1. Introduction
MRI has emerged as a cornerstone in biomedical imaging, utilizing the fundamental principles of magnetic resonance to provide details into the structure and function of tissues within living organisms. At the heart of MRI technology is the phenomenon where atomic nuclei, especially those with non-zero spin such as protons, align their magnetic moments in response to an external magnetic field. Upon exposure to radiofrequency (RF) pulses, these nuclei undergo energy transitions, which leads to RF signals emission that can be detected and processed to construct high-resolution images [1]. This unique method underpins the wide-ranging applications of MRI, allowing for an in-depth exploration of tissues without the harmful effects associated with ionizing radiation found in other imaging modalities
The growing reliance on MRI in clinical settings can be attributed to its numerous advantages. Predominantly, MRI’s non-invasive nature significantly reduces patient risk, making it a suitable option for repeated use and longitudinal studies. Furthermore, its ability to produce detailed soft tissue contrast enhances the diagnostic utility in various medical fields, from neurology to oncology [2]. However, despite its widespread adoption and remarkable capabilities, MRI does present limitations, such as long scanning times, susceptibility to motion artifacts, and higher operational costs compared to other imaging methods. Understanding both the strengths and challenges of MRI is essential for optimizing its use in diverse applications.
Beyond its traditional medical applications, MRI has recently found innovative uses in agricultural sciences, particularly in exploring plant physiology and food examinations. The non-destructive capability of MRI allows researchers to investigate complex root systems in crops such as corn, soybeans, and eggplants without causing harm [3]. This novel application is critical for understanding nutrient uptake processes, thereby enhancing agricultural productivity and sustainability. Moreover, the application of MRI extends into the realm of food safety and quality examination. With increasing global focus on food health hazards, MRI serves as a sophisticated tool for detecting contaminants and analyzing food components. Looking forward, the landscape of MRI technology is poised for significant advancements. Enhancements such as Ultra-High Field MRI (UVMRI) promise to revolutionize imaging, particularly in the context of cardiac diagnostics in which heightened sensitivity and resolution can yield critical insights into pathologies. Furthermore, the integration of artificial intelligence (AI) stands to markedly improve MRI applications, offering enhanced image interpretation, thus it can overcome existing limitations and facilitating more accurate diagnoses [4].
This paper will explore the fundamental principles of MRI, its advantages and limitations, and its multifaceted applications in root-detection, and food safety and quality before the UVMRI combined with AI in enhancing MRI effectiveness.
2. Basic knowledge of MR
2.1. Basic Principles of MRI
MRI is fundamentally based on the principles of magnetic resonance, which occurs when the magnetic moments of atomic nuclei within a substance become fragmented under the influence of an external magnetic field. In the presence of radiofrequency (RF) pulses, these nuclei can experience energy level jumps, leading to a phenomenon essential for MRI. The nuclei of certain atoms, notably hydrogen, carbon, and fluorine—as found in organic compounds—play a crucial role in MRI. When analyzing MRI in the human body, hydrogen atoms are particularly significant due to their abundance. A hydrogen atom consists of a proton and an electron, and because hydrogen is prevalent in body tissues, particularly in water, the signals generated from hydrogen nuclei are strong. Consequently, modern clinical MRI primarily employs hydrogen proton imaging. The varying hydrogen content across different tissues and organs leads to differences in the intensity of magnetic resonance signals, allowing for the distinction between tissues through a method known as proton density-weighted imaging.
By applying an external magnetic field, protons process and generate a magnetic vector. In a balanced state, most protons align with the direction of the external magnetic field, while the components in the transverse magnetic field direction cancel each other out due to phase differences, resulting in a net effect of only longitudinal magnetization at a macroscale. However, the alignment with the external magnetic field cannot be measured directly; thus, to obtain this signal, a disturbance is required. This is where an essential component of the magnetic resonance system comes into play, known as the radiofrequency (RF) system, which primarily functions to emit RF pulses that can excite the imaging region.
After the application of the RF pulse, protons undergo two main changes. First, there is a transfer of energy, where some protons gain energy, moved from a lower energy state to a higher energy state, increasing the overall number of protons in the high-energy state. Second, due to the presence of the RF pulse, the precession of protons becomes synchronized, resulting in them being aligned with the same phase. This means that the axis of rotation, previously uniformly distributed, now converges to form a collective state akin to “one proton” undergoing precession, which is the essence of nuclear magnetic resonance (NMR).
At this point, the processing protons possess components in both the vertical and horizontal planes. If the RF field power is further increased, the angle of precession will continue to grow while the angular frequency remains constant. When the RF pulse is at 90°, all protons will be in the horizontal plane, processing without any component in the vertical direction. Subsequently, when the RF pulse is no longer turned on, the states of all protons will inevitably return to their original state, resulting in a net magnetization vector that transitions “from none to some” in the (Y-axis) direction and “from some to none” in the horizontal (X-axis) direction. This process is referred to as “relaxation.”
Relaxation is divided into transverse (T2) relaxation, which is also known as spin-spin relaxation and longitudinal (T1) relaxation which is also known as spin-lattice relaxation [5]. The former presents a decaying signal of magnetization vector in the horizontal direction, with the time taken for the signal to decay from its maximum to 37% being termed the transverse relaxation time (T2). In contrast, the latter exhibits an enhanced signal of magnetization vector in the vertical direction, takingthe time for the signal to recover from 0 to its initial state at 63% being termed the longitudinal relaxation time (T1) [3]. Under normal conditions, magnetic moments within a substance cancel each other out, resulting in no observable macroscopic magnetization. However, the application of an external magnetic field in figure 1 [3].
