1. Introduction
Adult neurogenesis in the hippocampus appears in the subgranular zone (SGZ), where neural stem cells (NSCs) form various kinds of new cells through proliferation, differentiation, and migration [1-13]. In the adult neurogenesis process of dentate granule cells (DGCs), horizontal-to-radial repositioning is crucial to their survival and follow-up circuit integration [2, 13-15]. Reelin and sphingosine-1-phosphate (S1P) are found to facilitate this repositioning process [1-3, 5, 7, 11, 13-18].
Previous research shows that Reelin is encoded by the RELN gene as an extracellular matrix protein. It regulates the repositioning of newborn DGCs and contributes to their dendritic spine development [1, 3, 7, 11, 16, 18, 19]. In the meantime, as a bioactive lipid molecule, S1P's synthesis process is modulated by Sphingosine Kinase 1 and 2, which are encoded by the SPHK1 and 2 genes. The repositioning, neurite growth, and circuit integration of new DGCs can be promoted by S1P signaling [1, 2, 5, 13-15, 17].
The effects of Reelin and S1P on the repositioning of newborn DGCs have significant similarities, suggesting the potential overlapping of functioning pathways. Nevertheless, no studies have investigated whether Reelin and S1P signaling processes share the same pathway. Hence, this study focuses on the relationship between Reelin and S1P in the repositioning of newborn DGCs, using retroviral vectors, immunostaining, electrophysiological recording, and RNA sequencing to investigate the following two research questions:
1) Do Reelin and S1P work cooperatively in the same pathway or independently in two different pathways in the repositioning process of newly generated DGCs in the hippocampus?
2) If they work in the same pathway, what is the upstream/downstream relationship between Reelin and S1P in the repositioning process of newly generated DGCs in the hippocampus?
2. Methods and Expected Results
To answer these research questions, two experiments were conducted with adult mice, examining the relationship between Reelin and S1P in the horizontal-to-radial repositioning of newly generated DGCs.
• Experiment 1: Whether Reelin and S1P Work in the Same Pathway
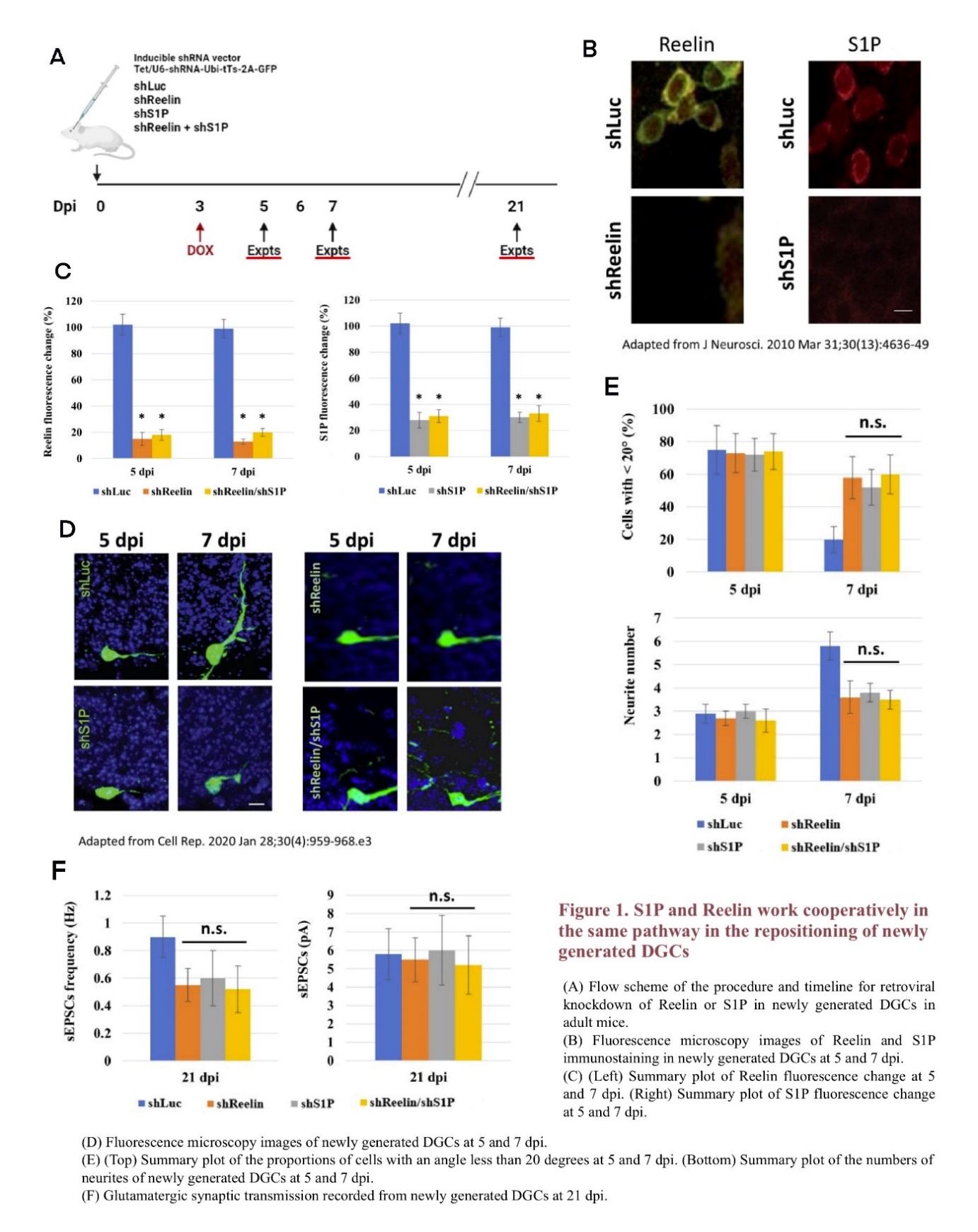
