1. Introduction
Cancer is one of the leading causes of death worldwide. According to a report by the World Health Organization (WHO), the number of deaths caused by cancer has been increasing annually. China is currently undergoing a transitional phase in cancer, with both the mortality and incidence rates approaching those of developed countries [1]. Although traditional treatment methods, such as surgery, radiotherapy, and chemotherapy, have delayed tumor growth to some extent, their general shortcomings include a lack of selectivity, often leading to severe side effects and reduced quality of life for patients. In response to these issues, scientists are dedicated to developing more precise and efficient treatment strategies, with nanotechnology-based targeted drug delivery systems gradually becoming a research focus in the field of cancer treatment.
Nanomedicine-based targeted delivery systems are drug delivery technologies based on nanomaterials, with the core principle being the use of the properties of nanoparticles to achieve drug accumulation at tumor sites while reducing drug distribution in healthy tissues. This system relies on the Enhanced Permeation and Retention (EPR) effect, which allows drugs to enter cancer cells through the highly permeable blood vessels in the tumor region, while avoiding uptake by normal cells [2]. For example, carriers such as liposomes [3] and polysaccharide nanoparticles not only improve the solubility and stability of drugs but also achieve active targeted delivery through surface modification [4].
In recent years, with the deepening research into the cancer microenvironment, environment-responsive nanotechnology systems have become a hot topic of study. These systems can respond to the local acidic environment of cancer cells, temperature changes, or external magnetic field stimuli, enabling directional drug release. For example, research by Li Kunzhao and others has shown that gold nanoparticles (AuNPs) can be used not only for photodynamic therapy but also for triggering drug release through external light exposure, thus reducing damage to healthy tissues [5]. However, most existing research focuses on single-response systems, and the development of multi-responsive systems remains to be explored.
The complexity of cancer necessitates a combined drug treatment strategy, which also places higher demands on the drug compatibility and toxic side effects of nanotechnology systems. Current research lacks sufficient understanding of the long-term stability of multi-drug delivery systems and the potential side effects of mixing drugs. This paper aims to summarize the recent advancements in nanomedicine-based targeted drug delivery systems and explore their application in combined drug therapy. The focus will be on drug compatibility, stability after mixing, and toxic side effects, with the goal of addressing the shortcomings of current systems and improving the precision and effectiveness of cancer treatment. Furthermore, this paper also discusses the preparation processes of various nanocarriers and their applications in the treatment of multiple types of cancer, providing theoretical foundations and references for the development of more efficient targeted delivery systems.
2. Basic Concept of Liposomes
2.1. Definition and Composition.
Liposomes are spherical lipid vesicles composed of one or more lipid bilayers (with a typical diameter range of 50–500 nm). They result from the emulsification of natural or synthetic lipids in an aqueous medium (see Figure 1) [6, 7].
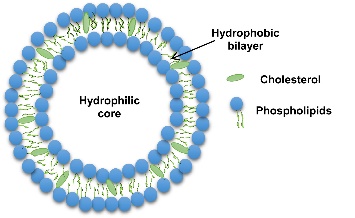
Figure 1: Schematic Diagram of Liposomes
2.2. Advantages of Liposomes in Anticancer Drug Delivery.
Liposomes are widely used as carriers in nanomedicine primarily due to their biocompatibility, stability, ease of synthesis, high drug-loading efficiency [8, 9], high bioavailability [10], and the safety of excipients used in these formulations [11].
Based on their structure, liposomes are categorized into four types according to the size and number of bilayers: small unilamellar vesicles (SUV), large unilamellar vesicles (LUV), multilamellar vesicles (MLV), and multivesicular vesicles (MVV). Liposomes have a single lipid bilayer in unilamellar structures, whereas they possess an onion-like structure in multilamellar forms. MVVs form multilayer arrangements with concentric phospholipid spheres, as many unilamellar vesicles are generated within larger liposomes [12]. The encapsulation efficiency of liposomes increases with the size of the liposome, whereas for hydrophilic compounds, the encapsulation efficiency decreases as the number of bilayers increases [13]. The size of the vesicle is a key factor in controlling the circulation half-life of liposomes. Both the size and the number of bilayers affect the amount of encapsulated drug.
