1. Introduction
Adolescence is a critical period of development phase between late childhood and adulthood, characterized by significant structural changes in brain development that impact cognition and behavior. Adolescent brains demonstrate a continual increase in white matter [1], which is presumed to be caused by myelination of axons by oligodendrocytes [2], enhancing the speed and efficiency of neural communication. Synaptic pruning also occurs [3], where excess synapses formed in childhood are eliminated in response to changing environmental demands and neural network refinement, rewiring existing neuronal connections. These processes contribute to changes in cortical volume and thickness in adolescence, often observed as a reduction in cortical gray matter volume, speculated to be due to the combined effects of pruning and increased myelination [4]. These dynamic structural transformations are speculated to link closely with the radial unit hypothesis, which posits that the cortical volume and thickness increase disproportionately during the cerebral cortex's organization and development.
Although high malleability is necessary for the adolescent brain to mature and adapt, brain structures may also be more impacted by adverse events in an individual’s early childhood. Childhood trauma is a term defined by the Diagnostic and Statistical Manual of Mental Disorders V as exposure to actual or threatened death, serious injury, or sexual violence [5]. It includes many early life adversity experiences, such as neglect, various forms of physical and sexual abuse, or emotional ill-treatment. Exposure to traumatic experiences during late childhood or adolescence is extremely common, with studies indicating that up to 60% of the youth experience at least one traumatic event before adulthood [6]. These experiences can profoundly impact brain development and cause morphological changes within the brain, including impaired synaptic potentiation and loss of synapses, as well as the reduction in the volume of hippocampal regions [7]. These structural changes have been indicated to bring about impairments in cognition and behavior, such as memory deficits [8] and greater developmental delays [9]. The argument has also been raised that structural brain changes due to stress is an adaptive response, instead of damage or impairment. The Stress Acceleration Hypothesis posits that early-life adversity can accelerate the development of neural structures necessary for survival and independence, particularly prioritizing limbic structures to help children adapt to adverse environments. As a result, maturation of brain regions responsible for higher brain function may be overlooked, resulting in thinner cortices and reduced structural integrity of association cortices [10]. Various kinds of traumatic early-life adversity events are also associated with an increased risk of internalizing and externalizing behaviors, including depression, anxiety, and attention-deficit/hyperactivity disorder (ADHD) [11], as well as suicidal ideation. The repercussions of adolescent trauma extend beyond the individual, often destabilizing family dynamics and placing substantial demands on societal resources, including healthcare and social services.
Current research faces several limitations in understanding the impact of traumatic experiences on adolescents. Firstly, many have associated childhood trauma with structural alterations of various regions, particularly prominently the prefrontal cortex, structures within the limbic system, and the parietal lobe [12]. However, there is significant variability in outcomes across different research teams: while some studies indicate trauma leading to a reduction in the volume or thickness of these regions [13], others indicate no significant difference or even greater volume and thickness [14]. Inconsistent results, potentially due to differing methodologies and interpretations, pose a significant obstacle to elucidating particular regions of interest impacted by childhood trauma. Secondly, many previous studies have limited sample sizes, hindering the replicability and widespread applicability of results. This necessitates larger datasets that integrate structural brain imaging data and clinical assessments of trauma and pathophysiological consequences. This current study builds on prior work, elucidating how traumatic experiences influence the maturation of the teenage brain through measuring cortical thickness and gray matter volume. We further assess the sensitivity of both brain developmental modalities on trauma and identify potential biomarkers associated with a reduction in volume and thinner cortices. Addressing these challenges will advance our knowledge of trauma's structural impacts on adolescent brains and aid in developing targeted intervention strategies for affected individuals.
2. Methods
2.1. Participants
Subjects were 9 to 10-year-old adolescents (n=7386) from Wave 1 of the Adolescent Brain Cognitive Development (ABCD, Release 3.0) study® (ABCD, Release 2.0.1) collected from 21 research sites distributed across the United States [15]. This multi-site, population-representative longitudinal cohort provides comprehensive clinical, behavioral, cognitive, and multimodal neuroimaging data from baseline, 1-year, and 2-year follow-up (age 12) assessments.
2.2. Brain Imaging Data, Processing
Subjects were imaged on a 3T magnetic resonance scanner (Siemens Prisma, General Electric MR 750, Philips). High-resolution T1-weighted structural MRI scans (1 mm isotropic voxels) were completed on a 3T MRI scanner (Siemens Prisma, General Electric MR 750, Philips). Structural MRI data pre-processing was done using FreeSurfer 5.3.0, following a standardized processing procedure [16]. Participants who did not pass T1 image visual inspection and FreeSurfer quality control were excluded from subsequent analysis. The current study used processed cortical thickness data according to the Desikan-Killiany Atlas, which was mapped to 34 cortical subdivisions in each hemisphere, totaling 68 regions of interest (ROIs) [17].
2.3. Trauma Measure
Trauma was assessed using the Kiddie Schedule for Affective Disorders and Schizophrenia (KSADS) scale [18] completed by a parent or guardian, particularly the Post-Traumatic Stress Disorder criteria A traumatic events checklist. This checklist contains 17 items assessing the occurrence of different traumatic events in children (Table 1). Each item was assessed based on participant's answer of “Yes”, documented as 1, or “No”, which was documented as 0. The extent of participant traumatic exposure was quantified as the sum of traumatic event occurrence for each participant.