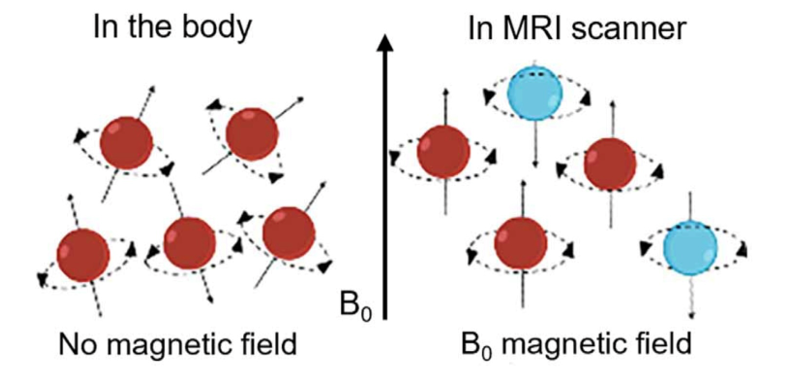
Figure 1: Proton alignment with or without a magnetic field [3]
Aligns the spins of protons or neutrons, inducing a processional motion of their spin axes. This alignment can be disrupted by applying a radiofrequency pulse that matches the precession frequency accurately, thus achieving resonance—a crucial step in magnetic resonance.
Each type of tissue exhibits unique T1 and T2 values, reflective of its specific chemical environment. In addition to T1 and T2, proton density—defined as the quantity of hydrogen protons per unit volume—is also a critical parameter integral to MRI. By manipulating the excitation and echo times in an MRI sequence, imaging can be weighted to emphasize T1, T2, or proton density characteristics, leading to distinct imaging modalities identified as T1-weighted, T2-weighted, and proton density-weighted imaging.
\( S∝N(H)*(1-{e^{-TR/{T_{1}}}})*{e^{-TE/{T_{2}}}} \) (1)
In this formula, N(H) represents the proton density, TR is the repetition time, and TE is the echo time, both of which can be set during the experiment. As can be seen from the formula, the contributions of the signal TI, T2, or proton density to the total signal S can be determined by adjusting the values of TR and TE. This is commonly referred to as T1T2-weighted, or proton density-weighted imaging. The fundamental contrast in nuclear magnetic resonance images arises from the fact that different tissues in biological organisms have varying T1 values, T2 values, and proton densities [3].
Magnetic resonance imaging (MRI) utilizes gradient magnetic fields for slice selection and spatial encoding. Under the influence of the gradient field, the proton spin frequencies differ across various layers in the direction of the gradient field. By applying radiofrequency pulses at specific frequencies, it is possible to excite protons in particular layers, allowing for tomographic imaging. During the signal acquisition process, the presence of the gradient field affects the frequency and signal phase of the protons rotating around the external magnetic field. By applying gradient fields in different directions, a correspondence between position and signal (frequency and phase) can be established. Finally, by using two-dimensional Fourier transformation, the original image can be reconstructed.
2.2. The contrast agents in MRI
T1 and T2 contrast agents can enhance the sensitivity of MRI in detecting changes in tissue anatomical structure and function, leading to more accurate disease diagnosis.
The relaxation rate is one of the most elementary properties of magnetic resonance contrast agents, representing the efficiency of the agent in enhancing the relaxation rate of water in tissues. The relaxation rate of water depends on the longitudinal relaxation time (T1) or the transverse relaxation time (T2). The degree to which contrast agents shorten the T1 or T2 of water is referred to as the longitudinal relaxation rate (r) and the transverse relaxation rate (r2), respectively. Contrast agents with a higher r1 value typically increase the signal, making MRI images brighter, while agents with a higher r2 value reduce the signal, resulting in darker MRI images [2].
The core mechanism of paramagnetic materials as T1 contrast agents lies in the interaction between protons in water molecules and the electrons of metal center ions, which serves as the primary mechanism for paramagnetic materials functioning as T1 contrast agents. In the classical model, this interaction is primarily categorized into the inner sphere mechanism, second sphere mechanism, and outer sphere mechanism. Water directly coordinated with the central metal ion which is inner sphere water, water interacting with groups on the complex, and water rapidly diffusing in the outer sphere waterfall help to enhancing longitudinal relaxation (r1) [2].
Currently, the inner sphere mechanism has been established to describe the relaxation processes of water molecules directly coordinated with the paramagnetic center and those interacting with groups on the complex. The primary mechanism for T1 relaxation enhancement in paramagnetic contrast agents is the inner sphere mechanism, described by the Solomon-Bloembergen-Morgan (SBM) theory, represented by equations.
\( \begin{array}{c} r_{1}^{IS}=\frac{{P_{M}}}{c}\frac{q}{{T_{1M}}+{τ_{M}}} \\ {T_{1M}}={\lbrace \frac{2}{15}\frac{{γ^{2}}{g^{2}}S(S+1)μ_{B}^{2}}{r_{M-H}^{6}}[\frac{3{τ_{C1}}}{1+ω_{H}^{2}τ_{C1}^{2}}+\frac{7{τ_{C2}}}{1+ω_{S}^{2}τ_{C2}^{2}}]\rbrace ^{-1}} \\ {τ_{Ci}}={(\frac{1}{{τ_{R}}}+\frac{1}{{T_{ie}}}+\frac{1}{{τ_{M}}})^{-1}}(i=1,2) \end{array} \) (2)
In these equations, c represents the concentration of metal ions (mM), PM is the molar fraction of metal ions, q is the number of coordinated water molecules in the inner sphere, zM is the residence lifetime of bound water, and TIM is the relaxation time of bound water. When the magnetic field strength exceeds 20 MHz, the values of TIM can be obtained from equations. In equation, γ refers to the gyromagnetic ratio of hydrogen protons, g is the g-factor of electrons, µB is the Bohr magneton, S is the spin quantum number, r-H is the distance between the protons and the metal ions, τci is the correlation time (i=1,2), and ωH is the Larmor precession frequency, whereas ωS is the angular electron frequency. In equation, T1e is the electron relaxation time, and TR is the rotational correlation time of the complex [5].