3. Research Progress on Liposomes
3.1. Development of Novel Liposome Materials.
Liposomes can be categorized into conventional liposomes, charged liposomes, stealth stable liposomes, active-targeting liposomes, stimulus-responsive liposomes, and bubble liposomes, depending on their composition and application. In recent years, scientists have also developed novel liposomes for clinical use, including long-circulating liposomes, precursor liposomes, and polymeric membrane liposomes [14].
3.2. Liposome Surface Modification and Functionalization.
Common strategies for liposome surface modification are divided into active-targeting carriers and passive-targeting formulations. Active-targeting carriers functionalize the surface of liposomes with corresponding ligands based on tumor-specific markers, including peptides, antibodies, aptamers, small molecules, and other types of liposomes. In contrast, passive-targeting formulations are represented by traditional liposomes and long-circulating liposomes [15].
4. Nanoparticle Delivery Systems
4.1. Classification of Nanoparticles
Nanoparticle delivery systems can be divided into inorganic nanoparticles and organic nanoparticles. Inorganic nanoparticles include gold nanoparticles and mesoporous silica nanoparticles, while organic nanoparticles include protein nanoparticles, polysaccharide nanoparticles, and composite nanoparticles.
1) Gold Nanoparticles (AuNPs) (see Figure 2): AuNPs can be modified with different chemical groups due to their unique surface properties, allowing for targeted release in tumor tissues. Currently, scientists have developed spherical, shell-like, star-shaped, cage-like, flower-like, and linear gold nanoparticles. Compared to traditional anticancer drug carriers, AuNPs offer advantages such as high stability, high permeability, improved drug solubility, and targeted drug delivery. These can be applied in clinical chemotherapy, photothermal therapy, photodynamic therapy, immunotherapy, and gene therapy [5].

Figure 2: TEM Image of Gold Nanoparticles
2) Mesoporous Silica Nanoparticles (MSNs): MSNs possess a unique mesoporous structure, good biocompatibility, non-toxicity, high drug-loading capacity, and can control drug release for targeted delivery. Therefore, they have gained significant attention from scholars in the biomedical field in recent years. MSNs can be functionalized with targeting peptides, stimulus-responsive peptides, or multifunctional chimeric peptides (see Figure 3), enabling their application in immunotherapy and other clinical scenarios. In addition, researchers have functionalized MSNs with antibodies or nucleic acid aptamers for tumor-targeted therapy [16].
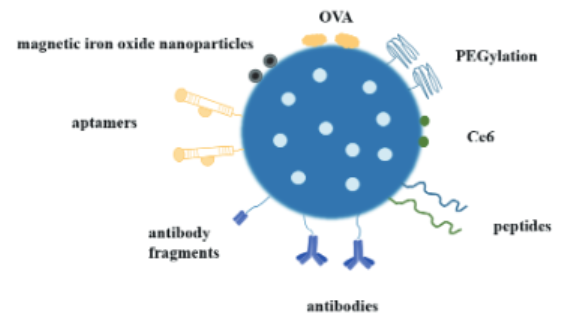
Figure 3: Molecular Modification of Mesoporous Silica for Nanodelivery Systems
3) Protein Nanoparticles (PNP): PNPs exhibit high biocompatibility, low immunogenicity, multifunctionality, and degradability. In the field of tumor drug delivery, protein nanoparticles can form complexes with drugs, targeting tumor sites, extending the drug’s circulation time in the body, improving drug development properties, reducing toxicity, and enhancing solubility [17].
4) Polysaccharide Nanoparticles: Polysaccharide nanoparticles have low toxicity and high safety. They can enhance the body’s immune response and increase the bioavailability of anticancer drugs, promote tumor cell apoptosis, and reduce the damage of anticancer drugs to normal cells. Natural polysaccharides play roles in reducing drug toxicity, regulating the tumor microenvironment, improving drug targeting, enhancing immune modulation for better therapeutic effects, and serving as drug delivery carriers [4].
5) Solid Lipid Nanoparticles: Solid lipid nanoparticles are an emerging protein delivery nanotechnology with good biodegradability and biocompatibility. Compared to liposomes, solid lipid nanoparticles are more stable for biological applications [18].
4.2. Preparation and Application of Nanoparticles
Regarding the preparation and application of nanoparticles, the mainstream methods include solvent precipitation, pH-shift methods, and solvent evaporation.