Table 1. Traumatic events items from the Kiddie Schedule for Affective Disorders and Schizophrenia (K-SADS) PTSD Criteria A checklist
KSADS item | |
1 | A car accident in which your child or another person in the car was hurt bad enough to require medical attention |
2 | Another significant accident for which your child needed specialized and intensive medical treatment |
3 | Witnessed or caught in a fire that caused significant property damage or personal injury |
4 | Witnessed or caught in a natural disaster that caused significant property damage or personal injury |
5 | Witnessed or present during an act of terrorism (e.g., Boston marathon bombing) |
6 | Witnessed death or mass destruction in a war zone |
7 | Witnessed someone shot or stabbed in the community |
8 | Shot, stabbed, or beaten brutally by a non-family member |
9 | Shot, stabbed, or beaten brutally by a grown up in the home |
10 | Beaten to the point of having bruises by a grown up in the home |
11 | A non-family member threatened to kill your child |
12 | A family member threatened to kill your child |
13 | Witness the grownups in the home push, shove or hit one another |
14 | A grown up in the home touched your child in their privates, had your child touch their privates, or did other sexual things to your child |
15 | An adult outside your family touched your child in their privates, had your child touch their privates or did other sexual things to your child |
16 | A peer forced your child to do something sexually |
17 | Learned about the sudden unexpected death of a loved one |
2.4. Statistical Analysis
Statistical analysis in this study was conducted using the MATLAB ‘lme’ package, implementing linear mixed models (LMMs) to perform correlational analysis of regional cortical thickness (CT) between individuals with association of traumatic event exposure and the control group (HC). In the LMMs, regional CT was the dependent variable, and trauma-exposed individuals (HC defined as 0 and patients defined as 1) was the independent variable. Site-level random intercepts were used to accommodate correlation from clustering of individuals within study sites. Random effect variables included age, sex assigned at birth, race/ethnicity, parental educational level, and family income as covariates. The general form of the mixed linear model is shown in formula (1):
\( Y=Xβ+ZΓ+ ε \) (1)
\( Z \) is the matrix constructed from the random effect variables listed above. \( Γ \) is the random effect parameter vector, which follows a normal distribution with a mean vector of 0 and a variance-covariance matrix of \( G \) , expressed as Γ ∼ \( N \) (0, \( G \) ). \( ε \) is a random error vector. For the comparison between a subgroup and HC, if the subject belongs to the subgroup, the variable is equal to 1; if the subject belongs to the HC group, the variable is equal to 0. Those subjects belonging to other subgroups are deleted. Intracranial volume is included as a covariate of participant gray matter volume; average framewise motion is included. LMM provides t-test results of regression coefficients and p-values indicating significance between traumatic exposure and CT.
3. Results
3.1. Cortical Area
There are 2 significantly altered regions of cortical area: the paracentral lobule (t=2.58, p= 9.90E-03) and the caudal portion of the anterior cingulate cortex (ACC) (t=-2.08, p=0. 3.77E-02). Adversity-specific area changes are also hemispheric, exerting significant effects on the frontal and parietal lobe cortices of the left hemisphere and the cingulate cortex of the right hemisphere. Whole-brain t-value maps of the correlation between adversity events and cortical area alteration (Fig. 1) showed a positive correlation in the area of the superior frontal gyrus (SFG), paracentral lobule, and precuneus cortex of the right hemisphere of participants and adversity. On the contrary, increased adversity was associated with a decrease in area in the cingulate cortex, including the rostral anterior cingulate cortex, the ACC, and the posterior cingulate cortex. Results indicating patients with elevated adversity in early childhood or adolescence had altered cortical areas in the cingulate cortex regions were consistent with the results of previous research [19].
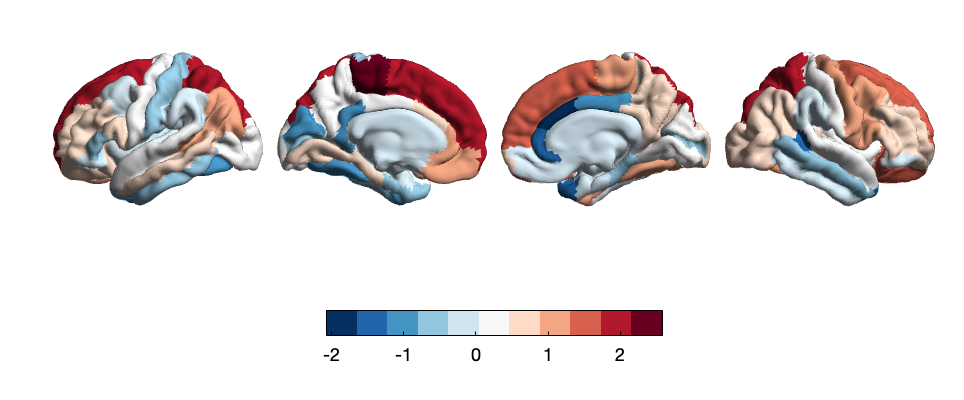
Figure 1. Whole-brain map of regional t-values correlating between cortical area and adverse life events. Color bars represent t-values of LMM-derived regression coefficients.
3.2. Cortical Sulcus
Increased adversity was associated with significant (p<0.05) cortical sulcus alteration in 4 areas. Of which, regional cortical sulcus decrease was most significant in the inferior temporal gyrus (IFG) (t=-2.99, p=2.82E-03) and rostral anterior cingulate cortex (rACC) (t=-2.91, p=3.57E-03). Conversely, cortical sulcus increase was shown in the fusiform gyrus (t= 2.35, p=1.90E-02) and the pars opercularis (t= 2.24, p=2.50E-02). T-value correlation mapping of cortical sulcus and adversity (Fig. 2) also indicates a negative association between adversity and temporal sulcus, shown through decreased t-values of the superior, middle, and inferior temporal gyrus. As demonstrated by Figure 2, the cingulate cortex, including the rACC and ACC display lowered cortical sulcus in increased adversity, in agreement with negative correlations revealed between the cingulate region and cortical area of adolescent brain structure. On the contrary, decreased sulcus in cingulate regions is concentrated on the left hemisphere while ACC area decrease was concentrated in the right hemisphere.