Thus, based on the classical SBM theory, the common parameters used to enhance the relaxation performance of molecular T1 contrast agents include three main factors: g, TR, and aw. Specific strategies may involve anchoring magnetic metal ions onto macromolecules to achieve slower molecular tumbling, thereby significantly extending TR and enhancing proton relaxation, which is considered the most effective method for increasing the relaxation rates of contrast agents. The term tM represents the reciprocal of the exchange rate of coordinated water molecules, where rapid water exchange is favorable for proton relaxation. Therefore, increasing the hydrophilicity of the materials and shortening the exchange time of coordinated water molecules is also a common and effective strategy.
Most clinically relevant molecular contrast agents sacrifice the physiological stability of paramagnetic metal complexes to achieve a q value of 1 [2]. On the other hand, certain rules should be followed when selecting ions for constructing contrast agents. Essentially, a metal ion has both electronic orbital motion and electronic spin motion, and the speed of electronic orbital motion is significantly faster than that of water proton relaxation, which means only the magnetic moment generated by the electronic spin motion of the ions can effectively influence the longitudinal relaxation of water protons. The greater the contribution of the electronic spin angular momentum (S) of adjacent ions to the total electronic angular momentum (J), the higher the r value will be. If J consists solely of electronic orbital angular momentum (L), then r will be very small. The relaxation rate T2 of a contrast agent is typically proportional to the square of the ion's effective magnetic moment.
2.3. Advantages and Limitations of MRI
MRI has become a useful tool in clinical medicine for diagnosing a variety of diseases. One of its most significant advantages is that it does not involve ionizing radiation harmful to patients, making it a safer option for imaging compared to other modalities such as computed tomography (CT). MRI is non-invasive and poses minimal side effects, allowing it to be used frequently without the health risks associated with radiation exposure.
A standout feature of MRI is its capability for multiparametric imaging. This versatility ensures that pathological conditions are detectable through various imaging methodologies without leaving any structures concealed. By employing T1-weighted or T2-weighted imaging, MRI can distinguish between tissues based on their unique relaxation times. Since pathological changes within human tissues often alter T1 and T2 values, these imaging techniques can serve as effective tools for identifying lesions. Moreover, MRI can also utilize proton density-weighted imaging to further enhance diagnostic capabilities.
The technology allows for various imaging techniques based on chemical shifts, diffusion-weighted imaging, as well as functional MRI (fMRI). Diffusion-weighted imaging, for instance, is influenced by molecular thermal motion, which impacts the magnetic resonance signals. This aspect is particularly relevant in detecting lesions as the surrounding environment of these pathological areas often changes.
When compared to other imaging techniques, especially CT, MRI excels in visualizing soft tissues with high resolution. It can clearly differentiate between various types of soft tissue structures, such as muscles, tendons, and fat, while avoiding bone artifacts that can obscure images produced by CT. This makes MRI particularly valuable in diagnosing neurological diseases, where clear distinction between gray and white matter is crucial. Additionally, the ability to depict blood flow dynamics through techniques like “flow void effect” or “inflow enhancement” allows for enhanced visualization of cardiac structures and blood vessels without the need for contrast agents, thus avoiding potential discomfort and risks associated with intravenous injection.
Functional MRI expands upon the capabilities of traditional MRI by enabling real-time monitoring of brain activity. Since its inception in 1991, when Belliveau and colleagues utilized variations in blood volume to produce functional images, fMRI has opened a new realm of research into cerebral metabolism and physiology [1]. It can detect neural activation through changes in blood oxygen levels, providing insights into brain functions by analyzing regions of local metabolic changes.
Despite its advantages, MRI has some drawbacks. One notable drawback is its relatively long imaging times compared to CT scans, which results in poorer temporal resolution. This can contribute to higher operational costs and increases the sensitivity of the procedure to patient movement, potentially compromising image quality. Furthermore, because the MRI signal is derived primarily from water protons in the body, MRI is less effective in identifying calcified lesions and hollow organs. For instance, lung imaging presents challenges due to the low water content and air-filled spaces within the lungs, which can render conventional MRI approaches less effective for assessing lung structure or function.
Other constraints of MRI include potential artifacts that can affect image clarity, the loud noise produced during scanning, and the inappropriateness of MRI for patients with certain metal implants, which can interfere with the magnetic fields generated by the scanner.
3. MRI applications
3.1. Application of MRI in Non-Destructive Detection of Crop root and growth
One signifiant application of MRI is its use in the non-destructive detection of crop root systems. The three-dimensional architecture of crop roots is crucial for effective nutrient uptake, making it essential to study their configuration. Researchers have specifically investigated the root systems of three commonly cultivated crops—corn, soybeans, and eggplants—utilizing MRI technology for in-situ analysis without harming the plants. This innovative approach allows for the real-time observation of root structure while preserving its integrity, a stark contrast to traditional methods that often involve destructive techniques such as excavation and soil drilling. Internationally, Heeraman et al. conducted an in situ quantitative study of the roots of leguminous crops that grew for 14 days in PVC containers using X-CT technology, comparing the measurement results with actual data. Anders Kaestner et al. reconstructed root networks using X-CT data from alder roots that were 4 months old and grown in natural glacial till, and compared these reconstructions with actual measurement data of the roots. MacFall et al. studied 8-day-old soybean roots using MRI, obtaining relatively clear three-dimensional images [3]. In China, researchers conducted pioneering in situ visualization research on plant roots using multi-slice spiral X-CT equipment. However, the X-CT tomographic sequence images obtained contained significant noise from non-root materials, and there was no clear boundary between the gray level distribution of the roots and that of the surrounding medium; instead, they intersected and overlapped , leading to difficulties in subsequent processing [3]. Maize root tomography images (Layer 8) and 3D reconstruction image of maize root system is shown in Figure 2.
