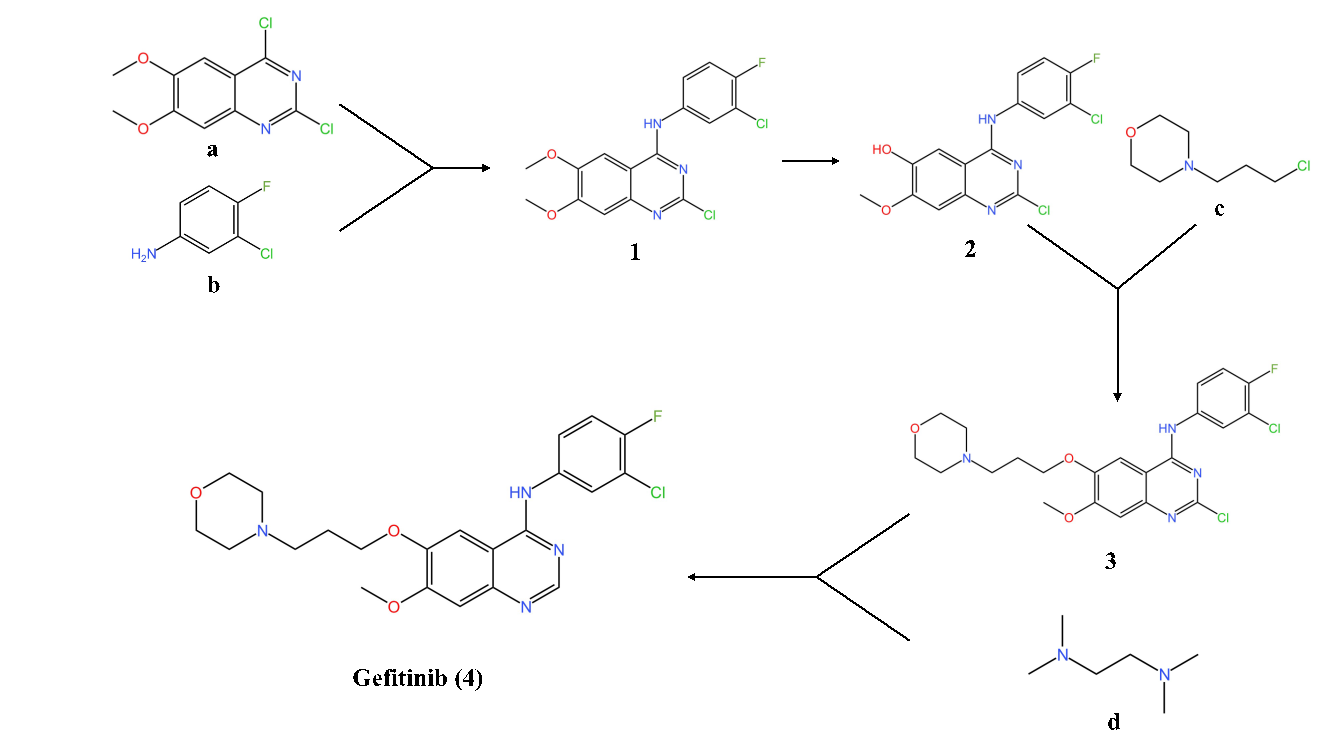
Figure 1. The synthesis of Gefitinib
Roughly 85 to 90% of all cases of lung cancer are non-small cell lung cancer (NSCLC). Based on the knowledge about the synthesis of Gefitinib, with the intention of avoiding using toxic reagents such as N, N-Dimethylformamide (DMF), SOCl2, POCl3 and trimethylsilyl iodide (TMSI) [2], the pathway as to its synthesis has been improved many times.