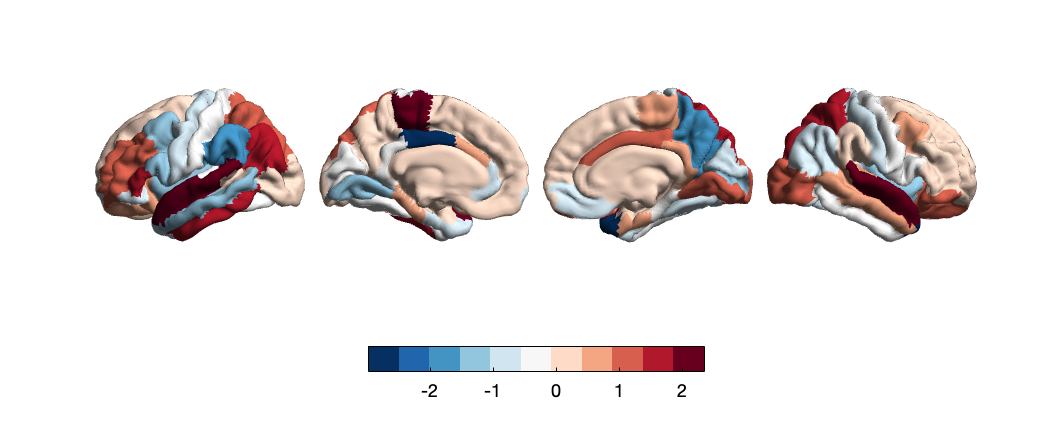
Figure 2. Whole-brain map of regional t-values correlating between cortical sulcus and adverse life events. Color bars represent t-values of LMM-derived regression coefficients.
3.3. Cortical Thickness
Of the three modal regions, cortical thickness shows the largest amount of 9 significant alterations associated with adversity. LMM significance analysis reveals adversity is predominantly associated with a decrease in cortical thickness. The insula (t=-2.83, p=4.71E-03), rostral middle frontal gyrus (t= -2.82, p=4.82E-03), lateral occipital cortex (t= -2.57, p=1.00E-02), paracentral lobule (t= -2.44, p=1.49E-02), lateral orbitofrontal cortex (t= -2.24, p=3.40E-02), superior frontal cortex (t= -2.07, p=3.81E-02) are all thinner in response to elevated adversity. However, the temporal pole (t= 2.74, p=6.19E-03) and the rACC (t= 2.05, p=4.02E-02) show thicker cortices. Hemispheric changes are less apparent in cortical thickness alterations: whole-brain t-value maps show an overall decrease in cortical thickness across both the left and right hemisphere, indicated by significantly negative t-values across multiple cortices, particularly in the frontal cortex and the occipital cortex (Fig. 3).
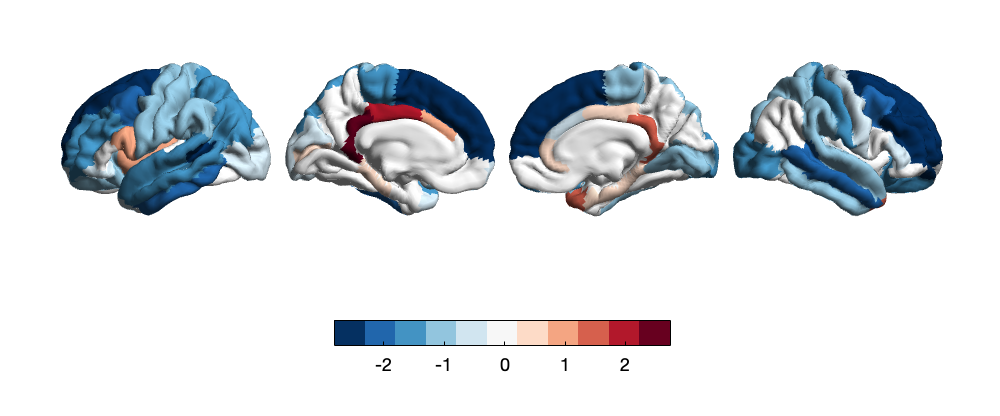
Figure 3. Whole-brain map of regional t-values correlating between cortical thickness and adversity. Color bars represent t-values of LMM-derived regression coefficients.
3.4. Network Connectivity
Individuals experiencing early-life trauma exhibited the most significant positive connectivity between the default and fronto-parietal network (t=2.95), and dorsal and ventral attention networks (t=2.02). Additionally, significant connectivity is demonstrated between sensory networks, such as between auditory and visual networks (t=2.23), cingulo-opercular and visual networks (t=2.26), and within-network connectivity of the cingulo-operator network (t=-2.22). No significant connectivity results were found for the salience network (Fig. 4), which has been indicated by previous literature to be altered by trauma exposure [20].