To explore whether Reelin and S1P work cooperatively in the same pathway or independently in two different pathways, newly generated DGCs in adult mice were labeled with green fluorescent protein (GFP) using retroviral vectors to observe their repositioning results [13, 20]. Cells in the mice DG were also infected with retroviral vectors carrying short-hairpin RNA to conduct gene knockdown. Four different vectors were designed, targeting RELN (shReelin), SPHK1/2(shS1P), both RELN and SPHK1/2 (shReelin/shS1P), and luciferase (shLuc) as a control. At 3 dpi, doxycycline (DOX) was added to the drinking water to induce the short-hairpin RNA expression (Figure 1A) [13, 21, 22]. The knockdown results were detectable at 5 and 7 dpi, when the levels of Reelin and S1P in the mice DG cells were analyzed under fluorescence microscopy after immunostaining. While both Reelin and S1P can be detected in the shLuc mice, their expression levels decrease significantly among shReelin, shS1P, and shReelin/shS1P mice, indicating a successful knockdown (Figure 1B, 1C) [23]. These results are consistent with retroviral knockdown conducted in previous research [13].
To determine the effects of shReelin, shS1P, and shReelin/shS1P on the repositioning and growth of newly generated DGCs, GFP-labeled newborn DGCs were observed under fluorescence microscopy. For new DGCs in shLuc mice, the horizontal-to-radial repositioning proceeds normally from 5 dpi to 7 dpi; however, in shReelin, shS1P, or shReelin/shS1P mice, the cells remain largely horizontal at 7 dpi, suggesting that the lack of Reelin or S1P may lead to deficiencies in newborn DGC repositioning, which is similar to findings from previous studies (Figure 1D) [13, 19]. To further investigate whether the absence of Reelin, S1P, or both results in the same or similar phenotypes, the percentage of newborn DGCs within 20 degrees and their neurite number at 5 and 7 dpi, as well as the spontaneous excitatory post-synaptic currents (sEPSCs) recording results in their glutamatergic synaptic transmission were summarized with histograms (Figure 1E, 1F) [13]. While shReelin, shS1P, and shReelin/shS1P mice all have more newborn DGCs remaining horizontally positioned, less neurite development, and lower sEPSCs frequency compared to shLuc mice, no significant difference is detected among these three groups, indicating that shReelin, shS1P, and shReelin/shS1P may result in similar phenotypes. These results suggest that Reelin and S1P possibly work in the same pathway during the repositioning of newly generated DGCs, as the knockdown of both does not lead to a greater deficiency than the knockdown of any one of these two genes.