Here displays the newsiest and latest one to share, which includes four steps in total. It is firstly synthesized from reactant (a, Figure 1), then mixed with reactant (b, Figure 1), acetic acid (20.4 equiv) to produce intermediate (1, Figure 1) under 55 °C for two hours [2]. Then product (1, Figure 1) is blended with Al2Cl7, tetramethylammonium hydroxide mixed ionic liquid, dichloromethane as reagent under 50 °C for two hours, thus removing a methyl group to generate product (2, Figure 1) [2]. In step three, material c, cesium carbonate, dimethyl sulfoxide (DMSO) as reagent is added with product (2, Figure 1) under 40 °C to undergo a condensation reaction, obtaining product (3, Figure 1) [2]. Reaction time as to 24 hours is the longest in the last step [2]. Mix product (3, Figure 1) with zinc, material (d, Figure 1) and reagents (acetic acid, formic acid) under 40 °C, then producing Gefitinib (4, Figure 1) [2]. As for the yield, it is proved to be able to crystallize the product from hot formic acid (MeOH) to provide Gefitinib (4, Figure 1) as colorless crystals in 82% yield [2], which is pretty efficient.
2.2. Sorafenib in liver cancer
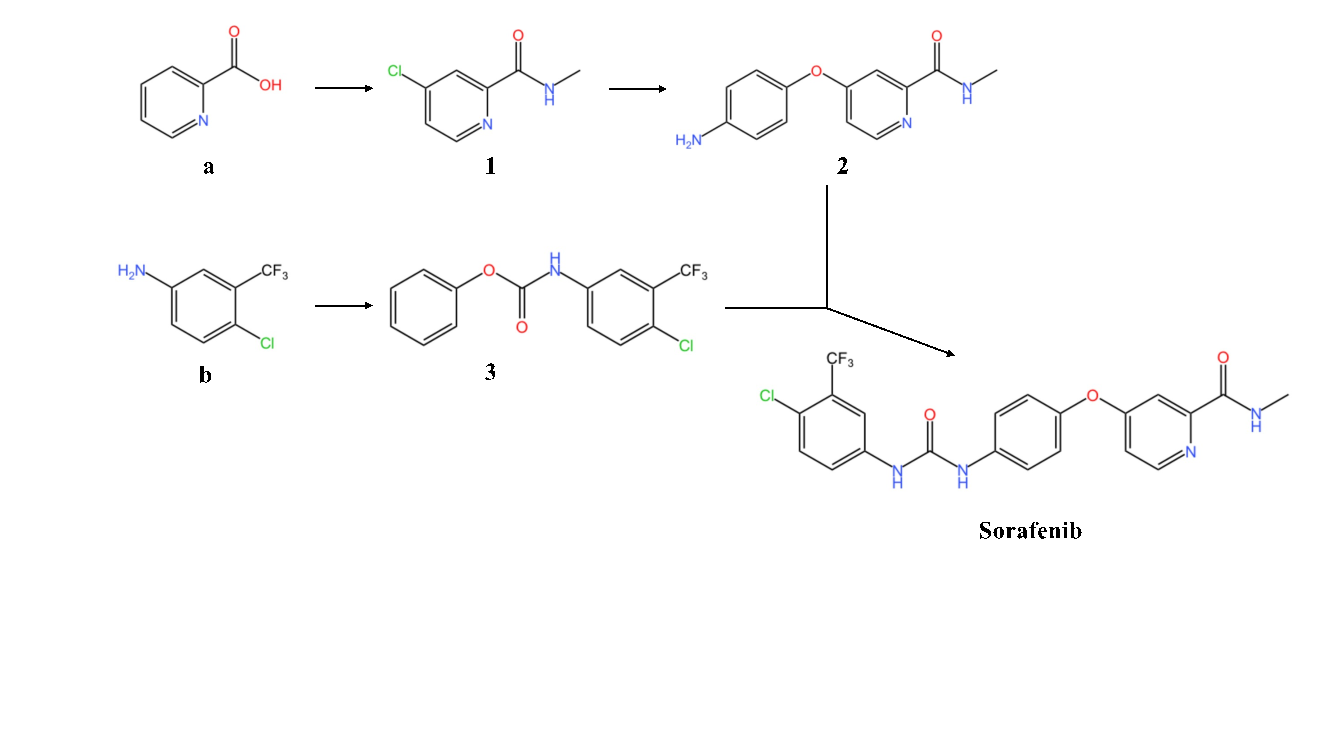
Figure 2. The synthesis of Sorafenib
Sorafenib is a type of kinase inhibitors that have been shown to inhibit tumor cell proliferation and anti-angiogenesis. The common synthesis of Sorafenib has disadvantages such as: use of toxic chemical materials like haloformates and isocyanates, which are commonly used in making phosgene gas; complicated steps for reactions [3]. Therefore, here exhibits another safer and more efficient way as to obtaining the product wanted.