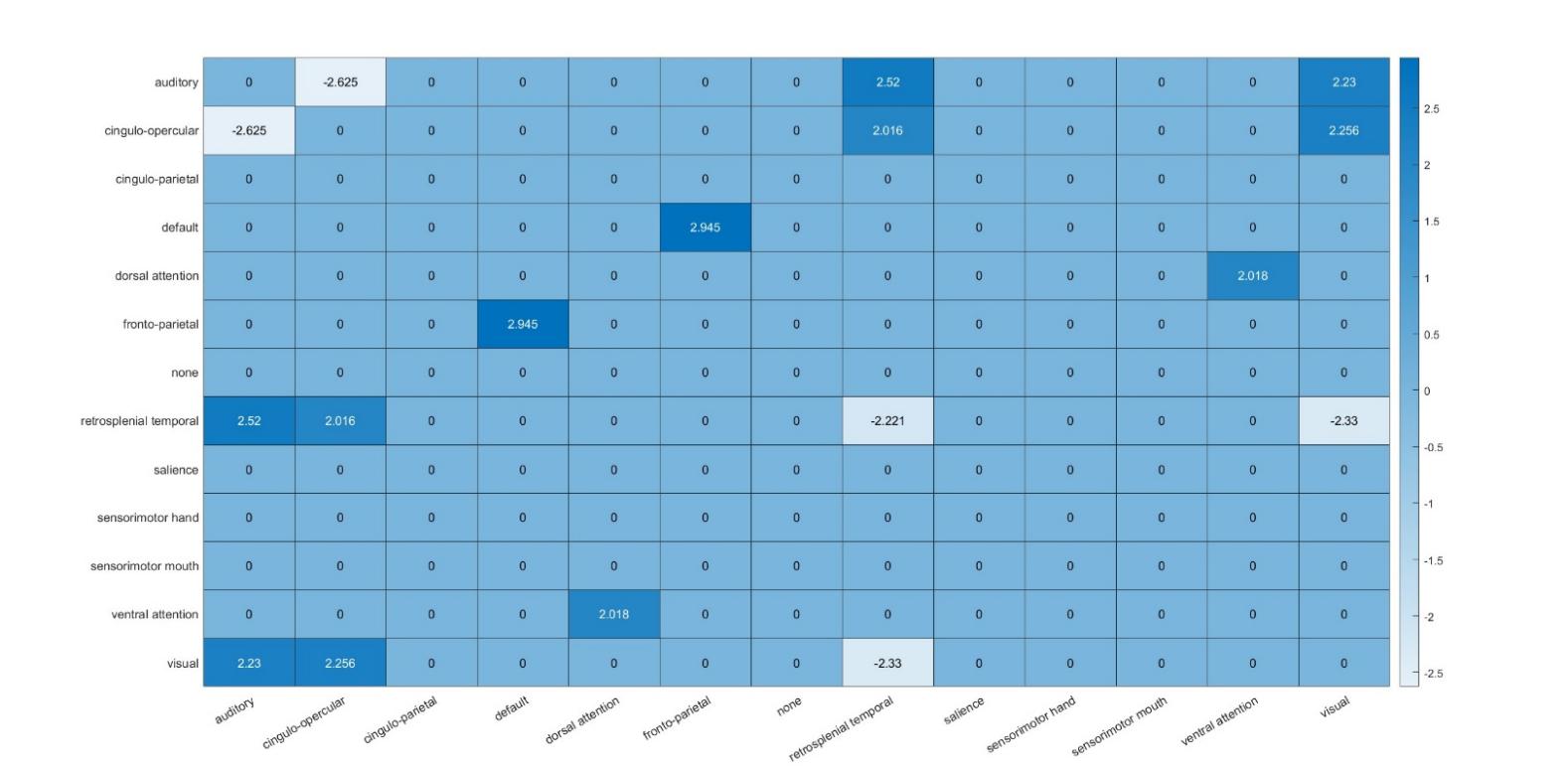
Figure 4. Heat map of connectivity between brain networks in individuals with trauma exposure. Color bars indicate significant t-values of regression coefficients from mixed linear models.
4. Discussions
The results of the present study, which investigated the association of trauma exposure on brain structures of adolescents, demonstrated significant alteration of cortical volume, sulcus, and thickness of multiple brain regions as a potential adaptive response to unfavorable environmental conditions. Supporting the Stress Acceleration Hypothesis, many brain regions exhibit premature structural and functional development relative to normal early adolescent development.
Firstly, there has been a significant increase in the volume, thickness, and sulcus observed in multiple subregions of the anterior cingulate cortex. Previous literature has conflicted findings on the effect of adversity on cingulate cortices thickness [21], but previous analysis on the ABCD study confirms findings that indicate the average cortical thickness of the cingulate cortex, which plays a role in the DMN, is elevated as a response to threat [22]. As the cingulate cortex is a key modulator of the DMN, involved in self-referential processing, thicker, and larger cingulate cortices in all modalities may be explained by a heightened awareness of self and surroundings adolescents feel as a result of PTSD developed from traumatic exposure. Previous research has linked pediatric PTSD patients to higher connectivity of the DMN, which may suggest trauma-related-sensory acceleration [23].
Cortical thickness increase of the lateral occipital region and sulcus increase of the fusiform gyrus indicate strong alterations of sensorimotor cortices in response to trauma events. These results are especially important considering previously conflicting findings. Childhood trauma has been indicated by previous literature to result in a thinner fusiform gyrus [24]; reduction in thickness of visual processing areas have also been reported in adolescents who have been victims of domestic violence as children, including reductions to somatosensory cortex [25]. This study conjectures increase in the development of sensorimotor regions may interact with limbic regions of emotion discrimination and facial processing in response to elevated aggression and a necessity for premature development of survival tactics. As the Stress Acceleration Hypothesis posits, limbic regions and visuo-emotional interactions mature quicker in children with traumatic events [26], explaining the elevated thickness of visual discrimination areas. Previous literature has shown physically abused children elicit a stronger response towards emotions, especially hostile ones such as anger and frustration — this may be accompanied by increased development of facial recognition structures such as the fusiform face area [27].
There were no significant alterations found between traumatic exposure in adolescents and cortical volume changes, which may indicate other modalities have more reactive associations with trauma and adversity. The discrepancy between thickness change and volume change indicates two modalities may develop differently throughout the process of adolescence, with thickness more closely reflecting functional connectivity and network reactivity.
Cortical thickness represents the most significant areas altered by elevated trauma. This correlates with previous literature indicating trauma may have a larger association with cortical thickness than volume [28]. Throughout the course of brain development, cortical thickness in brain areas cortices reaches its peak in adolescence and decreases linearly after adulthood, with the exception of frontal and parietal lobes, which only mature in late adolescence [29]. In this present study, brain regions become thicker in response to traumatic events and reach peak thickness in the present traumatic condition of ABCD sample individuals, where participants are in early stages of adolescence (age 9 - 12). Abnormal thickness development suggests accelerated structural maturation relative to healthy adolescents.
An exception to general cortical thickness increase is the insula. The insula has been implicated in pain pathways, risk-reward behavior, and greater association to abuse and adversity, especially in conditions of threat, where a structural alteration to the insula is associated with a skewed assessment of risk and pain awareness [30]. The insula is typically associated as the salience processing area, involved in self-perception and reflection, and also programmed as a signal to rapid responses of the environment. Youth who experience violence prioritize information that elicits fear and threat responses, so cortical thinning could represent accelerated functional development in the salience network. Another conclusion may be that premature synaptic pruning occurred as a result of early utilization of these salience regions [31], which normally develop in adolescence. However, more research is needed to elucidate specific effects of different traumatic events on insula cortical and salience network development.