• Experiment 2: The Upstream/Downstream Relationship of Reelin and S1P
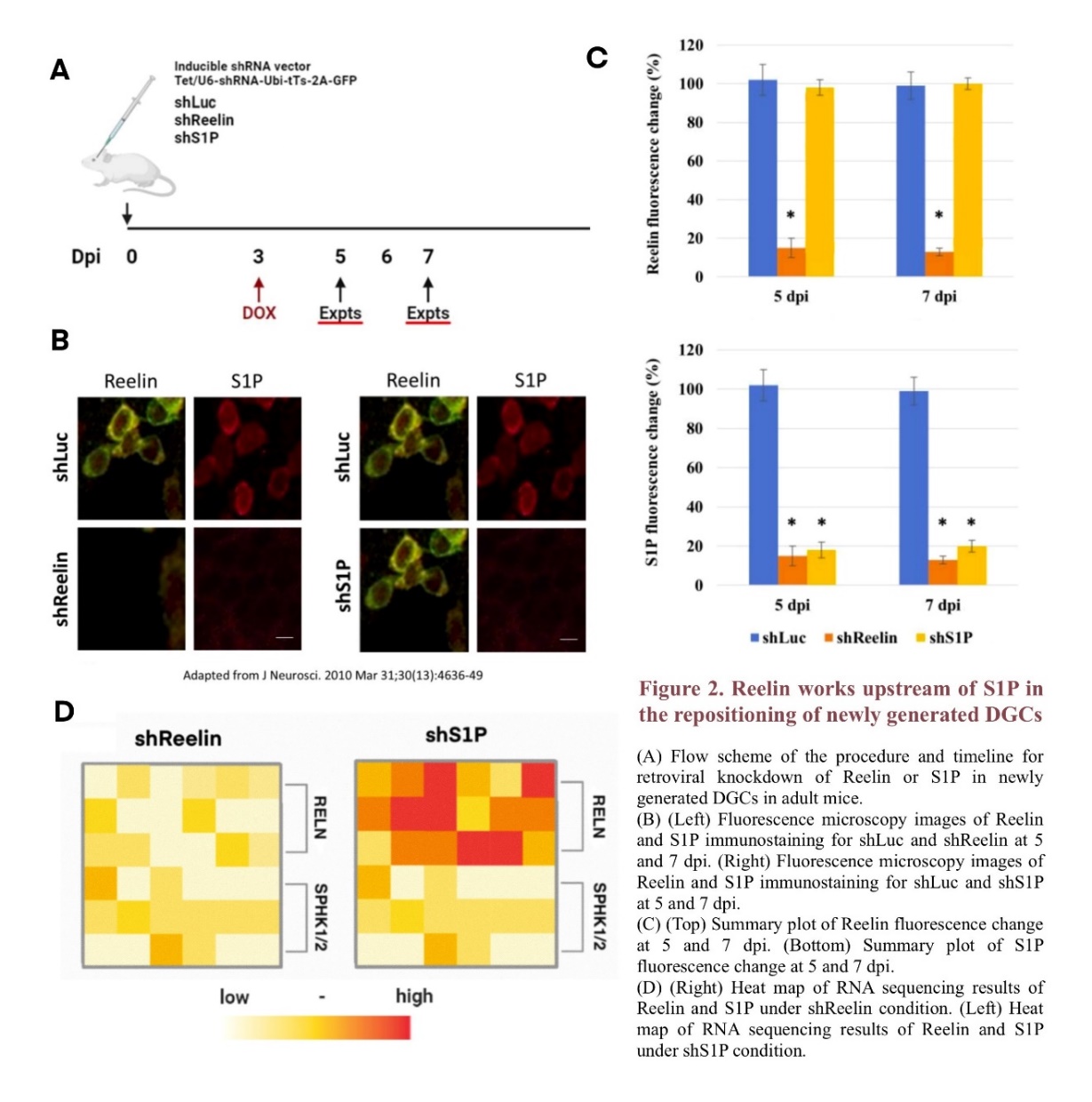
A similar method of retroviral knockdown was employed to examine the upstream/downstream relationship between Reelin and S1P in the horizontal-to-radial repositioning of newborn DGCs. Mice were infected with DOX-inducable retroviral vectors targeting RELN (shReelin) or SPHK1/2(shS1P), and the knockdown was induced at 3 dpi (Figure 2A) [13, 21, 22].