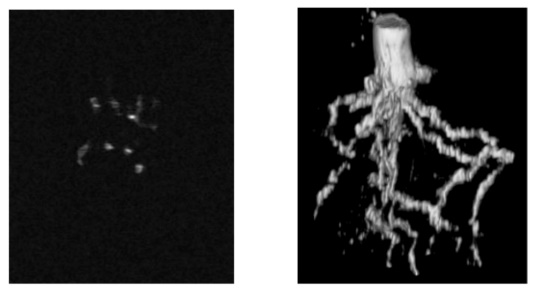
Figure 2: Maize root tomography and 3D reconstruction image of maize root system [4]
The application of MRI in this context resolves several challenges typically faced in the study of root systems. Conventional methods are not only invasive but also often lead to significant inaccuracies in data collection due to the physical disruption of the root systems. Traditional techniques include the excavation method, root chamber method, and isotope tracing. These methods have inherent limitations that can negatively influence plant growth and yield, thus complicating the understanding of root behavior in its natural environment. By employing MRI, researchers can quantitatively describe and analyze crop root system configurations without disturbing their natural state.
Researchers compared MRI imaging of soybean root systems grown in two types of soil: wet soil (i.e., soil volumetric water content of 40%) and dry soil (i.e., soil volumetric water content of 5%). The imaging results indicated that the soil volumetric water content largely great affects root imaging. The three-dimensional reconstruction results of the two samples are shown in Figure 2, clearly illustrating the differences between them. In the wet soil sample with higher volumetric water content, the main lateral roots of the soybean roots were able to form a complete and continuous root system image, as shown in Figure 2b. In contrast, the dry soil sample with lower volumetric water content failed to provide a complete root system image for the main lateral roots, resulting in a series of discontinuous breakpoints, as illustrated in Figure 2. However, the actual measurement results indicated that the diameters of the main lateral roots from both root system samples were very similar, suggesting that low volumetric water content in the root growth medium is detrimental to the nuclear magnetic resonance imaging of the root system [3]. Reconstructed images of soybean root in media with different moisture content is shown in Figure 3 [3].
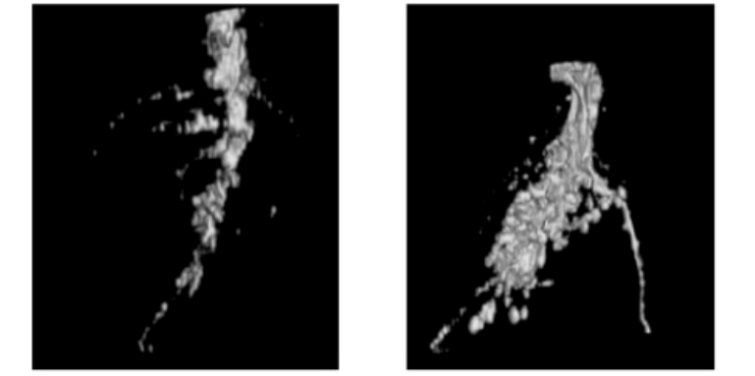
Figure 3: Volume moisture content 5% soil and Volume moisture content 40% soil [3]
Researches use two 3 cm long wheat leaf segments which exhibited significant background noise in their nuclear magnetic resonance (NMR) signals, whereas four segments of leaves demonstrated better signal-to-noise ratio (SNR) and analytical stability (as shown in Figure 2-2). In this study, four segments of leaves were selected for detection to enhance the SNR. Figure 2-3(a) displays the echo peak values of T2 relaxation decay for the leaves, stems, and grains of wheat variety 439. The initial signal intensity of the grains is the highest, followed by the leaves, and the stems have the lowest intensity. Given that the length of the leaf segments used for NMR detection is twice that of the stems, if we consider the signal values of wheat tissue organs per unit length, the signal values can be ranked from highest to lowest as follows: grains, stems, and leaves (with the height of the grain in the test tube taken into account.
3.2. Applications of MRI in Food Examination
3.2.1. MRI Non-destructive detection in fruit quality
Nuclear Magnetic Resonance (NMR) technology is based on the magnetic properties of atomic nuclei. NMR technology involves Nuclear Magnetic Resonance spectroscopy and Magnetic Resonance Imaging (MRI). The NMR spectroscopy technique analyzes the internal structure and properties of samples by measuring characteristic parameters of NMR spectral lines (such as line width, profile shape, area, and position), achieving extremely high resolution and accuracy while not damaging the structure and properties of the tested material. Based on the frequency of the radiofrequency field, NMR spectroscopy can also be categorized into two types: high-resolution NMR spectroscopy and low-resolution NMR spectroscopy. In recent years, there has been a significant amount of research utilizing NMR technology for non-destructive testing of agricultural products. MRI technology can be used for non-destructive testing of the internal quality, internal defects, surface defects, damage, ripeness, and quality changes of fruits.
Researchers employed MRI equipment to scan damaged Ya Pears, capturing coronal images of the fruits. They pre-processed the acquired images using MATLAB software and ultimately used corner detection methods to determine whether the pears were damaged [6]. The study concluded that MRI technology could achieve an accuracy of 92.1% in detecting fruit damage, with a recognition accuracy of 100% for identifying deformities.
Additionally, researchers utilized MRI technology to detect internal browning in Kuerle fragrant pears. They applied a feature extraction method based on browning characteristics, achieving detection accuracy rates of 83.3%, 82.6%, and 95.0% for the three different storage stages of the pears [6]. The results indicated the improvement in the accuracy of detecting browning in fragrant pears during the later storage stages.
