1. Introduction
Cancer is one of the significant causes of death in the world, and many different types of cancer can be caused by bad habits, bad habits, and so on. Cancer is a general term under which there are many different parts of the tumor. Its primary pathogenesis is that the virus cells first enter the body, then spread rapidly in the body, and it is difficult to control and cure the spread to a certain extent, leading to death. In modern technology, many ways exist to avoid or prevent the state in the early stage. Many cancers can be cured if detected early and treated effectively [1]. Cancer can gradually turn from benign to malignant. There are three different directions that lead to cancer in the body. The first is physical cancer; long-term exposure to ultraviolet light or radiation will lead to cancer. Secondly, chemical aspects, asbestos, tobacco smoke, alcohol, and so on. Finally, it comes from microbes and other bacteria.
One transcription factor that serves as a gene defender is p53, encoded by TP53 gene. The genome can be preserved as much as feasible. P53 initiates cell repair by halting the cell cycle. If that fails, p53 triggers the death of cells. This crucial tumor suppressor gene, which keeps an eye on DNA damage and signals cells to behave correctly, may stop the spread of damage to DNA during cell division and lower the risk of cancer. On the other hand, the TP53 gene on chromosome 17 pauses the cell cycle when DNA is broken, and TP53 is mutated in at least 50% of human cancers. Since many of the most tp53 altered proteins have characteristics that cause cancer, they may control the quantity of cancer cells as well as their capacity to invade and spread. Both future treatments focused on restoring tumor suppressive p53 function and biological pathways may be used by mutated p53 proteins to exercise their carcinogenic activities. It has been shown that CP-31398, a p53 modulator, may treat cancer by stabilizing the DNA-binding core domain of the human tumor suppressor protein p53 in vitro. A tiny chemical known as CP-31398 has been shown to stabilize the human tumor suppressor protein p53's DNA-binding core domain in vitro. Additionally, it was suggested that the molecule might potentially treat cancer by restoring the defective p53 protein's ability to bind DNA and, as a result, activate transcription in mice and mammalian tissue culture cells [2].
This review summarizes mechanisms and applications of CP-31398 in cancer, aiming to facilitate future clinical applications of CP-31398.
2. Role of p53 in cancer
TP53 is the gene that is most often mutated in human malignancies [3]. The idea that transcriptional control is essential to tumor suppression capabilities is supported by the mutation, which results in a total loss of TP53 transcriptional activity and reduces its capacity to inhibit tumor development. A mutation in the TP53 protein causes the cell to become uncontrollable and causes the damaged DNA to be reproduced, which results in unchecked cell division and malignant tumors. Tumor-associated TP53 mutations often vary from specific TP53 types. Numerous of these p53-mutated proteins are carcinogenic and have easy control on the orientation and motion of cells.
Due to the fact that human malignancies often have TP53 deficiency [4]. Numerous cells, including those involved in gene expression, DNA damage, hypoxia, metabolic failure, and so forth, activate the TP53 tumor suppressor. It then puts suitable countermeasures in place to stop the onset of cancer. Numerous cellular responses, such as senescence, death, non-functioning, DNA repair, and differentiation states, are brought on by the TP53 protein's activity [3]. The TP53 protein ensures that genomic integrity is maintained, which is crucial for tumor suppression, and performs vital roles in the cell's response to different stimuli. TP53 is the most often found genetic mutation because human malignancies have shown how important it is in preventing tumors. Even with all of the precise research and knowledge that scientists conduct, there is still a great deal of ambiguity with TP53.
There are many different pathways to TP53 to play their functions, different p53 mutations and their potential effects on its function and carcinogenic activity. TP53 gene mutations are associated with wild-type TP53 gene inactivation, and 75% of P53 mutations result in loss of P53 function. Wild TP53 causes many different morphologies. The effect of TP53 mutations is caused by the interaction between molecules. These pathways include inhibition of mTOR kinase, inhibition of Cip1, activation of NF-KB, etc. [4]. The effect of TP53 mutations refers to the loss of the protective effect of the wild p53 protein, which leads to the progression of cancer.