Immunostaining shows that both shReelin and shS1P lead to decreased fluorescence levels of the corresponding molecule at 5 and 7 dpi. However, while the fluorescence level of S1P also decreases under shReelin conditions, no significant difference is observed between the fluorescence level of Reelin under shS1P conditions and that under shLuc conditions, which suggests that the lack of Reelin may have an adverse impact on the expression level of S1P-related genes (Figure 2B) [23]. These observation results are further quantified in Figure 2C, where the fluorescence changes in Reelin and S1P are graphed in histograms respectively. For shS1P mice, only the fluorescence level of S1P changes significantly compared to shLuc mice; however, for shReelin mice, the fluorescence levels of both Reelin and S1P decrease greatly at 5 and 7 dpi, indicating Reelin's influence on S1P signaling. Similar results were found in the RNA sequencing of the mRNA of RELN, which produces Reelin, and Sphingosine Kinase 1/2, which produces Sphk 1/2 that regulates the synthesis of S1P, further confirming that the expression level of Reelin potentially plays a role in S1P regulation (Figure 2D) [14]. Overall, these results suggest that Reelin may work upstream of S1P in the repositioning and circuit integration of new DGCs, as Reelin exhibits a regulation effect on S1P level and S1P does not show a similar impact on Reelin.
3. Conclusion
In conclusion, this study investigates the relationship between Reelin and S1P in the horizontal-to-radial repositioning of newly generated DGCs. It is found that Reelin and S1P function cooperatively in the same signaling pathway during the new DGC repositioning process. Through retroviral knockdown experiments, it was observed that the absence of either Reelin or S1P results in similar deficiencies in DGC repositioning, neurite development, and synaptic activity, suggesting their interdependent roles. Additionally, the findings indicate that Reelin may act upstream of S1P, as knockdown of Reelin significantly affects S1P expression levels, whereas S1P knockdown does not alter Reelin expression.
While the study presents expected results suggesting a cooperative relationship between Reelin and S1P in the same pathway, it is important to acknowledge that disruptions in molecules within the same pathway do not always lead to identical phenotypes due to potential compensatory mechanisms or context-dependent effects. Consequently, the actual outcomes of this study may differ from the anticipated findings. Nevertheless, the insights from this study enhance the current understanding of the molecular mechanisms underlying adult neurogenesis and may inform future therapeutic strategies targeting cognitive disorders associated with impaired neurogenesis.
References
[1]. Abbott, L. C., & Nigussie, F. (2019). Adult neurogenesis in the mammalian dentate gyrus. Anatomia, Histologia, Embryologia, 49(1), 3–16. https://doi.org/10.1111/ahe.12496
[2]. Ahamad, A., Wang, J., Ge, S., & Kirschen, G. W. (2020). Early dendritic morphogenesis of adult-born dentate granule cells is regulated by FHL2. Frontiers in Neuroscience, 14(202). https://doi.org/10.3389/fnins.2020.00202
[3]. Bonafina, A., Paratcha, G., & Ledda, F. (2020). Deciphering new players in the neurogenic adult hippocampal niche. Frontiers in Cell and Developmental Biology, 8(548). https://doi.org/10.3389/fcell.2020.00548
[4]. Christian, K. M., Ming, G., & Song, H. (2020). Adult neurogenesis and the dentate gyrus: Predicting function from form. Behavioural Brain Research, 379(2020). https://doi.org/10.1016/j.bbr.2019.112346
[5]. Echten-Deckert, G. van, & Walter, J. (2012). Sphingolipids: Critical players in Alzheimer’s disease. Progress in Lipid Research, 51(2012), 378–393. http://dx.doi.org/10.1016/j.plipres.2012.07.001
[6]. Gage, F. H. (2019). Adult neurogenesis in mammals. Science, 364(6443), 827–828. https://doi.org/10.1126/science.aav6885
[7]. Kempermann, G., & Gage, F. H. (1999). New nerve cells for the adult brain. Scientific American.