Another team use MRI techniques are used in non-destructive detection in fruit. Nuclear magnetic resonance imaging technology and image processing techniques were used for non-destructive identification and classification of three different defects in pears: compression damage, drop damage, and internal browning [7]. Using medical nuclear magnetic resonance equipment, coronal T2-weighted images of the pears, including three types of pears, were collected. Through image transformation, image preprocessing, and feature extraction, the identification of compression damage and drop damage in Ya pears was achieved, along with the identification of internal browning in fragrant pears. Additionally, the stages of drop damage in pears and the severity of browning in fragrant pears were classified by statistically analyzing the Pearson correlation between the firmness of chrysanthemum pears and their NMR image texture coefficients.
3.2.2. MRI application in food safety
With the continuous innovation and improvement of NMR technology, this technique is widely applied in the analysis and detection of oils. Inspectors can effectively determine the liquid fat signals and solid fat signals in food samples using the direct NMR method. By performing computational processing and analysis, they can accurately obtain the solid fat content (SFC) of the samples [8]. Based on measurements taken at temperatures of 0°C and 10°C, food inspectors employed NMR technology to determine the SFC values of commonly used cooking oils, waste oil, and gutter oil. The results indicated that the SFC values of commonly consumed cooking oils were essentially 0, while the SFC values of waste oil and gutter oil were significantly higher. Using NMR technology to test cooking vegetable oil products, it is possible to successfully detect the presence of gutter oil if more than 1% is mixed into the vegetable oil product. Furthermore, the greater the quantity of waste oil mixed in, the higher the SFC value of the cooking oil product will be. Therefore, staff at food inspection institutions can scientifically utilize this characteristic of vegetable oil products to fully leverage the capabilities of NMR technology, clearly identifying any health and safety issues related to the adulteration of cooking oil products with waste oil [8].
In the detection of moisture content in food, the traditional Karl Fischer method is commonly employed by inspectors. However, this method is complex and cumbersome to operate, and it is destructive to solid samples, requiring inspectors to first crush the samples prior to testing. Another significant drawback is that traditional methods for measuring moisture content can only provide an average moisture level in the product. In reality, food is often a heterogeneous system, and moisture is not evenly distributed throughout. Modern inspectors, by scientifically applying nuclear magnetic resonance (NMR) and its imaging technology, can not only obtain comprehensive and accurate data on moisture content but also gain insights into the distribution, migration, and actual state of water molecules within food. The mobility and binding capacity of water molecules in food components can significantly affect the quality and stability of the food [9]. As a non-destructive testing and analysis technique, NMR is able to clearly and intuitively demonstrate the distribution, migration, and active state of moisture in food by measuring the relaxation time of hydrogen protons. This enables inspectors to clearly understand how moisture behaves in different types of food, allowing for a comprehensive assessment of food quality. Thus, NMR technology has found widespread application in the modern food quality testing sector, assisting food inspectors in analyzing and detecting the chemical and physical properties of food, thereby providing strong support for ensuring food safety and enhancing food quality.
In the testing of market dairy products, inspectors can apply nuclear magnetic resonance (NMR) technology in both quantitative and qualitative analysis. NMR technology can be effectively used to analyze the composition of fats in dairy fat, enabling inspectors to obtain relevant quantitative data. This primarily includes the mass ratio relationships of oleic acid, butyric acid, palm oil, and triglycerides, as well as the ability to scientifically and effectively determine the actual distribution of unsaturation at the sn-1 and sn-2 positions [10]. In the detection of active reactions in dairy products, inspectors can utilize NMR technology to successfully accomplish the scientific detection of microorganisms in dairy products without destroying the biological activity of the samples. First, inspectors need to place the dairy product microorganisms in an NMR detection tube for cultivation. Then, by conducting measurements of the sample spectra at different time intervals, they can effectively track the actual metabolic processes of the microorganisms in the detection tube, monitoring compounds clearly labeled with 13C. This approach enables the successful monitoring of the specific growth conditions of microorganisms within dairy products. Despite these benefits, there are challenges remain in the application in food examination, particularly concerning the detection of food doping and counterfeiting. The technology has some limitations in the sensitivity to identify isotope ratios so it becomes more difficult to detect trace contaminants or unknown ingredients in processed or imported foods [11]. For instance, the identification of adulterated oils or counterfeit products remains a significant hurdle. To overcome these challenges, it is important to establish a more comprehensive database based on magnetic resonance analysis which could help verify the provenance of food products, the duration of storage, and their quality attributes.
4. Improvement Potentials of MRI in medical imaging
4.1. Ultra-High Field MRI in Cardiac MRI(CMRI)
A pivotal improvement is evidenced in the implementation of Time-of-Flight Magnetic Resonance Angiography (TOF-MRA) at the ultra-high field. While 3.0 T systems exhibit limitations in visualizing detailed vascular structures, the Ultra-High Field MRI with 5.0 T and 7.0 T systems enhance the visibility of structures, leading to more precise clinical assessments. In comparative studies, the improvement in image quality at 5.0 T shows that it can distinctly delineate the location and morphology of arterial dissections, which are often obscured in lower-field systems.
Cardiac MRI (CMRI) can non-invasively and accurately assess myocardial tissue characteristics from different orientations and angles 6 [12]. It has advantages such as high soft tissue contrast and high level of details, as well as a large scanning field, making it suitable for examining cardiac structures, diagnosing heart diseases, and evaluating and monitoring cardiac function. Conventional 1.5T and 3.0T CMRI have been widely used in clinical practice; while emerging ultra-high-field MRI exhibits better resolution and signal-to-noise ratio (SNR) and shows finer anatomical structures more effectively [13]. However, its application in cardiac imaging may be constrained by issues such as unstable electrocardiographic signal triggering, inhomogeneities in the magnetic field, and radiofrequency power deposition [14]. Currently, 5.0T whole-body ultra-high-field MRI has achieved a better balance between imaging field strength and quality, and has been preliminarily applied to cardiac imaging [15].
4.2. Magnetic Field Inhomogeneity limitations
4.2.1. Causing Banding Artifacts
In ultra-high-field MRI, inhomogeneities which is in the main magnetic field (B0 field) and radiofrequency field (B1 field) can result in uneven image brightness and produce magnetic susceptibility artifacts, which limit its applications [4].