Firstly, it could be seen that Sorafenib has few functional groups such as amide groups, an ester group, which means it could be synthesized through condensation reaction. Hence, the target product could mainly be divided into two parts.
The first step involves generating product (1, Figure 2), getting prepared for the first part. 2-Pixolinic acid (a, Figure 2) is mixed with SOCl2(3.5 equiv), tetrahydrofuran (THF) and DMF (0.1mL) under 70°C for 16 hours, producing 4-chloro-N-methylpicolinamide [3]. Then this intermediate reacts with 40% aqeuous methylamine at 0-3°C for four hours, with the yield about 95% in getting product (1, Figure 2) [3]. Thereafter, p-aminophenol is added to react with product (1, Figure 2) to obtain product (2, Figure 2), with conditions containing DMF (dry), potassium tert-butoxide (KOtBu) and potassium carbonate under 80°C for eight hours [3]. However, this reaction is not satisfying owing to its low yield merely about 25% [3]. As for the second part, material (b, Figure 2), which is reacted with diphenyl carbonate (DPC), is used to generate product (3, Figure 2) [3].
In the final step, reactant (2, Figure 2) and reactant (3, Figure 2) are put in 0.2 mmol triethylenediamine (DABCO) and acetonitrile under 65°C [3]. Then we attain Sorafenib, with the yield about 54% [3].
2.3. Imatinib (Gleevec) in chronic myeloid leukemia (CML)
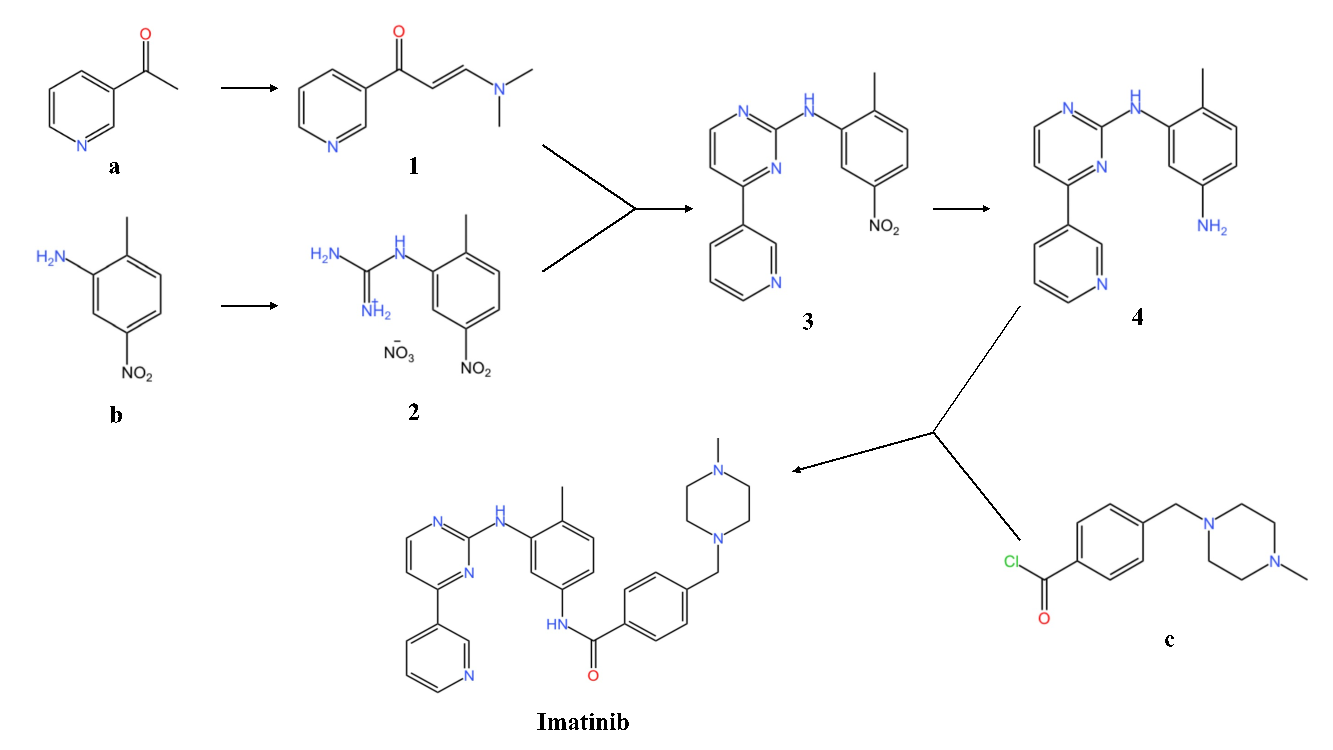
Figure 3. The synthesis of Imatinib
Imatinib is a synthetic tyrosine kinase inhibitor, which is used for the treatment of CML and gastrointestinal stromal tumors [4]. The original synthesis of imatinib was published by Zimmermann in a patent in1993. Here Zimmermann’s pathway would be displayed. Firstly, material (a, Figure 3) is mixed with ethyl formate in the