Solvent Precipitation is a commonly used technique for preparing nanoparticles. Its principle is based on the differences in distribution coefficients of substances between the solvent and the antisolvent. By adding an insoluble or slightly soluble liquid (antisolvent) to the solution of the target substance, the solubility of the solute in the solution is altered, leading to supersaturation and the precipitation of the solute to form nanoparticles or precipitates [19]. For example, Yin et al. used this method to prepare zein nanoparticles [20].
pH-Shift Method involves adjusting the pH of the reaction system to alter the ionic or molecular state of substances in the solution, thereby influencing the nucleation and growth of nanoparticles. Changes in pH can affect the solubility of reactants, reaction rates, and the stability of products, thus controlling the morphology and size of the nanoparticles.
Solvent Evaporation involves evaporating a solution containing a dissolved solute. As the solvent gradually evaporates, the concentration of the solute increases. When it reaches a supersaturated state, the solute molecules aggregate, nucleate, and grow into nanoparticles. During this process, the size, morphology, and dispersion of the nanoparticles can be controlled by adjusting the evaporation conditions. For example, Wang Weiguang et al. used the solvent evaporation method to prepare novel nanoparticles loaded with disulfiram [21].
5. Nanoemulsion Delivery System
5.1. Basic Concept of Nanoemulsion
Nanoemulsion is a type of kinetically stable colloidal system formed by two immiscible liquids, in which spherical droplets of one liquid are surrounded by another liquid, forming a continuous phase. The average particle size of nanoemulsions is less than 200 nm, and based on their stability and droplet size, nanoemulsions differ from traditional emulsions and microemulsions, among other types of emulsions [22]. Nanoemulsions can be classified into oil-in-water (O/W), water-in-oil (W/O), and double continuous phase (W/O/W or O/W/O) nanoemulsions [23], with the dispersing phase used as the distinguishing factor.
Compared with traditional emulsions, nanoemulsions have advantages such as kinetic stability [24], optical transparency [25], stability against gravity separation, high chemical reactivity, and a larger emulsifying layer [26]. When used as a drug delivery system, nanoemulsions also offer functions such as increasing the bioavailability of drug molecules [27], enhancing drug stability [28], and improving the solubility of poorly soluble drugs [29].
5.2. Preparation Methods of Nanoemulsions
The preparation methods for nanoemulsions are generally categorized into high-energy emulsification methods and low-energy emulsification methods based on the energy input used. High-energy emulsification methods include high-speed shear stirring and high-pressure homogenization, while low-energy emulsification methods include spontaneous emulsification and phase transition component methods [30].
1) High-speed shear stirring is a commonly used high-energy physical method for preparing nanoemulsions. A high-speed rotating stirrer generates a strong shear force in the liquid, allowing the oil phase and water phase to mix thoroughly, ultimately forming a nano-sized oil droplet dispersion system [31]. This method is simple to operate, fast, and pollution-free, making it suitable for small-scale production of nanoemulsions.
2) High-pressure homogenization is a commonly used high-energy technique for preparing nanoemulsions. It utilizes high pressure to force the initial emulsion through a narrow nozzle, generating intense shear force, collisions, and vortex effects, dispersing larger oil droplets into the nanoscale to form a stable nanoemulsion. This method offers advantages such as ease of operation, small particle size of the resulting sample, and good stability [32].
3) Spontaneous emulsification does not require external mechanical energy input; it relies on the physicochemical properties of the system components, especially the self-assembly and diffusion behavior of surfactants, to form a stable nanoemulsion. This method is relatively simple to use in preparing nanoemulsions, consumes less energy, and is particularly suitable for applications that require gentle preparation conditions [33]. It has advantages such as simple processing, mild conditions, and improved loading capacity.
4) The phase transition component method induces phase transitions between the oil phase and water phase by adjusting the ratios of the oil phase, water phase, and surfactant in the system, thereby forming a stable nanoemulsion. This method is suitable for a variety of systems, energy-efficient, and mild in condition [34]. For example, Zheng Xiaoyang [34] et al. used nanoemulsion technology to prepare sunscreen agents.