The inhomogeneity of the B0 field is primarily caused by defects in the magnet itself, differences in magnetic susceptibility among various human tissues, and the interactions between the magnet and the body. The degree of inhomogeneity increases with stronger field strengths, making imaging of complex structures such as the heart more challenging. Local magnetic field inhomogeneities can lead to phase mismatches, resulting in banding artifacts, particularly prominent in high-contrast sequences such as bSSFP and fast gradient echo sequences, as well as in regions near the lungs or diaphragm where there are significant differences in magnetic susceptibility. Due to artifacts, Figure 4 shows unable to distinguish the myocardial tissue of the diaphragm and unable to distinguish the outline of the heart.
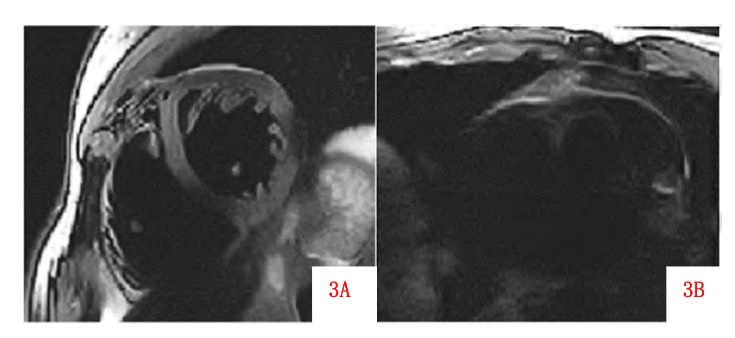
Figure 4: Image in diaphragm and hears image
B1 field inhomogeneity is primarily induced by short-wave radiofrequency effects. The wavelength of the radiofrequency is inversely proportional to the field strength; as the magnetic field strength increases, the wavelength of the radiofrequency waves decreases. In a 5.0T MR system, the radiofrequency wavelength is 16 cm, significantly lower than that of 1.5T (52 cm) or 3.0T (26 cm) and smaller than the width of the human body. This results in uneven distribution of the B1 field within the subject, limiting large field-of-view imaging. Additionally, standing wave effects can prevent effective excitation of myocardial tissue, leading to signal loss which is most commonly seen in FSE sequences and the generation of banding artifacts which is most commonly seen in GRE sequences.
4.2.2. High SAR Due to Radiofrequency Power Deposition
The safety issues of ultra-high-field MRI applications is of utmost importance for patients. High specific absorption rate (SAR) indicates that human tissues absorb more radiofrequency energy, which is converted into heat, potentially leading to localized or overall temperature increases within the body, causing discomfort or even tissue damage. As the field strength increases, radiofrequency power deposition also increases, resulting in the risk of localized overheating in the body; therefore, it is crucial to check to SAR levels. However, accurately monitoring and adjusting SAR is relatively complex.
4.2.3. Imaging Optimization for Ultra-High Field MRI
The domestically produced 5.0T whole-body ultra-high-field MR equipment has been preliminarily used for cardiac imaging. By incorporating artificial intelligence (AI) technology and optimizing imaging sequences, imaging quality and safety can be improved, ultimately resulting in higher resolution and SNR for CMRI [16].
The introduction of AI technology can improve the uniformity of the B0 field, which helps enhance imaging quality and reduce scan time [16]. By utilizing AI technology, it is possible to mitigate the interference of magnetic fluid effects on electrocardiogram waveforms, thereby reducing imaging limitations [17]. The combination of CMRI and AI has the potential to simplify imaging workflows, increase imaging speed, and shorten acquisition times without compromising image quality, all while improving magnetic field uniformity [18]. AI-based general uniformity tools can accurately segment cardiac regions and specifically enhance magnetic field uniformity. AI technology can accurately segment cardiac structures, and this advantage can also be applied to B1 uniform fields. Combined with parallel transmission techniques and artificial intelligence, B1 field inhomogeneity which involves simultaneously transmitting radiofrequency pulses using multiple independently controlled RF coils to enhance imaging performance [19]. Compared to traditional volume uniformity methods, AI-based uniformity results in images with greater uniformity as shown in Figure 5.
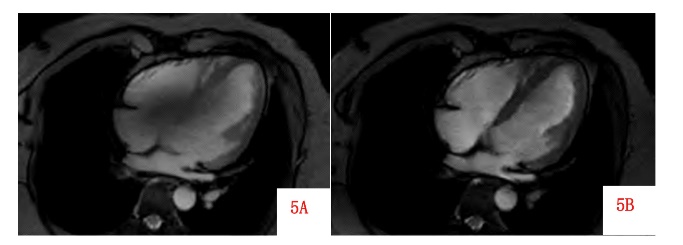
Figure 5: AI-based uniformity results in images
The 5.0T CMR can provide higher signal strength and resolution; however, the prolongation of T1 relaxation time and shortening of T2 relaxation time presents challenges [20]. The former can lead to insufficient magnetization vector recovery in modified Look-Locker inversion recovery (MOLLI) [21] sequences, significantly underestimating T1 values, while the latter can decrease the SNR of T2 quantitative sequence images. Both factors can contribute to reduced image quality and contrast. Nevertheless, the aforementioned characteristics of 5.0T CMR are not entirely disadvantages; the increased T1 time can reveal more information in T1 quantitative imaging. Utilizing special imaging sequences and post-processing techniques, such as Look-Locker techniques with continuous GRE acquisition, or combining readout corrections and other methods, could further enhance the quantitative imaging performance of 5.0T CMR [22].