3. Structure of CP-31398
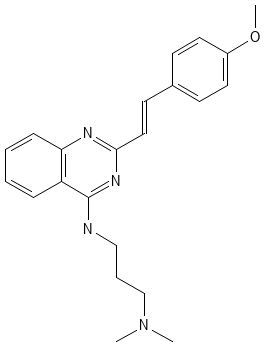
CP-31398 (PubChem CID 9950868) has become another topic and research object. Quick recall CP-31398, a styrylquinazoline, emerged from a high throughput screen for therapeutic agents. CP-31398 has a more professional name, which is Unii-IN3WH41H3A. It’s mainly made up of three different chemical compounds: Carbon, Hydrogen, and Oxygen. The molecular weight is about 362.5 g/mol, compared with water’s molecular weight (18.01528 g/mol) is a larger number. The structure of CP-31398 is very typical, with the capital letters N, O, and H at the nodes to form a hexagonal model of matter [4]. Figure 1 shows the chemical structure of CP-31398. Hydrogen bonding is where a molecule can form hydrogen bonds through its nitrogen and oxygen atoms, which is important for its interaction with biological targets such as p53. Biologically speaking, CP-31398's ability to stabilize p53 protein makes it an important molecule in cancer research. TP53 is often referred to as the guardian of the genome because it plays an important role in preventing DNA damage and proliferation. Mutations in p53 are common in many types of cancer, and small molecules like CP-31398 can restore its function.
|
Figure 1. Chemical structure of CP-31398. |
4. Applications of CP-31398 on cancers
CP-31398 has been found to have anti-cancer effects in multiple cancers, including hepatocellular carcinoma (HCC), colorectal cancer, pancreatic cancer, breast cancer, bladder cancer, neck squamous cell carcinoma (HNSCC), soft-tissue sarcoma etc. (Table 1).
4.1. HCC
The mutated p53 protein cannot back activate its downstream target genes, which in fact have a strong relationship with the activation and decay of TP53. UVB radiation exposure over time may cause p53 mutations. These MPT alterations are caused by CP-31398-induced p53 mitochondrial translocation. The release of cytochrome, which may impede MPT, can lessen p53 translocation and apoptosis. These mice often get UVB signature mutations as a result of chronic UVB exposure. The protein that codes for p53 loses its tumor suppressor function when the TP53 gene is mutated. Consider the case of liver cancer in particular. In HCC, the tumor suppressor p53 gene is among the most frequently altered genes. Researchers discovered CP-31398's effects on p53-mutant HCC cells [5]. Cell cycle analysis, cell death, cell reproduction, and the specific expression of TP53 have all been studied, as has the impact of CP-31398 on the properties of HCC cells with p53 mutations. PLC/PRF/5 cell culture tumor xenografts were used to evaluate the inhibitory impact of CP-31398 on tumor development using growth trend, general pattern, and expression of p53-related genes [5]. Initially, it was discovered that CP-31398 and TP53 had the same econometric dependency. Further research then revealed that in p53-mutant HCC cells, CP-31398 restored wild-type p53 function, which could successfully reduce the quantity of cell deaths and modify the cycle. CP-31398 ultimately prevented the formation of xenograft tumors by transactivating p53 response downstream molecules. The research indicates that CP-31398 may be developed as a potential treatment agent for HCC with p53 mutations as it causes the phenotypic alterations necessary for p53 mutant HCC cells both in vitro and in vivo [5].
4.2. Colorectal cancer
Scientists then begin to concentrate on distinct types of cancer. Colorectal cancer is the first condition. The effects of CP-31398, a p53-modulating drug, both alone and in combination with celecoxib on colon adenocarcinomas and aberrant crypt foci (ACF) generated by azoxymethane in F344 rats are the main focus of this study [6]. This is a control experiment that regularly changes the amount of food. Because of the azoxymethane treatment, the possibility for mice to get the disease is way lower than usual. At the same time, diet also can effectively reduce the possibility of incidence rate. The most incredible low-dose CP-31398 can almost reduce 90% of the incidence rate. This shows that CP-31398 has a strong inhibitory effect [6].
4.3. Pancreatic cancer
PNCs, which include pancreatic adenocarcinoma, often include TP53 mutations that impact the transcriptional activity of TP53. Two of these chemicals, CP-31398 and RITA, were shown to activate p53/DNA binding and p53 phosphorylation, which in turn triggered distinct behaviors, including cell proliferation and death, without having any effect on total numbers. Thr172 is phosphorylated by AMPK, and this is how CP-31398 and RITA control the axis SESN1-2/AMPK/mTOR. Thr172 is important for the autophagic response. By preventing cell development, some chemical reactions may protect CP-31398 from harm. Based on autophagy suppression linked to p53 activation, the results encourage the development of anticancer medicines and highlight the function autophagy produced by p53 reactivating molecules plays in survival [7].