Fronto-Parietal regions, including the Lateral Orbitofrontal, Rostral middle frontal, Superior Frontal cortices, also thin as a result of traumatic exposure. The frontal cortex develops the latest and matures in late adolescence, as higher order processing matures in an individual. Brain network hubs also center around the primary cortices to support sensorimotor development to the association cortex around the advance of adolescence [32] — thinner cortices in PFC regions may indicate that premature development of sensorimotor cortex caused by adversity and trauma has caused weaker structural integrity to the brain networks normally maturing in the frontal cortices. This may explain the lowered cortical thickness in frontal and parietal cortices, and may provide an explanation for the observed cognitive problems that follow children with childhood victimization and unstable social environments [33].
However, in later network connectivity analysis, elevated functional connectivity is observed between the default and fronto-parietal network in individuals experiencing traumatic events. The Default Mode Network (DMN) is a group of brain regions involved in the development of introspection and self-referential processing. Studies have indicated that the DMN network in infants highly resembles its adult counterpart [34]. The DMN’s early maturity makes its connectivity extremely susceptible to early life stress events, including childhood abuse [35] and interparental conflict [36]. However, conflicting results exist on the specific effects of trauma on DMN connectivity. The fronto-parietal network (FPN), as part of the brain regions responsible for executive functioning, develops later during adolescence [37]. Hence, exposure of early-life traumatic events results in inadequate development of these networks, exhibited by lowered functional connectivity of the FPN in adulthood [38], as well as thinner cortices in FPN brain regions demonstrated by correlational analysis. Connectivity analysis reveals surprising results of interaction between these two networks. The specific reasons for this elevated connectivity remain unclear and pose new questions for further research of its significance in trauma response.
Connectivity analysis also suggests a strong association between trauma exposure and elevated connectivity of sensory networks, particularly the visual network. This suggests greater maturation of functional visual processes, results in line with previous literature indicating greater sensitivity in identifying and processing visual information in children with early life trauma [39]. In particular, children experiencing abuse-related traumatic events develop stronger emotion detection and facial recognition abilities [40], further supported by results of elevated fusiform gyrus development suggested from previous cortical sulcus correlational analysis. The development of visual cortices and networks may be explained as a consequence of survival pressure, where visual emotion attention maturation is prioritized in unfavorable developmental conditions to aid children in identifying and avoiding threatening emotional cues from the environment [41]. Differentiated development of visual attention pathways related to threat can also be supported by the significantly altered connectivity between the ventral attention network (VAN) and dorsal attention network (DAN). The attention network is responsible for attention orientation and continuous automatic processing of external environmental stimuli, and previous studies revealed trauma-induced PTSD altered orienting attention abilities of adult veterans [42], as well as impairing sustained attention behavior [43]. Empirical studies have also supported emotional attention bias as a significant cognitive feature of children with adversity events [44], and stronger stimulus-driven attention towards threatening stimuli [45]. Hence, results conclude a potential role of traumatic events in adolescents and the development of visual and attention networks to achieve greater attention bias toward dangerous visual stimuli.
This study has several limitations that warrant consideration. Firstly, while the use of the ABCD study, which has a large sample, provided valuable, representative insight into consequences of early-life trauma on child development, participants were exclusively adolescents aged 9 to 12, which restricts the observation of traumatic experiences and their effects to a relatively narrow developmental window. Traumatic experiences during childhood can have profound impacts on individuals across their entire lifespan, and future research utilizes long-term observational studies with participants of diverse age cohorts to capture the full spectrum of trauma effects across adulthood and through aging processes. Additionally, the current study incorporated correlational analysis to generate findings. Hence, establishing a causal relationship between trauma and specific structural alterations or disease biomarkers remains a challenge. Future research would benefit from incorporating machine learning techniques to perform large-scale analyses of diverse datasets and identify more robust causal links between traumatic experiences and mental health outcomes. This approach could enhance the precision of predictions and deepen our understanding of the mechanisms through which trauma influences mental health.
5. Conclusions
In summary, the study is consistent with prior research, demonstrating an association with childhood traumatic exposure and significant alterations in cortical thickness, volume, sulcus, and network connectivity of brain regions related to higher order functioning, attention, introspection, and emotion recognition. The study demonstrates a significant correlation between traumatic experiences and alterations in brain regions that could serve as biomarkers for early detection. By identifying these biomarkers, clinicians can potentially recognize individuals at higher risk of developing internalizing and externalizing disorders before they fully manifest. This allows for timely and precise interventions, which could not only prevent the onset of mental disorders but also provide insights into targeted treatment for affected individuals. This paper is particularly significant, as it lays the groundwork for integrating biomarkers into clinical practice, offering a novel approach to early detection, intervention, and also improving therapeutic outcomes in mental healthcare.
References
[1]. Perrin, J. S., Hervé, P. Y., Leonard, G., Perron, M., Pike, G. B., Pitiot, A., Richer, L., Veillette, S., Pausova, Z., & Paus, T. (2008). Growth of white matter in the adolescent brain: Role of testosterone and androgen receptor. The Journal of Neuroscience, 28(38), 9519–9524.
[2]. Yakovlev, P. A., & Lecours, I. R. (1967). The myelogenetic cycles of regional maturation of the brain. In A. Minkowski (Ed.), Regional development of the brain in early life, 3–70.
[3]. Huttenlocher, P. R. (1994). Synaptogenesis in human cerebral cortex. In G. Dawson & K. W. Fischer (Eds.), Human behavior and the developing brain, 137–152.
[4]. Giedd, J., Blumenthal, J., Jeffries, N., et al. (1999). Brain development during childhood and adolescence: A longitudinal MRI study. Nature Neuroscience, 2, 861–863.
[5]. American Psychiatric Association. (2013). Trauma- and stressor-related disorders. Diagnostic and statistical manual of mental disorders (5th ed.). American Psychiatric Publishing, 10.