5. Conclusion
In summary, Magnetic Resonance Imaging (MRI) is an essential diagnostic tool that rests on the profound principles of magnetic resonance, providing unparalleled insights into the structural and functional aspects of biological tissues. The advantages of MRI, notably its non-invasive nature and absence of ionizing radiation, position it as a safer alternative compared to conventional imaging techniques like computed tomography (CT). As highlighted in this paper, MRI not only excels in medical diagnostics, but also finds innovative applications in agricultural research by enabling the non-destructive analysis of crop root systems and food examination. The integration of MRI into the food safety sector illustrates its growing versatility, as it enhances the detection of quality parameters and contaminants, ultimately contributing to public health. The advancements in Ultra-High Field MRI technology have further elevated its diagnostic capabilities, particularly in cardiac imaging, where high-resolution and rapid image acquisition are paramount for effective patient care.
As it looks forward, it is clear that the synergy between MRI technology and AI will amplify the effectiveness of this imaging modality across diverse fields, from healthcare to agricultural science and food safety. MRI's ongoing evolution is driven by technological advancements and the integration of AI, underscores its significance not only as a vital imaging technique but also as an innovative tool poised to address the complexities of modern challenges in diagnostics and beyond. As researchers and practitioners continue to explore its capabilities, making endeavors in improving imaging quality, MRI is likely to play an increasingly pivotal role in enhancing our understanding of health, nutrition, and safety issues in the future
References
[1]. Ruan Weiwei. Research on the application of hyperpolarized 129Xe diffusion MRI in visualizing COPD. University of Chinese Academy of Sciences, 2017.
[2]. Zhou Z, Bai R, Munasinghe J, et al. Ti-Tz Dual-Modal Magnetic Resonance Imaging: From Molecular Basis to Contrast Agents. ACS Nano, 2017, 11: 5227-5232.
[3]. Zhang Jianfeng, Wu Di, Gong Xiangyang, et al. Non-destructive detection of plant roots based on magnetic resonance imaging technology. Transactions of the Chinese Society of Agricultural Engineering (Transactions of the CSAE), 2012, 28(8): 181-185.
[4]. Barisano G, Sepehrband F, Ma S, et al. Clinical 7 T MRI: Are we there yet? A review about magnetic resonance imaging at ultra-high field. British Journal of Radiology, 2019, 92(1094): 20180492.
[5]. Foster D, Larsen J. Polymeric Metal Contrast Agents for Ti-Weighted Magnetic Resonance Imaging of the Brain. ACS Biomaterials Science & Engineering, 2023, 9: 1224-1242.
[6]. Zhou Shuiqin. Research on nondestructive detection methods for pear quality based on magnetic resonance imaging. Zhejiang University, 2013.
[7]. Yin Yong, Chu Taotao, Zhang Hong. Progress in non-destructive detection techniques for fruit quality. Guangxi Agricultural Mechanization, 2021(05): 31-33.
[8]. Du Guangyuan. Research on the dynamic distribution of water and sugar in wheat during the filling stage using nuclear magnetic resonance. Northwest A&F University, 2013.
[9]. Feng Cuiping, Liu Yinuo, Xu Ziwei, et al. Determination of glucose, fructose, and sucrose in fruit juice by 1H-NMR. Food and Fermentation Industries, 2022, 48(10): 226-233.
[10]. Qi Yinxia, Cheng Jian, Wang Qin. Applications of nuclear magnetic resonance technology in food testing. Food and Machinery, 2012(06): 117-120.
[11]. Wang Xiaohua, et al. Applications of magnetic resonance technology in food quality and safety research. Journal of Spectroscopy, 2017, 34(02): 245-256.
[12]. Holtackers R J, Wildberger J E, Winterspreger B J, et al. Impact of field strength in clinical cardiac magnetic resonance imaging. Investigative Radiology, 2021, 56(11): 764-772.
[13]. Leiner T, Bogaert J, Friedrich M G, et al. SCMR Position Paper (2020) on clinical indications for cardiovascular magnetic resonance. Journal of Cardiovascular Magnetic Resonance, 2020, 22(1): 76.
[14]. Guo R, Weingärtner S, Šiurytė P, et al. Emerging techniques in cardiac magnetic resonance imaging. Journal of Magnetic Resonance Imaging, 2022, 55(4): 1043-1059.
[15]. Demirkiran A, Everaars H, Amier R P, et al. Cardiovascular magnetic resonance techniques for tissue characterization after acute myocardial injury. European Heart Journal - Cardiovascular Imaging, 2019, 20(7): 723-734.
[16]. Ladd M E, Bachert P, Meyerspeer M, et al. Pros and cons of ultra-high-field MRI/MRS for human application. Progress in Nuclear Magnetic Resonance Spectroscopy, 2018, 109: 1-50.
[17]. Lott J, Platt T, Niesporek S C, et al. Corrections of myocardial tissue sodium concentration measurements in human cardiac 23Na MRI at 7 Tesla. Magnetic Resonance in Medicine, 2019, 82(1): 159-173.
[18]. Reiter T, Lohr D, Hock M, et al. On the way to routine cardiac MRI at 7 Tesla: A pilot study on consecutive 84 examinations. PLoS One, 2021, 16(7): e0252797.
[19]. Lin L, Liu P, Sun G, et al. Bi-ventricular assessment with cardiovascular magnetic resonance at 5 Tesla: A pilot study. Frontiers in Cardiovascular Medicine, 2022, 9: 913707.
[20]. Curtis A D, Cheng H M. Primer and historical review on rapid cardiac CINE MRI. Journal of Magnetic Resonance Imaging, 2022, 55(2): 373-388.
[21]. Holtackers R J, Van de Heyning C M, Chiribiri A, et al. Dark-blood late gadolinium enhancement cardiovascular magnetic resonance for improved detection of subendocardial scar: A review of current techniques. Journal of Cardiovascular Magnetic Resonance, 2021, 23(1): 96.