6. Targeted Delivery Mechanism of Nano Delivery Systems
6.1. Passive Targeted Delivery
Passive targeted delivery refers to the accumulation and retention of drugs in specific tissues through the physicochemical properties of nanoparticles (such as particle size, shape, and surface characteristics) and the unique physiological and pathological features of the target site (such as increased vascular permeability and imperfect lymphatic drainage), without relying on molecular-level targeting recognition mechanisms [35]. In cancer treatment, passive targeted delivery can enhance the therapeutic efficiency of drugs and reduce toxicity to normal tissues. Currently, passive targeted delivery has become a relatively mature method applied in research. Rajan et al. [36] used this technology to target curcumin delivery to the human colon to inhibit cancer cell proliferation; Zhang et al. [37] utilized chitosan nanoparticles for passive targeted delivery of curcumin to human colorectal cancer cells to suppress cell proliferation.
6.2. Active Targeted Delivery
Active targeted delivery refers to the modification of nanoparticles’ surfaces with specific targeting molecules (such as antibodies, ligands, peptides, sugars, or small molecule drugs), enabling them to bind to specific receptors or antigens on the surface of target cells or tissues, thereby achieving precise drug delivery. This method utilizes the specificity of molecular recognition mechanisms, allowing nanoparticles to actively locate and selectively accumulate in cancerous cells [38]. Active targeted delivery can significantly increase the drug’s concentration at the target site, enhance therapeutic effects, and reduce toxicity to normal tissues. Therefore, active targeted delivery has become one of the research hotspots in the field of drug delivery in recent years. Wang et al. [39] used hyaluronic acid-modified ribonuclease A (RNase A) to create nanoparticles that significantly increased the uptake of RNase A by lung cancer cells.
6.3. Environment-Responsive Targeted Delivery
Environment-responsive targeted delivery refers to the design of nanoparticles that can respond to specific pathological or physiological microenvironments (such as acidity, redox state, enzyme activity, temperature, or external stimuli like light, electromagnetic fields, etc.), triggering drug release or altering their distribution behavior in the body. This targeted delivery method utilizes the differences in the microenvironments of cancerous regions and normal tissues, enabling the nanoparticles to release drugs at specific sites and reduce toxicity to normal tissues [40]. Fang Xueyang et al. [41] designed multifunctional targeted nano-selenium particles, using environment-responsive targeted delivery to transport baicalin to liver cancer cells for treatment.
7. Biocompatibility of Nano Delivery Systems
The potential of nano delivery systems in drug delivery has attracted extensive attention, but their biocompatibility remains a major obstacle to clinical application. The toxic side effects of nanocarriers and the metabolic and clearance pathways of nano drugs are core considerations in ensuring their safety.
7.1. Toxic Side Effects of Nanocarriers
Currently, the toxic side effects of nanocarriers are still a key issue in evaluating their biocompatibility. The chemical composition, surface properties, size, and shape of nanomaterials can cause cytotoxicity or inflammatory responses. Studies have shown that certain metal nanoparticles, such as silver nanoparticles, generate reactive oxygen species (ROS), which can lead to cell damage and DNA mutations [42]. Even organic nanomaterials can induce toxic reactions. For instance, Lee et al. found in their study on lipid nanoparticles (LNPs) used in mRNA vaccine delivery that, although LNPs have good biocompatibility, the polyethylene glycol (PEG) portion of their surface modification could trigger rare immune allergic reactions [43].
7.2. Metabolism and Clearance of Nano Drugs
The metabolism and clearance of nano drugs are another important aspect of ensuring their biocompatibility. Some nanocarriers, especially non-degradable or poorly degradable inorganic materials, may persist in the body for long periods, leading to toxic accumulation. For example, Khlebtsov et al. found that gold nanoparticles, due to their chemical stability, are difficult to clear through biological pathways, making the design of degradable or surface-modified nanocarriers that facilitate clearance an urgent challenge [44]. In contrast, degradable polymer nanoparticles (such as PLGA) have shown better safety. These carriers can gradually degrade in the body into non-toxic metabolites, which are then eliminated through normal metabolic pathways [45]. However, how to maintain the stability of nanoparticles in different biological environments while ensuring their rapid metabolism and clearance after completing drug delivery remains a key direction for future research.
8. Applications and Challenges of Nano Delivery Systems
Nano delivery systems have shown great potential in modern medicine, particularly in areas such as cancer treatment, gene therapy, and vaccine development. However, with the deepening of their applications, nano delivery systems also face many challenges.