4.4. Breast cancer
Breast cancer cells originate in the milk ducts, or lobules, of the breast. Cancer cells can spread to nearby breast tissue and eventually form tumors that can lead to lumps or thickening [8]. People found that in breast cancer cells with p53 mutations, drugs with the small molecule CP-31398 for WT-like TP53 function increase their sensitivity to NK-mediated breakdown. Cp31398-induced autophagy can isolate many forms of autophagy, specifically Bcl-XL and XIAP, promoting a good penetration effect of the particles. There are certain types of cells that cause death. Conclusion The TP53-dependent autophagy approach has effectively increased the cytotoxic lymphocyte-mediated cleavage of p53 mutated breast cancer cells [8].
4.5. Bladder cancer
Bladder cancer is the next research topic. Common cancers that start in the bladder's cells are bladder cancers [9]. Urine is stored in the bladder, a hollow, muscular structure in your lower belly. Tumor suppressor TP53 mutation and elevated polyamine levels may both significantly impact the growth and metastasis of urothelial carcinomas. DFMO, an inhibitor drug, and CP-31398 as a stable drug can reduce the spread of epithelial tumors and successfully inhibit them. Scientists have taken mice as experimental objects, and in the process of feeding them food, DFMO element was added into the food of one group as a control group. Mice were fed with food with different percentages of DFMO. At either level, urothelial tumors were more than 50% suppressed. Therefore, they can successfully detect the reduction of tumors [9].
4.6. HNSCC
PRIMA-1, CP-31398, RITA, and nutlin-3 were tested in four human HNSCC cell lines differing in TP53 status. It assesses cell death, survival, change, division etc. p53 was significantly activated in both mutant and wild-type tp53 tumor cell lines treated with CP-31398. Nutlin-3 has a strong inhibitory effect on the growth of MDM2-dependent p53-degraded tumor cells [10].
4.7. Soft-tissue sarcoma
When TP53 is in the wild form, the small molecule p53RA can restore the cancer-inhibiting effect of TP53. The last research direction is the resistance of TP53 to TNF-α cytotoxicity in tissue sarcomas especially limb sarcomas. For the treatment of incurable limb sarcoma, intrathecal administration of TNF-α and melphalan proved efficacious. TNF-α's capacity to cause sarcoma cells to undergo direct apoptosis was examined [11]. Naturally, TP53 is also a major factor in this. The majority of skin cells are dead after staining, making it possible to examine how sensitive human sarcoma cell lines (n = 9) with varying TP53 and MDM2 status are to TNF-α. If employed, CP-31398 may increase and promote TP53(Mut) and TP53(Wt)/MDM2(Ampl) cell death. Specifically, in TP53(Mut) cells, CP-31398 may trigger p53 and a few of its apoptotic target genes. The Nutlin-3a effect in TP53(Wt) or MDM2(Ampl) cells was linked to decreased TNF-α-induced NF-κb-dna binding as well as changed, encouraged, and modified differential regulation of genes such fam, TP53BP2, GADD45, and TGF-β1. Thus, there is a high correlation between the restoration of p53 function in sarcoma cells and their sensitivity to TNF-α, indicating that this might be a crucial factor in determining the efficacy of TNF-α-based sarcoma treatment [11].
Table 1. Applications of CP-31398 on cancers.
Cancer | Model | Treatments | Results | Ref(s) |
HCC | PLC/PRF/5 cell culture tumor xenografts | CP-31398 reactivates wild-type p53 function in p53 mutated HCC cells | Transactivating downstream molecules of the p53 response. | [5] |
Colorectal cancer | F344 rats | CP-31398 alone and combined with celecoxib | Effectively reduced the possibility of incidence rate | [6] |
Pancreatic cancer | Pancreatic cancer cells | C-31398 and RITA | Increased autophagy; significant anti-cancer effects | [7] |
Breast cancer | Cytotoxic Tlymphocytes (CTL) and natural killer cells (NK) | Granzyme B- or NK cell induced apoptosis | Relatively effectively reduce the possibility of accident rate | [8] |
Bladder cancer | DFMO, an ODC-inhibiting agent | Molecular analysis of the urothelial tumors suggested a modulation | A combination of CP and DFMO | [9] |
5. Conclusions
CP-31398 has been proven its anti-cancer effects, making progress in cancer resistance drugs, increasing the treatment options for more patients. CP-31398 is an effective anticancer drug by stabilizing the core domain of tumor suppressor p53 in vitro and by rescuing unstable mutants of p53. CP-31398 can mainly stabilize the activity of p53 in vitro or in vivo to ensure the normal operation of cells, reducing the severity of cancer. CP-31398 has been approved its anti-cancer effects in multiple cancers, including hepatocellular carcinoma (HCC), colorectal cancer, pancreatic cancer, breast cancer, bladder cancer, neck squamous cell carcinoma, etc. Future research is required to further understand the targets of CP-31398. In vivo studies and clinical trials are required to optimize the pharmacological effects and promote its clinical applications.