reaction system. Thereafter, it is first dissolved in NaOMe and toluene under 25°C for 16 hours. Then put the product in HNMe2, acetic acid and toluene (reflux) for one hour, generating intermediate (1, Figure 3). Secondly, 2-methyl-5-nitroaniline (b, Figure 3) reacts with NH2CN, nitric acid and alcohol (reflux) for 21 hours, producing intermediate (2, Figure 3). Intermediate (1, Figure 3) and intermediate (2, Figure 3) are subsequently mixed with sodium hydroxide, isopropanol (reflux) for 12 hours, generating intermediate (3, Figure 3). Intermediate (4, Figure 3) is produced after that reactant (3, Figure 3) undergoes hydrogenation (Pd/C, H2, THF, room temperature) for 21 hours. Ultimately, reactant (c, Figure 3) reacts with intermediate (4, Figure 3) to generate Imatinib in a basic environment (add pyridine) at room temperature for 24 hours.
3. Pathology & efficacy & side effects
3.1. Gefitinib in non-small cell lung cancers (NSCLC)
3.1.1. Pathology of NSCLC and mechanism of Gefitinib
Research has shown that the signaling pathway of the epidermal growth factor receptor (EGFR), which is a cell-surface receptor, is activated in more than 50% patients with NSCLC [5]. Four members of the ERBB (or HER) family (namely EGFR, HER2, HER3, HER4) all have tyrosine kinase activity, except HER3 [5]. These proteins can secrete growth factors, such as the epidermal growth factor (EGF) and other EGF-like growth factors, which contributes to the phosphorylation of tyrosine residues in the kinase domain [5].
Gefitinib, designed as highly specific small molecule tyrosine kinase inhibitors, can competitively barrier the binding of adenosine triphosphate to its binding point in the tyrosine kinase domain of EGFR. Therefore, it could help prevent the autophosphorylation.