8.1. Applications of Nano Delivery Systems
Due to their unique physical and chemical properties, such as high surface area, good biocompatibility, and ease of surface functionalization, nano delivery systems have become an ideal platform for drug delivery. One of the most common applications is the delivery of anticancer drugs. Compared with traditional drug delivery systems, nanomaterials can precisely target tumor sites through the enhanced permeability and retention (EPR) effect, reducing the damage to healthy tissues and improving the targeted therapy and bioavailability of the drug [46]. In addition, the application of nano delivery systems in gene therapy is also increasing. By effectively delivering gene editing tools such as CRISPR/Cas9 to target cells, these systems are expected to play a major role in the treatment of genetic diseases [47].
Moreover, nano delivery systems hold great promise in vaccine development. Studies have shown that nanoparticles, as antigen carriers, can significantly improve the immunogenicity of vaccines. For example, Zhang et al. made significant progress in the development of a COVID-19 mRNA vaccine using nano delivery technology, providing technical support for the rapid market release of the vaccine [48]. In other medical applications, nano delivery systems have also shown great potential for precise drug release, such as in the delivery of anti-inflammatory drugs, antibiotics, and immunomodulatory drugs.
8.2. Challenges Facing Nano Delivery Systems
Despite the great potential of nano delivery systems, their clinical application still faces many challenges. Biocompatibility remains a primary concern for nano delivery systems. While many nanomaterials show good biocompatibility in vitro, their long-term toxicity in vivo has not been fully studied. Some nanoparticles may accumulate in the body, triggering immune responses or causing irreversible side effects [49]. Therefore, designing delivery carriers that can efficiently deliver drugs while avoiding toxicity remains an urgent challenge for researchers.
The stability and delivery efficiency of nano delivery systems in vivo also pose significant challenges. For example, Lee et al. found that in blood circulation, nanoparticles are prone to plasma protein adsorption, forming a protein corona, which affects their delivery efficiency and targeting ability [50]. To overcome this issue, researchers are developing various surface modification techniques, such as polyethylene glycol (PEG) modification, to reduce protein adsorption and extend the circulation time of nanoparticles in the body. However, these modifications may, to some extent, reduce the interaction between nanoparticles and target cells, thereby affecting the drug release efficacy.
9. Conclusion
Research on nano systems for targeted drug delivery has shown that the differences in structural design and surface modifications of various nano carriers determine their effectiveness and applications in drug delivery. Carriers such as liposomes and gold nanoparticles demonstrate excellent biocompatibility and drug encapsulation efficiency, and are widely used in cancer treatment. However, how to design environment-responsive delivery systems based on the tumor microenvironment remains an important focus of current research.
By designing nano systems that respond to the acidic environment, temperature, or light of tumors, precise drug release can be achieved, reducing damage to normal tissues. The application of nano systems in combination drug therapy has shown great potential, but the compatibility between drugs and the toxic side effects after mixing still remain unresolved issues. Stable nano systems must not only possess good biocompatibility but also ensure the metabolism and clearance of drugs within the body.
Future research needs to further explore how to optimize the structure and surface modification of nano carriers to improve their targeting ability and delivery efficiency. Additionally, combination therapy strategies targeting multiple cancers and viral infections are expected to become a research hotspot. In conclusion, the development of nano systems provides new pathways and directions for cancer treatment, and their application potential in precision medicine is enormous.
References
[1]. Cao, W., Chen, H. D., Yu, Y. W., et al. (2021). Changing profiles of cancer burden worldwide and in China: A secondary analysis of the global cancer statistics 2020. Chinese Medical Journal. Advance online publication. https://doi.org/10.1097/CM9.0000000000001474
[2]. Ong, S., Sandy, O., Long, et al. (2016). Influence of the encapsulation efficiency and size of liposome on the oral bioavailability of griseofulvin-loaded liposomes. Pharmaceutics, 8(3). https://doi.org/10.3390/pharmaceutics8030025
[3]. Noble, G. T., Stefanick, J. F., Ashley, J. D., et al. (2014). Ligand-targeted liposome design: Challenges and fundamental considerations. Trends in Biotechnology, 32(1), 32-45. https://doi.org/10.1016/j.tibtech.2013.09.007
[4]. Yang, R., He, Y., Lu, Z., et al. (2023). Progress in the study of natural polysaccharide-enhanced drug delivery systems for anti-tumor effects. Chinese Medicinal Materials, 46(3), 789-795.
[5]. Li, K., Liu, Y., Dai, Y., et al. (2022). Research progress on gold nanoparticles as anti-tumor drug carriers. Chinese Journal of Pharmaceutical Sciences, 4(004), 20.