[22]. Shao J, Nguyen K L, Natsuaki Y, et al. Instantaneous signal loss simulation (InSiL): An improved algorithm for myocardial T1 mapping using the MOLLI sequence. Journal of Magnetic Resonance Imaging, 2015, 41(3): 721-729.
Cite this article
Cheng,G. (2025). Research on Innovative Applications of MRI Technology in Medicine and Agriculture. Theoretical and Natural Science,80,7-19.
Data availability
The datasets used and/or analyzed during the current study will be available from the authors upon reasonable request.
Disclaimer/Publisher's Note
The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of EWA Publishing and/or the editor(s). EWA Publishing and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
About volume
Volume title: Proceedings of the 4th International Conference on Computing Innovation and Applied Physics
© 2024 by the author(s). Licensee EWA Publishing, Oxford, UK. This article is an open access article distributed under the terms and
conditions of the Creative Commons Attribution (CC BY) license. Authors who
publish this series agree to the following terms:
1. Authors retain copyright and grant the series right of first publication with the work simultaneously licensed under a Creative Commons
Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this
series.
2. Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the series's published
version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial
publication in this series.
3. Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and
during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See
Open access policy for details).
References
[1]. Ruan Weiwei. Research on the application of hyperpolarized 129Xe diffusion MRI in visualizing COPD. University of Chinese Academy of Sciences, 2017.
[2]. Zhou Z, Bai R, Munasinghe J, et al. Ti-Tz Dual-Modal Magnetic Resonance Imaging: From Molecular Basis to Contrast Agents. ACS Nano, 2017, 11: 5227-5232.
[3]. Zhang Jianfeng, Wu Di, Gong Xiangyang, et al. Non-destructive detection of plant roots based on magnetic resonance imaging technology. Transactions of the Chinese Society of Agricultural Engineering (Transactions of the CSAE), 2012, 28(8): 181-185.
[4]. Barisano G, Sepehrband F, Ma S, et al. Clinical 7 T MRI: Are we there yet? A review about magnetic resonance imaging at ultra-high field. British Journal of Radiology, 2019, 92(1094): 20180492.
[5]. Foster D, Larsen J. Polymeric Metal Contrast Agents for Ti-Weighted Magnetic Resonance Imaging of the Brain. ACS Biomaterials Science & Engineering, 2023, 9: 1224-1242.
[6]. Zhou Shuiqin. Research on nondestructive detection methods for pear quality based on magnetic resonance imaging. Zhejiang University, 2013.
[7]. Yin Yong, Chu Taotao, Zhang Hong. Progress in non-destructive detection techniques for fruit quality. Guangxi Agricultural Mechanization, 2021(05): 31-33.
[8]. Du Guangyuan. Research on the dynamic distribution of water and sugar in wheat during the filling stage using nuclear magnetic resonance. Northwest A&F University, 2013.
[9]. Feng Cuiping, Liu Yinuo, Xu Ziwei, et al. Determination of glucose, fructose, and sucrose in fruit juice by 1H-NMR. Food and Fermentation Industries, 2022, 48(10): 226-233.
[10]. Qi Yinxia, Cheng Jian, Wang Qin. Applications of nuclear magnetic resonance technology in food testing. Food and Machinery, 2012(06): 117-120.
[11]. Wang Xiaohua, et al. Applications of magnetic resonance technology in food quality and safety research. Journal of Spectroscopy, 2017, 34(02): 245-256.
[12]. Holtackers R J, Wildberger J E, Winterspreger B J, et al. Impact of field strength in clinical cardiac magnetic resonance imaging. Investigative Radiology, 2021, 56(11): 764-772.
[13]. Leiner T, Bogaert J, Friedrich M G, et al. SCMR Position Paper (2020) on clinical indications for cardiovascular magnetic resonance. Journal of Cardiovascular Magnetic Resonance, 2020, 22(1): 76.
[14]. Guo R, Weingärtner S, Šiurytė P, et al. Emerging techniques in cardiac magnetic resonance imaging. Journal of Magnetic Resonance Imaging, 2022, 55(4): 1043-1059.
[15]. Demirkiran A, Everaars H, Amier R P, et al. Cardiovascular magnetic resonance techniques for tissue characterization after acute myocardial injury. European Heart Journal - Cardiovascular Imaging, 2019, 20(7): 723-734.
[16]. Ladd M E, Bachert P, Meyerspeer M, et al. Pros and cons of ultra-high-field MRI/MRS for human application. Progress in Nuclear Magnetic Resonance Spectroscopy, 2018, 109: 1-50.
[17]. Lott J, Platt T, Niesporek S C, et al. Corrections of myocardial tissue sodium concentration measurements in human cardiac 23Na MRI at 7 Tesla. Magnetic Resonance in Medicine, 2019, 82(1): 159-173.
[18]. Reiter T, Lohr D, Hock M, et al. On the way to routine cardiac MRI at 7 Tesla: A pilot study on consecutive 84 examinations. PLoS One, 2021, 16(7): e0252797.
[19]. Lin L, Liu P, Sun G, et al. Bi-ventricular assessment with cardiovascular magnetic resonance at 5 Tesla: A pilot study. Frontiers in Cardiovascular Medicine, 2022, 9: 913707.
[20]. Curtis A D, Cheng H M. Primer and historical review on rapid cardiac CINE MRI. Journal of Magnetic Resonance Imaging, 2022, 55(2): 373-388.
[21]. Holtackers R J, Van de Heyning C M, Chiribiri A, et al. Dark-blood late gadolinium enhancement cardiovascular magnetic resonance for improved detection of subendocardial scar: A review of current techniques. Journal of Cardiovascular Magnetic Resonance, 2021, 23(1): 96.
[22]. Shao J, Nguyen K L, Natsuaki Y, et al. Instantaneous signal loss simulation (InSiL): An improved algorithm for myocardial T1 mapping using the MOLLI sequence. Journal of Magnetic Resonance Imaging, 2015, 41(3): 721-729.