3.1.2. Clinical statics & efficacy
The Iressa Survival Evaluation in Lung Cancer (ISEL) trial included patients who aged 18 years old or older with histologically or cytologically proven, metastaic NSCLC that was not curable with surgery or radiotherapy and so forth [6]. Thereafter, patients were randomly given Gefitinib (250 mg/day) or placebo tablets in a ratio of two to one. The result turned out that gefitinib was superior in terms of the time to treatment failure to placebo (3.0 to 2.6 months, p=0.0006). However, the difference was not significant in context of median survival between Gefitinib and placebo (5.6 to 5.1 months, respectively) [6], which indicated that Gefitinib was dissatisfying in overall survival [6].
3.1.3. Side effects
According to the research, it was found that almost half of the volunteers jad rash. Among the patients, the onset of rash occurs seven to fourteen days after the initiation of therapy [8]. Frequently, the rash usually appears on face, scalp, chest, and back, and it is often follicular and papulopustular [6]. Diarrhea is another common adver event among the population using Gefitinib, which is around 35% [7]. If serious diarrhea appears, it is suggested that therapy oughts to be stopped for up to 14 days until the symptoms have relieved [6]. Besides rash and diarrhea, asthenic disorders (25%), nausea (20.3%), anorexia (21.8%), vomiting (15.0%) and so forth are other common symptoms of the adverse events [7].
3.2. Sorafenib in liver cancer
3.2.1. Pathology of liver cancer and mechanism of Sorafenib
Hepatocellular carcinoma (HCC) is the most common primary maligancy of the liver, accounting for about 75-85% of cases of liver cancer [9]. The etiology and exect molecular of primary liver cancer are still not completely clear till now. It is believed that the pathogenesis of primary liver cancer is a complex process of multi-factors and multi-steps, and it is influenced by the environment and diet. Genetics, epigenetic changes, hepatitis B virus, hepatitis C virus, aflatoxin exposure, smoking, obesity and diabetes are the critical causes for the cancer.
Sorafenib, the most commonly utilized chemotherapeutic agent to treat HCC recently, is an oral multikinase inhibitor which helps prohibit tumor cell proliferation and angiogenesis, promoting tumor cell apoptosis as well [9]. Furthermore, Sorafenib is proved to be able to decline tumor cell proliferation and tumor agiogenesis through the inhibition of serine-threonine kinases Raf-1 and B-Raf and receptor tyrosine kinase activity of vascular endothelial growth factor receptor (VEGFR) 1,2,3 and platelet-derived growth factor receptor β (PDGFR-β) [10].
3.2.2. Clinical statics & efficacy
The experiment involved patients with a certain diagnosis of unresectable HCC and without prior radical treatment [10]. All the volunteers underwent physical examination, laboratory evaluation and collected whole blood samples [10]. Sorafenib was continuously administered orally at a dose of 400 mg twice every day [10]. After 2 month, it was found that the percentages of total CD56dimCD16+ NK cells increased (mean, 91.187.00% versus 93.815.36%, p=0.009), while the percentage of CD56brightCD16-NK cells decreased (mean, 2.942.92% versus 1.941.99%, p=0.004), resulting in a significantly declined ratio of CD56bright and CD56dim NK cells (ratiobri/dim) (p=0.004)[10]. And patients with low raio tended to perform better in Modified Response Evaluation Criteria in Solid Tumors (mRECIST) and longer median Overall survival (OS) [10]. Therefore, it proved that Sorafenib has direct effect on tumor cells.
3.2.3. Side effects
Adverse events, such as diarrhea, weight loss, and handfoot skin reactions [9] are linked to Sorafenib and it leads to drug resistance [11]. The mechanism of drug resistance in HCC to Soferanib mainly includes liver caner cells themselves and the tumor microenvironment [11]. Many pateints have experienced resistance and disease relapse during the treatment [11].