[6]. Sun, T., Zhang, Y. S., Pang, B., et al. (2014). Engineered nanoparticles for drug delivery in cancer therapy. Angewandte Chemie International Edition. https://doi.org/10.1002/anie.201403036
[7]. Jha, S., Sheetal, K., et al. (2016). Liposomal drug delivery system for cancer therapy: Advancement and patents. Recent Patents on Drug Delivery & Formulation.
[8]. Noble, G. T., Stefanick, J. F., Ashley, J. D., et al. (2014). Ligand-targeted liposome design: Challenges and fundamental considerations. Trends in Biotechnology, 32(1), 32-45. https://doi.org/10.1016/j.tibtech.2013.09.007
[9]. Hafner, A., Lovrić, J., Perina Lakoš, G., et al. (2014). Nanotherapeutics in the EU: An overview on current state and future directions. International Journal of Nanomedicine, 9(1), 1005-1023. https://doi.org/10.2147/IJN.S55359
[10]. McClements, D. J., & Rao, J. (2011). Food-grade nanoemulsions: Formulation, fabrication, properties, performance, biological fate, and potential toxicity. Critical Reviews in Food Science & Nutrition, 51(4), 285-330. https://doi.org/10.1080/10408398.2011.559558
[11]. Chen, T., Gong, T., Zhao, T., et al. (2018). A comparison study between lycobetaine-loaded nanoemulsion and liposome using nRGD as a therapeutic adjuvant for lung cancer therapy. European Journal of Pharmaceutical Sciences. https://doi.org/10.1016/j.ejps.2017.09.041
[12]. Maja, L., Eljko, K., & Mateja, P. (2020). Sustainable technologies for liposome preparation. Journal of Supercritical Fluids, 165, 104984. https://doi.org/10.1016/j.supflu.2020.104984
[13]. Ong, S. G. M., Ming, L. C., Lee, K. S., et al. (2016). Influence of the encapsulation efficiency and size of liposome on the oral bioavailability of griseofulvin-loaded liposomes. Pharmaceutics, 8(3). https://doi.org/10.3390/pharmaceutics8030025
[14]. Wang, X., Dai, Y., Wang, D., et al. (2024). Research progress on the preparation methods and applications of liposomes. Chinese Journal of Pharmaceutical Sciences (Online Edition), (01).
[15]. Yip, A. G., Wang, Z., He, H., et al. (2023). Surface modification strategies for liposomes in anti-tumor drug delivery. West China Journal of Pharmacy, (02).
[16]. Yao, L. T., Liu, Y. T., Liu, Y. J., et al. (2019). Research progress of mesoporous silica in tumor treatment. Biotechnology Bulletin, 35(2), 10. https://doi.org/10.13560/j.cnki.biotech.bull.1985.2018-0404
[17]. Mao, Y. L., Zhu, Z., Wang, J. (2022). Nanomaterials promoting protein-loaded nanocarriers to escape endosomes/lysosomes: Current status and future directions. Chinese Tissue Engineering Research, 26(34), 9.
[18]. Xu, Y., Zheng, Y., Wu, L., et al. (2018). A novel solid lipid nanoparticle with endosomal escape function for oral delivery of insulin. ACS Applied Materials & Interfaces. https://doi.org/10.1021/acsami.8b00507
[19]. Zhao, H., Wang, J., Zhang, H., et al. (2009). Rapid preparation of hydrophobic drug nanoparticles using the ultracentrifugation anti-solvent precipitation method. Chinese Journal of Chemical Engineering (English Edition), 17(2), 318-323.
[20]. Yin, S. W., Cao, X. X., Huang, X. N., et al. (2024). Antibacterial nanoparticles and their preparation methods and applications. CN202210008131.9, CN114451511B.
[21]. Wang, W. G., Wang, Z. P., Bian, X. W. (2024). Preparation of double disulfiram-loaded nanoparticles by solvent evaporation method: CN202210031136.3, CN202210031136.3.