[8]. Ognibene, E. , Adriani, W. , Simone Macrì, & Laviola, G. . (2007). Neurobehavioural disorders in the infant reeler mouse model: interaction of genetic vulnerability and consequences of maternal separation. Behavioural Brain Research, 177(1), 142-149.
[9]. Buuse, M. V. D. , Halley, P. , Hill, R. , Labots, M. , & Martin, S. . (2012). Altered n-methyl-d-aspartate receptor function in reelin heterozygous mice: male–female differences and comparison with dopaminergic activity - sciencedirect. Progress in Neuro-Psychopharmacology and Biological Psychiatry, 37( 2), 237-246.
[10]. Li, Y., Xu, N.-N., Hao, Z.-Z., & Liu, S. (2023). Adult neurogenesis in the primate hippocampus. Zoological Research, 44(2), 315−322. https://doi.org/10.24272/j.issn.2095-8137.2022.399
[11]. Stranahan, A. M., Erion, J. R., & Wosiski-Kuhn, M. (2013). Reelin signaling in development, maintenance, and plasticity of neural networks. Ageing Research Reviews, 12(3). https://doi.org/10.1016/j.arr.2013.01.005
[12]. Tuncdemir, S. N., Lacefield, C. O., & Hen, R. (2019). Contributions of adult neurogenesis to dentate gyrus network activity and computations. Behavioural Brain Research, 374(112112). https://doi.org/10.1016/j.bbr.2019.112112
[13]. Yang, C.-H., Di Antonio, A., Kirschen, G. W., Varma, P., Hsieh, J., & Ge, S. (2020). Circuit Integration Initiation of New Hippocampal Neurons in the Adult Brain. Cell Reports, 30(4), 959-968.e3. https://doi.org/10.1016/j.celrep.2019.12.084
[14]. Mendelson, K., Evans, T., & Hla, T. (2014). Sphingosine 1-phosphate signalling. Development, 141(1), 5–9. https://doi.org/10.1242/dev.094805
[15]. PébayA., & Turksen, K. (Eds.). (2018). Sphingosine-1-phosphate: Methods and protocols. Humana Press.
[16]. Förster, E. (2014). Reelin, neuronal polarity and process orientation of cortical neurons. Neuroscience, 269(2014), 102–111. https://doi.org/10.1016/j.neuroscience.2014.03.004
[17]. Hannun , Y. A., & Obeid, L. M. (2008). Principles of bioactive lipid signalling: Lessons from sphingolipids. Nature Reviews Molecular Cell Biology, 9(2008), 139–150. https://www.nature.com/articles/nrm2329
[18]. Ranaivoson, F. M., von Daake, S., & Comoletti, D. (2016). Structural insights into reelin function: Present and future. Frontiers in Cellular Neuroscience, 10(137). https://doi.org/10.3389/fncel.2016.00137
[19]. Teixeira, C. M., Kron, M., Núria Masachs, Zhang, H., Lagace, D. C., Martı́nezA., Reillo, I., Duan, X., Bosch, C., Lluı́s Pujadas, Brunso, L., Song, H., Eisch, A. J., BorrellV., Howell, B. W., Parent, J. M., & Soriano, E. (2012). Cell-Autonomous inactivation of the reelin pathway impairs adult neurogenesis in the hippocampus. The Journal of Neuroscience, 32(35), 12051–12065. https://doi.org/10.1523/jneurosci.1857-12.2012
[20]. Wang, J., Shen, J., Kirschen, G. W., Gu, Y., Jessberger, S., & Ge, S. (2019). Lateral dispersion is required for circuit integration of newly generated dentate granule cells. Nature Communications, 10(1). https://doi.org/10.1038/s41467-019-11206-9
[21]. Kumamoto, N., Gu, Y., Wang, J., Janoschka, S., Takemaru, K.-I., Levine, J., & Ge, S. (2012). A role for primary cilia in glutamatergic synaptic integration of adult-born neurons. Nature Neuroscience, 15(3), 399–405. https://doi.org/10.1038/nn.3042
[22]. Rao, S., Kirschen, G. W., Szczurkowska, J., Adrian Di Antonio, Wang, J., Ge, S., & Shelly, M. (2017). Repositioning of somatic golgi apparatus is essential for the dendritic establishment of adult-born hippocampal neurons. Journal of Neuroscience, 38(3), 631–647. https://doi.org/10.1523/jneurosci.1217-17.2017
[23]. Pujadas, L., Gruart, A., Bosch, C., Delgado, L., Teixeira, C. M., Rossi, D., de Lecea, L., Martinez, A., Delgado-Garcia, J. M., & Soriano, E. (2010). Reelin regulates postnatal neurogenesis and enhances spine hypertrophy and long-term potentiation. Journal of Neuroscience, 30(13), 4636–4649. https://doi.org/10.1523/jneurosci.5284-09.2010
Cite this article
Xie,Y. (2025). The Relationship Between Reelin and Sphingosine-1- Phosphate in the Repositioning of Adult-Generated Dentate Granule Cells. Theoretical and Natural Science,73,76-81.