[6]. McLaughlin, K. A. (2016). Future directions in childhood adversity and youth psychopathology. Journal of Clinical Child & Adolescent Psychology, 45(3), 361–382.
[7]. Short, A. K., & Baram, T. Z. (2019). Early-life adversity and neurological disease: Age-old questions and novel answers. Nature Reviews Neurology, 15(11), 657–669.
[8]. Lupien, S. J., McEwen, B. S., Gunnar, M. R., & Heim, C. (2009). Effects of stress throughout the lifespan on the brain, behaviour, and cognition. Nature Reviews Neuroscience, 10 (6), 434–445.
[9]. Karcher, N. R., Loewy, R. L., Savill, M., et al. (2022). Persistent and distressing psychotic-like experiences using adolescent brain cognitive development study data. Molecular Psychiatry, 27(3), 1490–1501.
[10]. Callaghan, B. L., & Tottenham, N. (2016). The stress acceleration hypothesis: Effects of early-life adversity on emotion circuits and behavior. Current Opinion in Behavioral Sciences, 7, 76–81.
[11]. adversity on emotion circuits and behavior. Current Opinion in Behavioral Sciences, 7, 76–81. Karcher, N. R., Loewy, R. L., Savill, M., et al. (2022). Persistent and distressing psychotic-like experiences using adolescent brain cognitive development study data. Molecular Psychiatry, 27(3), 1490–1501.
[12]. Hart, H., & Rubia, K. (2012). Neuroimaging of child abuse: A critical review. Frontiers in Human Neuroscience, 6, 52.
[13]. Lim, L., et al. (2018). Grey matter volume and thickness abnormalities in young people with a history of childhood abuse. Psychological Medicine, 48(6), 1034–1046.
[14]. Jeong, H. J., Durham, E. L., Moore, T. M., et al. (2021). The association between latent trauma and brain structure in children. Translational Psychiatry, 11, 240.
[15]. Casey, B., Cannonier, T., Conley, M. I., et al. (2018). The adolescent brain cognitive development (ABCD) study: Imaging acquisition across 21 sites. Developmental Cognitive Neuroscience, 32, 43–54.
[16]. Hagler, D. J. Jr., Hatton, S., Cornejo, M. D., et al. (2019). Image processing and analysis methods for the Adolescent Brain Cognitive Development Study. NeuroImage, 202, 116091.
[17]. Desikan, R. S., Segonne, F., Fischl, B., et al. (2006). An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. NeuroImage, 31 (3), 968–980.
[18]. Kaufman, J., et al. (1997). Schedule for affective disorders and schizophrenia for school-age children-present and lifetime version (K-SADS-PL): Initial reliability and validity data. Journal of the American Academy of Child & Adolescent Psychiatry, 36 (7), 980–988.
[19]. Graham, A. M., Pfeifer, J. H., Fisher, P. A., Carpenter, S., & Fair, D. A. (2015). Early life stress is associated with default system integrity and emotionality during infancy. Journal of Child Psychology and Psychiatry, 56 (11), 1212–1222.
[20]. Zhang, W., Kaldewaij, R., Hashemi, M. M., et al. (2022). Acute-stress-induced change in salience network coupling prospectively predicts post-trauma symptom development. Translational Psychiatry, 12, 63.
[21]. Grieve, S. M., et al. (2011). Regional heterogeneity in limbic maturational changes: Evidence from integrating cortical thickness, volumetric, and diffusion tensor imaging measures. NeuroImage, 55 (3), 868–879.
[22]. Peverill, M., et al. (2023). Childhood trauma and brain structure in children and adolescents. Developmental Cognitive Neuroscience, 59, 101180.
[23]. Patriat, R., et al. (2016). Default-mode network abnormalities in pediatric posttraumatic stress disorder. Journal of the American Academy of Child & Adolescent Psychiatry, 55(4), 319–327.
[24]. Peverill, M., et al. (2023). Childhood trauma and brain structure in children and adolescents. Developmental Cognitive Neuroscience, 59, 101180.
[25]. Kelly, P. A., et al. (2015). Sex differences in socioemotional functioning, attentional bias, and gray matter volume in maltreated children: A multilevel investigation. Development and Psychopathology, 27(4 Pt 2), 1591–1609.
[26]. Dayton, C. J., Huth-Bocks, A. C., & Busuito, A. (2016). The influence of interpersonal aggression on maternal perceptions of infant emotions: Associations with early parenting quality. Emotion, 16(4), 436–448.
[27]. Bérubé, A., et al. (2023). Emotion recognition in adults with a history of childhood maltreatment: A systematic review. Trauma, Violence, & Abuse, 24 (1), 278–294.
[28]. Madden, R. A., et al. (2023). Structural brain correlates of childhood trauma with replication across two large, independent community-based samples. European Psychiatry, 66 (1), 19.
[29]. Gilmore, J. H., et al. (2018). Imaging structural and functional brain development in early childhood. Nature Reviews Neuroscience, 19(3), 123–137.
[30]. Peverill, M., et al. (2023). Childhood trauma and brain structure in children and adolescents. Developmental Cognitive Neuroscience, 59, 101180.
[31]. Tau, G. Z., & Peterson, B. S. (2010). Normal development of brain circuits. Neuropsychopharmacology, 35 (1), 147–168.
[32]. Oldham, S., & Fornito, A. (2019). The development of brain network hubs. Developmental Cognitive Neuroscience, 36, 100607.
[33]. Everson-Rose, S. A., Mendes de Leon, C. F., Bienias, J. L., Wilson, R. S., & Evans, D. A. (2003). Early life conditions and cognitive functioning in later life. American Journal of Epidemiology, 158 (11), 1083–1089.
[34]. Gao, W., Alcauter, S., & Smith, J. K. (2014). Development of human brain cortical network architecture during infancy. Brain Structure and Function, 220, 1173-1186.
[35]. Philip, N. S., Sweet, L. H., Tyrka, A. R., Price, L. H., Bloom, R. F., & Carpenter, L. L. (2013). Decreased default network connectivity is associated with early life stress in medication-free healthy adults. European Neuropsychopharmacology, 23(1), 24–32.