3.3. Imatinib (Gleevec) in chronic myeloid leukemia (CML)
3.3.1. Pathology of CML and mechanism of Imatinib
Chronic myeloid leukemia is a hematopoietic stem cell disorder [12], which is caused by a reciprocal translocation between chromosomes 9 and 22. The symbol of the disease is the Philadelphia chromosome, which refers to the shortened version of chromosome 22. The molecular consequence of this inter-chromosomal exchange is the creation of the BCR-ABL gene, which encodes a protein with elevated tyrosine kinase activity [13], leading to cells dividing uncontrollably and leukemia cells proliferating widly, thereby resulting in CML disease. Imatinib, a tyrosine kinase inhibitor, can bind with high specificity to an inactive form of the ABL kinase [13] to inhibit the kinase.
3.3.2. Clinical statics & efficacy
Patients were assessed with CML receiving Imatinib for the effect of the early molecular response (EMR) on the long-term result [13]. They were divided into two groups as to their age, gender, Sokal risk score, and optimal response [13]. Patients were evaluated in terms of achieving an optimal response (OR) at 3 and 6 months [13]. It was demonstrated that patients with EMR at 3 months had apparently greater complete cytogenetic response and major molecular response (MMR) rates compared with patients without it [13]. The percentage of an OR at 6 months was 93% and 95% for two groups (P=0.553), respectively [13]. The result turned out that patients with an OR at 3 and 6 months had superrior event-free survival rates compared with the ones without it in groups.
3.3.3. Side effects
STI571(Imatinib mesylate) caused common adverse effects include mild to moderate nausea, myalgias, edema and so forth [14].
4. Discussion
4.1. Pros and Cons of synthesis
4.1.1. Gefitinib
The four steps involved in the pathway efficiently avoid using protective groups and risky materials such as DMF, SOCl2, POCl3, TMSI and so on [2]. Moreover, it simplifies the process of producing Gefitinib and get a satisfying yield (82%) as well [2]. Also, the temperature of the whole process is controlled under 55°C [2]. The thing is the potential cost of the use of ionic liquid reagents (trimethylammonium heptachlorodialuminate) so as to mass production in chemical factories.
4.1.2. Sorafenib
The common synthesis of Sorafenib has demerits such as: use of toxic chemical materials like haloformates and isocyanates, which are commonly used in making phosgene gas, and they are usually pretty complex [3]. Therefore, this pathway is safer and more efficient. However, there is one thing needed to be concerned, which mentions about the pretty low yield (25%) when getting one intermediate (2) [3]. To resolve this, more measures as to reducing unnecessary side reactions in the condensation reaction, such as temperature, reagents and so forth to get higher selectivity, need to be considered.
4.1.3. Imatinib
It was reported that the last step in this pathway yield at 72%, which was pretty noticeable [4]. However, purification of the product requires column chromotography, resulting in potential pricy cost, thereby it may not be suitable for large scale production [4]. This could be regarded as a reminder that the subsequent improvement should take into consideration that the potential cost, whether the synthesis is appropriate for mass production (temperature, reducing the use of toxic materials, pressure, time and so forth).
4.2. Development tendency
4.2.1. Resistance
Most small molecule drugs are prone to resistance mutaitons, especially protease inhibitors. Take Sorafenib as an instance, it can remarkably extend the median survival time of patients, but only by 3-5 months owing to the occurance of resistance mutations. Besides, small molecule drugs are prone to multi-drug resistance sites.
4.2.2. Combination therapy
Recent years, people have devoted to the research of synergistic effects of using two or multiple kinds of drugs to reach better treatment effects, such as utilizing statins and erlotinib in non-small cell lung cancer cell (NSCLC) lines to induce cell death.
5. Conclusion
The development of small molecule drugs has temporarily stuck in recent years, with the annual development rate being fixed in a range, which means that its development may begin to steadily advance. However, with the lasting development of these drugs with low resistance, high efficacy and few side effects, as well as the reasearch of new drug combination therapies, it can be ensured that small molecule drugs will bring us new breakthoughs in the treatment of a large number of refractory diseases in the coming future.
References
[1]. Schlicher, L., Green, L. G., Romagnani, A., & Renner, F. (2023). Small molecule inhibitors for cancer immunotherapy and associated biomarkers - the current status. Frontiers in immunology, 14, 1297175.