Data availability
The datasets used and/or analyzed during the current study will be available from the authors upon reasonable request.
Disclaimer/Publisher's Note
The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of EWA Publishing and/or the editor(s). EWA Publishing and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
About volume
Volume title: Proceedings of the 4th International Conference on Biological Engineering and Medical Science
© 2024 by the author(s). Licensee EWA Publishing, Oxford, UK. This article is an open access article distributed under the terms and
conditions of the Creative Commons Attribution (CC BY) license. Authors who
publish this series agree to the following terms:
1. Authors retain copyright and grant the series right of first publication with the work simultaneously licensed under a Creative Commons
Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this
series.
2. Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the series's published
version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial
publication in this series.
3. Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and
during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See
Open access policy for details).
References
[1]. Abbott, L. C., & Nigussie, F. (2019). Adult neurogenesis in the mammalian dentate gyrus. Anatomia, Histologia, Embryologia, 49(1), 3–16. https://doi.org/10.1111/ahe.12496
[2]. Ahamad, A., Wang, J., Ge, S., & Kirschen, G. W. (2020). Early dendritic morphogenesis of adult-born dentate granule cells is regulated by FHL2. Frontiers in Neuroscience, 14(202). https://doi.org/10.3389/fnins.2020.00202
[3]. Bonafina, A., Paratcha, G., & Ledda, F. (2020). Deciphering new players in the neurogenic adult hippocampal niche. Frontiers in Cell and Developmental Biology, 8(548). https://doi.org/10.3389/fcell.2020.00548
[4]. Christian, K. M., Ming, G., & Song, H. (2020). Adult neurogenesis and the dentate gyrus: Predicting function from form. Behavioural Brain Research, 379(2020). https://doi.org/10.1016/j.bbr.2019.112346
[5]. Echten-Deckert, G. van, & Walter, J. (2012). Sphingolipids: Critical players in Alzheimer’s disease. Progress in Lipid Research, 51(2012), 378–393. http://dx.doi.org/10.1016/j.plipres.2012.07.001
[6]. Gage, F. H. (2019). Adult neurogenesis in mammals. Science, 364(6443), 827–828. https://doi.org/10.1126/science.aav6885
[7]. Kempermann, G., & Gage, F. H. (1999). New nerve cells for the adult brain. Scientific American.
[8]. Ognibene, E. , Adriani, W. , Simone Macrì, & Laviola, G. . (2007). Neurobehavioural disorders in the infant reeler mouse model: interaction of genetic vulnerability and consequences of maternal separation. Behavioural Brain Research, 177(1), 142-149.
[9]. Buuse, M. V. D. , Halley, P. , Hill, R. , Labots, M. , & Martin, S. . (2012). Altered n-methyl-d-aspartate receptor function in reelin heterozygous mice: male–female differences and comparison with dopaminergic activity - sciencedirect. Progress in Neuro-Psychopharmacology and Biological Psychiatry, 37( 2), 237-246.