[36]. Graham, A. M., et al. (2015). Early life stress is associated with default system integrity and emotionality during infancy. Journal of Child Psychology and Psychiatry, 56(11), 1212–1222.
[37]. Oldham, S., & Fornito, A. (2019). The development of brain network hubs. Developmental Cognitive Neuroscience, 36, 100607.
[38]. Liston, C., et al. (2014). Default mode network mechanisms of transcranial magnetic stimulation in depression. Biological Psychiatry, 76(7), 517–526.
[39]. Mueller-Pfeiffer, C., et al. (2014). Atypical visual processing in posttraumatic stress disorder. NeuroImage: Clinical, 3, 531–538.
[40]. Dodge, K. A., Pettit, G. S., Bates, J. E., & Valente, E. (1995). Social information-processing patterns partially mediate the effect of early physical abuse on later conduct problems. Journal of Abnormal Psychology, 104(4), 632.
[41]. Davis, J. S., et al. (2014). Attachment anxiety moderates the relationship between childhood maltreatment and attention bias for emotion in adults. Psychiatry Research, 217, 79–85.
[42]. Ely, S. L., et al. (2023). Attention, attention! Posttraumatic stress disorder is associated with altered attention-related brain function. Frontiers in Behavioral Neuroscience, 17, 1244685.
[43]. Hart, H., Lim, L., & Mehta, M. A. (2017). Reduced functional connectivity of fronto-parietal sustained attention networks in severe childhood abuse. PLoS ONE, 12(11), e0188744.
[44]. Romens, S. E., & Pollak, S. D. (2012). Emotion regulation predicts attention bias in maltreated children at-risk for depression. Journal of Child Psychology and Psychiatry, 53 (2), 120–127.
[45]. Mao, Y., et al. (2020). The role of attention in the relationship between early life stress and depression. Scientific Reports, 10(1), 6154.
Cite this article
Geng,J. (2024). The Impact of Childhood Trauma and Abuse on Adolescent Brain Structure and Function: An MRI-Based Neuroimaging Study. Theoretical and Natural Science,67,138-148.
Data availability
The datasets used and/or analyzed during the current study will be available from the authors upon reasonable request.
Disclaimer/Publisher's Note
The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of EWA Publishing and/or the editor(s). EWA Publishing and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
About volume
Volume title: Proceedings of the 4th International Conference on Biological Engineering and Medical Science
© 2024 by the author(s). Licensee EWA Publishing, Oxford, UK. This article is an open access article distributed under the terms and
conditions of the Creative Commons Attribution (CC BY) license. Authors who
publish this series agree to the following terms:
1. Authors retain copyright and grant the series right of first publication with the work simultaneously licensed under a Creative Commons
Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this
series.
2. Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the series's published
version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial
publication in this series.
3. Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and
during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See
Open access policy for details).
References
[1]. Perrin, J. S., Hervé, P. Y., Leonard, G., Perron, M., Pike, G. B., Pitiot, A., Richer, L., Veillette, S., Pausova, Z., & Paus, T. (2008). Growth of white matter in the adolescent brain: Role of testosterone and androgen receptor. The Journal of Neuroscience, 28(38), 9519–9524.
[2]. Yakovlev, P. A., & Lecours, I. R. (1967). The myelogenetic cycles of regional maturation of the brain. In A. Minkowski (Ed.), Regional development of the brain in early life, 3–70.
[3]. Huttenlocher, P. R. (1994). Synaptogenesis in human cerebral cortex. In G. Dawson & K. W. Fischer (Eds.), Human behavior and the developing brain, 137–152.
[4]. Giedd, J., Blumenthal, J., Jeffries, N., et al. (1999). Brain development during childhood and adolescence: A longitudinal MRI study. Nature Neuroscience, 2, 861–863.
[5]. American Psychiatric Association. (2013). Trauma- and stressor-related disorders. Diagnostic and statistical manual of mental disorders (5th ed.). American Psychiatric Publishing, 10.
[6]. McLaughlin, K. A. (2016). Future directions in childhood adversity and youth psychopathology. Journal of Clinical Child & Adolescent Psychology, 45(3), 361–382.
[7]. Short, A. K., & Baram, T. Z. (2019). Early-life adversity and neurological disease: Age-old questions and novel answers. Nature Reviews Neurology, 15(11), 657–669.
[8]. Lupien, S. J., McEwen, B. S., Gunnar, M. R., & Heim, C. (2009). Effects of stress throughout the lifespan on the brain, behaviour, and cognition. Nature Reviews Neuroscience, 10 (6), 434–445.
[9]. Karcher, N. R., Loewy, R. L., Savill, M., et al. (2022). Persistent and distressing psychotic-like experiences using adolescent brain cognitive development study data. Molecular Psychiatry, 27(3), 1490–1501.
[10]. Callaghan, B. L., & Tottenham, N. (2016). The stress acceleration hypothesis: Effects of early-life adversity on emotion circuits and behavior. Current Opinion in Behavioral Sciences, 7, 76–81.
[11]. adversity on emotion circuits and behavior. Current Opinion in Behavioral Sciences, 7, 76–81. Karcher, N. R., Loewy, R. L., Savill, M., et al. (2022). Persistent and distressing psychotic-like experiences using adolescent brain cognitive development study data. Molecular Psychiatry, 27(3), 1490–1501.
[12]. Hart, H., & Rubia, K. (2012). Neuroimaging of child abuse: A critical review. Frontiers in Human Neuroscience, 6, 52.
[13]. Lim, L., et al. (2018). Grey matter volume and thickness abnormalities in young people with a history of childhood abuse. Psychological Medicine, 48(6), 1034–1046.
[14]. Jeong, H. J., Durham, E. L., Moore, T. M., et al. (2021). The association between latent trauma and brain structure in children. Translational Psychiatry, 11, 240.