References
[1]. de Martel C, Georges D, Bray F, Ferlay J and Clifford GM 2020 Lancet Glob. Health. 8 e180-e190
[2]. World Health Organization, Cancer today (2024), Available online at: https://gco.iarc.fr/today.
[3]. Marei HE, Althani A, Afifi N, Hasan A, Caceci T, Pozzoli G, Morrione A, Giordano A and Cenciarelli C 2021 Cancer Cell Int. 21 703
[4]. Huang Y, Jiao Z, Fu Y, Hou Y, Sun J, Hu F, Yu S, Gong K, Liu Y and Zhao G 2024 Eur. J. Med. Chem. 265 116121
[5]. He XX, Zhang YN, Yan JW, Yan JJ, Wu Q and Song YH 2016 Tumour Biol. 37 807-15
[6]. Rao CV, Steele VE, Swamy MV, Patlolla JM, Guruswamy S and Kopelovich L 2009 Cancer Res. 69 8175-82
[7]. Fiorini C, Menegazzi M, Padroni C, Dando I, Dalla Pozza E, Gregorelli A, Costanzo C, Palmieri M and Donadelli M 2013 Apoptosis 18 337-46
[8]. Chollat-Namy M, Ben Safta-Saadoun T, Haferssas D, Meurice G, Chouaib S and Thiery J 2019 Cell Death Dis. 10 695
[9]. Madka V, Mohammed A, Li Q, Zhang Y, Kumar G, Lightfoot S, Wu X, Steele V, Kopelovich L and Rao CV 2015 Am. J. Cancer Res. 5 3030-41.
[10]. Roh JL, Kang SK, Minn I, Califano JA, Sidransky D and Koch WM 2011 Oral Oncol. 47 8-15
[11]. Muret J. et al. 2012 PLoS One 7 e38808
Cite this article
Cai,X. (2025). Applications of CP-31398 Targeting P53 on Cancers. Theoretical and Natural Science,74,206-210.
Data availability
The datasets used and/or analyzed during the current study will be available from the authors upon reasonable request.
Disclaimer/Publisher's Note
The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of EWA Publishing and/or the editor(s). EWA Publishing and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
About volume
Volume title: Proceedings of ICBioMed 2024 Workshop: Computational Proteomics in Drug Discovery and Development from Medicinal Plants
© 2024 by the author(s). Licensee EWA Publishing, Oxford, UK. This article is an open access article distributed under the terms and
conditions of the Creative Commons Attribution (CC BY) license. Authors who
publish this series agree to the following terms:
1. Authors retain copyright and grant the series right of first publication with the work simultaneously licensed under a Creative Commons
Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this
series.
2. Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the series's published
version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial
publication in this series.
3. Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and
during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See
Open access policy for details).
References
[1]. de Martel C, Georges D, Bray F, Ferlay J and Clifford GM 2020 Lancet Glob. Health. 8 e180-e190
[2]. World Health Organization, Cancer today (2024), Available online at: https://gco.iarc.fr/today.
[3]. Marei HE, Althani A, Afifi N, Hasan A, Caceci T, Pozzoli G, Morrione A, Giordano A and Cenciarelli C 2021 Cancer Cell Int. 21 703
[4]. Huang Y, Jiao Z, Fu Y, Hou Y, Sun J, Hu F, Yu S, Gong K, Liu Y and Zhao G 2024 Eur. J. Med. Chem. 265 116121
[5]. He XX, Zhang YN, Yan JW, Yan JJ, Wu Q and Song YH 2016 Tumour Biol. 37 807-15
[6]. Rao CV, Steele VE, Swamy MV, Patlolla JM, Guruswamy S and Kopelovich L 2009 Cancer Res. 69 8175-82
[7]. Fiorini C, Menegazzi M, Padroni C, Dando I, Dalla Pozza E, Gregorelli A, Costanzo C, Palmieri M and Donadelli M 2013 Apoptosis 18 337-46
[8]. Chollat-Namy M, Ben Safta-Saadoun T, Haferssas D, Meurice G, Chouaib S and Thiery J 2019 Cell Death Dis. 10 695
[9]. Madka V, Mohammed A, Li Q, Zhang Y, Kumar G, Lightfoot S, Wu X, Steele V, Kopelovich L and Rao CV 2015 Am. J. Cancer Res. 5 3030-41.
[10]. Roh JL, Kang SK, Minn I, Califano JA, Sidransky D and Koch WM 2011 Oral Oncol. 47 8-15
[11]. Muret J. et al. 2012 PLoS One 7 e38808