[2]. Maskrey, T. S. , Kristufek, T. , Laporte, M. G. , Nyalapatla, P. R. , & Wipf, P. . (2018). A new synthesis of gefitinib. Synlett, 30(4).
[3]. Prachi, R. , & Gill, M. S. . (2023). A practical and efficient method for the synthesis of sorafenib and regorafenib. SynOpen. 7, 422–429.
[4]. Deadman, B. J. , Hopkin, M. D. , Baxendale, I. R. , & Ley, S. V. . (2014). The synthesis of bcr-abl inhibiting anticancer pharmaceutical agents imatinib, nilotinib and dasatinib. Organic & Biomolecular Chemistry, 44(11), 1766-1800.
[5]. Li, W. Q., Li, L. Y., Chai, J., & Cui, J. W. (2021). Cost-effectiveness analysis of first-line treatments for advanced epidermal growth factor receptor-mutant non-small cell lung cancer patients. Cancer medicine, 10(6), 1964–1974.
[6]. Chen, R., & Sun, J. L. (2016). Zhonghua zhong liu za zhi [Chinese journal of oncology], 38(3), 239–240.
[7]. Kim, E. S. , Hirsh, V. , Mok, T. , Socinski, M. A. , Gervais, R. , & Wu, Y. L. , et al. (2008). Gefitinib versus docetaxel in previously treated non-small-cell lung cancer (interest): a randomised phase iii trial. Lancet (London, England), 372(9652), 1809-18.
[8]. Mahon, S. M., & Carr, E. (2021). Skin Toxicities: Common Side Effect. Clinical journal of oncology nursing, 25(6), 32.
[9]. Wang, H. , & Liu, J. . (2020). Exploration of sorafenib influences on gene expression of hepatocellular carcinoma. Frontiers in Genetics, 11, 577000.
[10]. Hu, J. , Wang, E. , Liu, L. , Wang, Q. , & Han, G. . (2020). Sorafenib may enhance antitumour efficacy in hepatocellular carcinoma patients by modulating the proportions and functions of natural killer cells. Investigational New Drugs, 38(3), 1-10.
[11]. Tang, W., Chen, Z., Zhang, W., Cheng, Y., Zhang, B., Wu, F., Wang, Q., Wang, S., Rong, D., Reiter, F. P., De Toni, E. N., & Wang, X. (2020). The mechanisms of sorafenib resistance in hepatocellular carcinoma: theoretical basis and therapeutic aspects. Signal transduction and targeted therapy, 5(1), 87.
[12]. Narayan, B., Buchete, N. V., & Elber, R. (2021). Computer Simulations of the Dissociation Mechanism of Gleevec from Abl Kinase with Milestoning. The journal of physical chemistry. B, 125(22), 5706–5715.
[13]. Capdeville, Renaud, Buchdunger, Elisabeth, Zimmermann, & Juerg, et al. (2002). Glivec (sti571, imatinib), a rationally developed, targeted anticancer drug. Nature Reviews Drug Discovery.
[14]. Teive, H. A. G., Germiniani, F. M. B., Munhoz, R. P., & Camargo, C. H. F. (2020). Blepharospasm and periorbital edema after imatinib mesylate: improvement with botulinum toxin. Arquivos de neuro-psiquiatria, 78(1), 58–59.
Cite this article
Sun,Y. (2024). Advances in the clinical application of small molecule chemical drugs. Theoretical and Natural Science,74,136-143.
Data availability
The datasets used and/or analyzed during the current study will be available from the authors upon reasonable request.