[15]. Casey, B., Cannonier, T., Conley, M. I., et al. (2018). The adolescent brain cognitive development (ABCD) study: Imaging acquisition across 21 sites. Developmental Cognitive Neuroscience, 32, 43–54.
[16]. Hagler, D. J. Jr., Hatton, S., Cornejo, M. D., et al. (2019). Image processing and analysis methods for the Adolescent Brain Cognitive Development Study. NeuroImage, 202, 116091.
[17]. Desikan, R. S., Segonne, F., Fischl, B., et al. (2006). An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. NeuroImage, 31 (3), 968–980.
[18]. Kaufman, J., et al. (1997). Schedule for affective disorders and schizophrenia for school-age children-present and lifetime version (K-SADS-PL): Initial reliability and validity data. Journal of the American Academy of Child & Adolescent Psychiatry, 36 (7), 980–988.
[19]. Graham, A. M., Pfeifer, J. H., Fisher, P. A., Carpenter, S., & Fair, D. A. (2015). Early life stress is associated with default system integrity and emotionality during infancy. Journal of Child Psychology and Psychiatry, 56 (11), 1212–1222.
[20]. Zhang, W., Kaldewaij, R., Hashemi, M. M., et al. (2022). Acute-stress-induced change in salience network coupling prospectively predicts post-trauma symptom development. Translational Psychiatry, 12, 63.
[21]. Grieve, S. M., et al. (2011). Regional heterogeneity in limbic maturational changes: Evidence from integrating cortical thickness, volumetric, and diffusion tensor imaging measures. NeuroImage, 55 (3), 868–879.
[22]. Peverill, M., et al. (2023). Childhood trauma and brain structure in children and adolescents. Developmental Cognitive Neuroscience, 59, 101180.
[23]. Patriat, R., et al. (2016). Default-mode network abnormalities in pediatric posttraumatic stress disorder. Journal of the American Academy of Child & Adolescent Psychiatry, 55(4), 319–327.
[24]. Peverill, M., et al. (2023). Childhood trauma and brain structure in children and adolescents. Developmental Cognitive Neuroscience, 59, 101180.
[25]. Kelly, P. A., et al. (2015). Sex differences in socioemotional functioning, attentional bias, and gray matter volume in maltreated children: A multilevel investigation. Development and Psychopathology, 27(4 Pt 2), 1591–1609.
[26]. Dayton, C. J., Huth-Bocks, A. C., & Busuito, A. (2016). The influence of interpersonal aggression on maternal perceptions of infant emotions: Associations with early parenting quality. Emotion, 16(4), 436–448.
[27]. Bérubé, A., et al. (2023). Emotion recognition in adults with a history of childhood maltreatment: A systematic review. Trauma, Violence, & Abuse, 24 (1), 278–294.
[28]. Madden, R. A., et al. (2023). Structural brain correlates of childhood trauma with replication across two large, independent community-based samples. European Psychiatry, 66 (1), 19.
[29]. Gilmore, J. H., et al. (2018). Imaging structural and functional brain development in early childhood. Nature Reviews Neuroscience, 19(3), 123–137.
[30]. Peverill, M., et al. (2023). Childhood trauma and brain structure in children and adolescents. Developmental Cognitive Neuroscience, 59, 101180.
[31]. Tau, G. Z., & Peterson, B. S. (2010). Normal development of brain circuits. Neuropsychopharmacology, 35 (1), 147–168.
[32]. Oldham, S., & Fornito, A. (2019). The development of brain network hubs. Developmental Cognitive Neuroscience, 36, 100607.
[33]. Everson-Rose, S. A., Mendes de Leon, C. F., Bienias, J. L., Wilson, R. S., & Evans, D. A. (2003). Early life conditions and cognitive functioning in later life. American Journal of Epidemiology, 158 (11), 1083–1089.
[34]. Gao, W., Alcauter, S., & Smith, J. K. (2014). Development of human brain cortical network architecture during infancy. Brain Structure and Function, 220, 1173-1186.
[35]. Philip, N. S., Sweet, L. H., Tyrka, A. R., Price, L. H., Bloom, R. F., & Carpenter, L. L. (2013). Decreased default network connectivity is associated with early life stress in medication-free healthy adults. European Neuropsychopharmacology, 23(1), 24–32.
[36]. Graham, A. M., et al. (2015). Early life stress is associated with default system integrity and emotionality during infancy. Journal of Child Psychology and Psychiatry, 56(11), 1212–1222.
[37]. Oldham, S., & Fornito, A. (2019). The development of brain network hubs. Developmental Cognitive Neuroscience, 36, 100607.
[38]. Liston, C., et al. (2014). Default mode network mechanisms of transcranial magnetic stimulation in depression. Biological Psychiatry, 76(7), 517–526.
[39]. Mueller-Pfeiffer, C., et al. (2014). Atypical visual processing in posttraumatic stress disorder. NeuroImage: Clinical, 3, 531–538.
[40]. Dodge, K. A., Pettit, G. S., Bates, J. E., & Valente, E. (1995). Social information-processing patterns partially mediate the effect of early physical abuse on later conduct problems. Journal of Abnormal Psychology, 104(4), 632.
[41]. Davis, J. S., et al. (2014). Attachment anxiety moderates the relationship between childhood maltreatment and attention bias for emotion in adults. Psychiatry Research, 217, 79–85.
[42]. Ely, S. L., et al. (2023). Attention, attention! Posttraumatic stress disorder is associated with altered attention-related brain function. Frontiers in Behavioral Neuroscience, 17, 1244685.
[43]. Hart, H., Lim, L., & Mehta, M. A. (2017). Reduced functional connectivity of fronto-parietal sustained attention networks in severe childhood abuse. PLoS ONE, 12(11), e0188744.
[44]. Romens, S. E., & Pollak, S. D. (2012). Emotion regulation predicts attention bias in maltreated children at-risk for depression. Journal of Child Psychology and Psychiatry, 53 (2), 120–127.
[45]. Mao, Y., et al. (2020). The role of attention in the relationship between early life stress and depression. Scientific Reports, 10(1), 6154.