[22]. Saxena, N., Kumar, A., & Mandal, A. (2022). Basic aspects of emulsion and nano-emulsion. In Advances in Emulsions and Nanomaterials (pp. 1-21). https://doi.org/10.1007/978-3-031-06689-4_1
[23]. Shi, F. F., Cao, J. H., Yu, S. L., et al. (2021). Preparation and properties of W/O/W-type Codonopsis pilosula polysaccharide nanoemulsions as immuno-enhancers. China Agricultural Science and Technology Bulletin, 23(8), 106-113. https://doi.org/10.13304/j.nykjdb.2020.0801
[24]. Wang, L., Cheng, X., Zhang, S., et al. (2023). The rheological/interfacial behavior and stability properties of nanoemulsions prepared using whey protein-carboxymethyl chitosan conjugates. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 662, 130924. https://doi.org/10.1016/j.colsurfa.2023.130924
[25]. Graves, S. M. (2008). The formation, optical properties, and structure of nanoemulsions. Dissertations & Theses - Gradworks.
[26]. McClements, D. J. (2011). Edible nanoemulsions: Fabrication, properties, and functional performance. Soft Matter, 7(6), 2297–2316. https://doi.org/10.1039/C0SM00549E
[27]. Gayoso, L., Ansorena, D., & Astiasarán, I. (2019). DHA-rich algae oil delivered by O/W or gelled emulsions: Strategies to increase its bioaccessibility. Journal of the Science of Food and Agriculture, 99(5), 2251–2258. https://doi.org/10.1002/jsfa.9420
[28]. Montes de Oca-Ávalos, J. M., Candal, R. J., & Herrera, M. L. (2017). Nanoemulsions: Stability and physical properties. Current Opinion in Food Science, 16, 1–6. https://doi.org/10.1016/j.cofs.2017.06.003
[29]. Thapa, R., Sai, K., Saha, D., et al. (2021). Synthesis and characterization of a nanoemulsion system for solubility enhancement of poorly water-soluble non-steroidal anti-inflammatory drugs. Journal of Molecular Liquids, Pt A, 115998. https://doi.org/10.1016/j.molliq.2021.115998
[30]. Luo, Y., Xiu, W., Wang, Z., et al. (2023). The preparation methods of nanoemulsions and their application research progress. Grain and Oil, 36(7), 5–9. https://doi.org/10.3969/j.issn.1008-9578.2023.07.002
[31]. Gazolu-Rusanova, D., Lesov, I., Tcholakova, S., et al. (2020). Food grade nanoemulsions preparation by rotor-stator homogenization. Food Hydrocolloids. https://doi.org/10.1016/J.FOODHYD.2019.105579
[32]. Chengxi, W., Rishuo, G., Quliang, G., et al. (2018). The prevention effects of cryptotanshinone nanoemulsion on postoperative peritoneal adhesions. Drug Development & Research, 1–24. https://doi.org/10.1080/03639045.2018.1529788
[33]. Li, X., Wang, L., & Dong, J. (2024). The low-energy emulsification preparation method and its application of nanoemulsions. In The 26th Academic Annual Meeting of the Chinese Chemical Society, Colloid and Interface Chemistry Session (pp. 5–9). https://doi.org/ConferenceArticle/5aa0b976c095d722207e01a3
[34]. Zheng, X. (2020). Preparation of sunscreen nanoparticles using phase transition component-based nanoemulsions as templates. (Master’s thesis, Shandong University).
[35]. Wu, J., Li, J., Zhou, X., et al. (2023). Research progress on silk fibroin nanoparticle-loaded anticancer drug delivery systems. Silk, 60(2), 24–34.
[36]. Rajan, S. S., Pandian, A., & Palaniappan, T. (2016). Curcumin loaded in bovine serum albumin–chitosan derived nanoparticles for targeted drug delivery. Bulletin of Materials Science, 39(3), 811–817. https://doi.org/10.1007/s12034-016-1213-z
[37]. Zhang, X., Yang, X., Ji, J., et al. (2016). Tumor targeting strategies for chitosan-based nanoparticles. Colloids and Surfaces B: Biointerfaces, 148, 460–473. https://doi.org/10.1016/j.colsurfb.2016.09.020
[38]. Yu, H., Wang, D., Yang, X., et al. (2018). Application of nanotechnology in tumor-targeted drug delivery systems. Science & Technology Guide, 36(22), 10. https://doi.org/CNKI:SUN:KJDB.0.2018-22-011
[39]. Wang, X., Li, Y., Li, Q., et al. (2017). Hyaluronic acid modification of RNase A and its intracellular delivery using lipid-like nanoparticles. Journal of Controlled Release, 39–45. https://doi.org/10.1016/j.jconrel.2017.01.037
[40]. Wu, H., Yan, H., & Xia, X. (2023). Research progress on gene delivery nanocarriers based on tumor microenvironment. Chinese Journal of Tumor Biological Therapy, 30(7), 639–644.