Disclaimer/Publisher's Note
The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of EWA Publishing and/or the editor(s). EWA Publishing and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
About volume
Volume title: Proceedings of ICBioMed 2024 Workshop: Computational Proteomics in Drug Discovery and Development from Medicinal Plants
© 2024 by the author(s). Licensee EWA Publishing, Oxford, UK. This article is an open access article distributed under the terms and
conditions of the Creative Commons Attribution (CC BY) license. Authors who
publish this series agree to the following terms:
1. Authors retain copyright and grant the series right of first publication with the work simultaneously licensed under a Creative Commons
Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this
series.
2. Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the series's published
version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial
publication in this series.
3. Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and
during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See
Open access policy for details).
References
[1]. Schlicher, L., Green, L. G., Romagnani, A., & Renner, F. (2023). Small molecule inhibitors for cancer immunotherapy and associated biomarkers - the current status. Frontiers in immunology, 14, 1297175.
[2]. Maskrey, T. S. , Kristufek, T. , Laporte, M. G. , Nyalapatla, P. R. , & Wipf, P. . (2018). A new synthesis of gefitinib. Synlett, 30(4).
[3]. Prachi, R. , & Gill, M. S. . (2023). A practical and efficient method for the synthesis of sorafenib and regorafenib. SynOpen. 7, 422–429.
[4]. Deadman, B. J. , Hopkin, M. D. , Baxendale, I. R. , & Ley, S. V. . (2014). The synthesis of bcr-abl inhibiting anticancer pharmaceutical agents imatinib, nilotinib and dasatinib. Organic & Biomolecular Chemistry, 44(11), 1766-1800.
[5]. Li, W. Q., Li, L. Y., Chai, J., & Cui, J. W. (2021). Cost-effectiveness analysis of first-line treatments for advanced epidermal growth factor receptor-mutant non-small cell lung cancer patients. Cancer medicine, 10(6), 1964–1974.
[6]. Chen, R., & Sun, J. L. (2016). Zhonghua zhong liu za zhi [Chinese journal of oncology], 38(3), 239–240.
[7]. Kim, E. S. , Hirsh, V. , Mok, T. , Socinski, M. A. , Gervais, R. , & Wu, Y. L. , et al. (2008). Gefitinib versus docetaxel in previously treated non-small-cell lung cancer (interest): a randomised phase iii trial. Lancet (London, England), 372(9652), 1809-18.
[8]. Mahon, S. M., & Carr, E. (2021). Skin Toxicities: Common Side Effect. Clinical journal of oncology nursing, 25(6), 32.
[9]. Wang, H. , & Liu, J. . (2020). Exploration of sorafenib influences on gene expression of hepatocellular carcinoma. Frontiers in Genetics, 11, 577000.
[10]. Hu, J. , Wang, E. , Liu, L. , Wang, Q. , & Han, G. . (2020). Sorafenib may enhance antitumour efficacy in hepatocellular carcinoma patients by modulating the proportions and functions of natural killer cells. Investigational New Drugs, 38(3), 1-10.
[11]. Tang, W., Chen, Z., Zhang, W., Cheng, Y., Zhang, B., Wu, F., Wang, Q., Wang, S., Rong, D., Reiter, F. P., De Toni, E. N., & Wang, X. (2020). The mechanisms of sorafenib resistance in hepatocellular carcinoma: theoretical basis and therapeutic aspects. Signal transduction and targeted therapy, 5(1), 87.
[12]. Narayan, B., Buchete, N. V., & Elber, R. (2021). Computer Simulations of the Dissociation Mechanism of Gleevec from Abl Kinase with Milestoning. The journal of physical chemistry. B, 125(22), 5706–5715.
[13]. Capdeville, Renaud, Buchdunger, Elisabeth, Zimmermann, & Juerg, et al. (2002). Glivec (sti571, imatinib), a rationally developed, targeted anticancer drug. Nature Reviews Drug Discovery.
[14]. Teive, H. A. G., Germiniani, F. M. B., Munhoz, R. P., & Camargo, C. H. F. (2020). Blepharospasm and periorbital edema after imatinib mesylate: improvement with botulinum toxin. Arquivos de neuro-psiquiatria, 78(1), 58–59.