[10]. Li, Y., Xu, N.-N., Hao, Z.-Z., & Liu, S. (2023). Adult neurogenesis in the primate hippocampus. Zoological Research, 44(2), 315−322. https://doi.org/10.24272/j.issn.2095-8137.2022.399
[11]. Stranahan, A. M., Erion, J. R., & Wosiski-Kuhn, M. (2013). Reelin signaling in development, maintenance, and plasticity of neural networks. Ageing Research Reviews, 12(3). https://doi.org/10.1016/j.arr.2013.01.005
[12]. Tuncdemir, S. N., Lacefield, C. O., & Hen, R. (2019). Contributions of adult neurogenesis to dentate gyrus network activity and computations. Behavioural Brain Research, 374(112112). https://doi.org/10.1016/j.bbr.2019.112112
[13]. Yang, C.-H., Di Antonio, A., Kirschen, G. W., Varma, P., Hsieh, J., & Ge, S. (2020). Circuit Integration Initiation of New Hippocampal Neurons in the Adult Brain. Cell Reports, 30(4), 959-968.e3. https://doi.org/10.1016/j.celrep.2019.12.084
[14]. Mendelson, K., Evans, T., & Hla, T. (2014). Sphingosine 1-phosphate signalling. Development, 141(1), 5–9. https://doi.org/10.1242/dev.094805
[15]. PébayA., & Turksen, K. (Eds.). (2018). Sphingosine-1-phosphate: Methods and protocols. Humana Press.
[16]. Förster, E. (2014). Reelin, neuronal polarity and process orientation of cortical neurons. Neuroscience, 269(2014), 102–111. https://doi.org/10.1016/j.neuroscience.2014.03.004
[17]. Hannun , Y. A., & Obeid, L. M. (2008). Principles of bioactive lipid signalling: Lessons from sphingolipids. Nature Reviews Molecular Cell Biology, 9(2008), 139–150. https://www.nature.com/articles/nrm2329
[18]. Ranaivoson, F. M., von Daake, S., & Comoletti, D. (2016). Structural insights into reelin function: Present and future. Frontiers in Cellular Neuroscience, 10(137). https://doi.org/10.3389/fncel.2016.00137
[19]. Teixeira, C. M., Kron, M., Núria Masachs, Zhang, H., Lagace, D. C., Martı́nezA., Reillo, I., Duan, X., Bosch, C., Lluı́s Pujadas, Brunso, L., Song, H., Eisch, A. J., BorrellV., Howell, B. W., Parent, J. M., & Soriano, E. (2012). Cell-Autonomous inactivation of the reelin pathway impairs adult neurogenesis in the hippocampus. The Journal of Neuroscience, 32(35), 12051–12065. https://doi.org/10.1523/jneurosci.1857-12.2012
[20]. Wang, J., Shen, J., Kirschen, G. W., Gu, Y., Jessberger, S., & Ge, S. (2019). Lateral dispersion is required for circuit integration of newly generated dentate granule cells. Nature Communications, 10(1). https://doi.org/10.1038/s41467-019-11206-9
[21]. Kumamoto, N., Gu, Y., Wang, J., Janoschka, S., Takemaru, K.-I., Levine, J., & Ge, S. (2012). A role for primary cilia in glutamatergic synaptic integration of adult-born neurons. Nature Neuroscience, 15(3), 399–405. https://doi.org/10.1038/nn.3042
[22]. Rao, S., Kirschen, G. W., Szczurkowska, J., Adrian Di Antonio, Wang, J., Ge, S., & Shelly, M. (2017). Repositioning of somatic golgi apparatus is essential for the dendritic establishment of adult-born hippocampal neurons. Journal of Neuroscience, 38(3), 631–647. https://doi.org/10.1523/jneurosci.1217-17.2017
[23]. Pujadas, L., Gruart, A., Bosch, C., Delgado, L., Teixeira, C. M., Rossi, D., de Lecea, L., Martinez, A., Delgado-Garcia, J. M., & Soriano, E. (2010). Reelin regulates postnatal neurogenesis and enhances spine hypertrophy and long-term potentiation. Journal of Neuroscience, 30(13), 4636–4649. https://doi.org/10.1523/jneurosci.5284-09.2010