[41]. Fang, X. (2017). Functionalized nano-selenium drug delivery system preparation and anticancer activity research. (Master’s thesis, Jinan University).
[42]. Foldbjerg, R., Dang, D. A., & Autrup, H. (2011). Cytotoxicity and genotoxicity of silver nanoparticles in the human lung cancer cell line, A549. Archives of Toxicology, 85(7), 743–750. https://doi.org/10.1007/s00204-010-0545-5
[43]. Lee, Y., Jeong, M., Park, J., et al. (2023). Immunogenicity of lipid nanoparticles and its impact on the efficacy of mRNA vaccines and therapeutics. Experimental & Molecular Medicine, 55(10), 2085–2096. https://doi.org/10.1038/s12276-023-01086-x
[44]. Khlebtsov, N., & Dykman, L. (2011). Biodistribution and toxicity of engineered gold nanoparticles: A review of in vitro and in vivo studies. Cheminform, 42(3), 1647–1671. https://doi.org/10.1002/chin.201127274
[45]. Kapoor, D. N., Bhatia, A., Kaur, R., et al. (2015). PLGA: A unique polymer for drug delivery. Therapeutic Delivery, 6(1), 41–58. https://doi.org/10.4155/tde.14.91
[46]. Maeda, H. (2013). The enhanced permeability and retention (EPR) effect in tumor vasculature: The key role of tumor-selective macromolecular drug targeting.
[47]. Yin, H., Song, C. Q., Dorkin, J. R., et al. (2016). Therapeutic genome editing by combined viral and non-viral delivery of CRISPR system components in vivo. Nature Biotechnology, 34(3), 328–333. https://doi.org/10.1038/nbt.3471
[48]. Zhang, N. N., Li, X. F., Deng, Y. Q., et al. (2020). A thermostable mRNA vaccine against COVID-19. Cell. https://doi.org/10.1016/j.cell.2020.07.024
[49]. Fadeel, B., Bussy, C., Merino, S., et al. (2018). Safety assessment of graphene-based materials: Focus on human health and the environment. ACS Nano, 12. https://doi.org/10.1021/acsnano.8b04758
[50]. Lee, Y. K., Choi, E. J., Webster, T. J., et al. (2015). Effect of the protein corona on nanoparticles for modulating cytotoxicity and immunotoxicity. International Journal of Nanomedicine, 10, 97–113. https://doi.org/10.2147/IJN.S72998.
Cite this article
Jin,Y. (2025). Nanotechnology-Based Targeted Drug Delivery Systems for Cancer Treatment. Theoretical and Natural Science,69,61-70.
Data availability
The datasets used and/or analyzed during the current study will be available from the authors upon reasonable request.
Disclaimer/Publisher's Note
The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of EWA Publishing and/or the editor(s). EWA Publishing and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
About volume
Volume title: Proceedings of the 3rd International Conference on Modern Medicine and Global Health
© 2024 by the author(s). Licensee EWA Publishing, Oxford, UK. This article is an open access article distributed under the terms and
conditions of the Creative Commons Attribution (CC BY) license. Authors who
publish this series agree to the following terms:
1. Authors retain copyright and grant the series right of first publication with the work simultaneously licensed under a Creative Commons
Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this
series.
2. Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the series's published
version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial
publication in this series.
3. Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and
during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See
Open access policy for details).
References
[1]. Cao, W., Chen, H. D., Yu, Y. W., et al. (2021). Changing profiles of cancer burden worldwide and in China: A secondary analysis of the global cancer statistics 2020. Chinese Medical Journal. Advance online publication. https://doi.org/10.1097/CM9.0000000000001474
[2]. Ong, S., Sandy, O., Long, et al. (2016). Influence of the encapsulation efficiency and size of liposome on the oral bioavailability of griseofulvin-loaded liposomes. Pharmaceutics, 8(3). https://doi.org/10.3390/pharmaceutics8030025
[3]. Noble, G. T., Stefanick, J. F., Ashley, J. D., et al. (2014). Ligand-targeted liposome design: Challenges and fundamental considerations. Trends in Biotechnology, 32(1), 32-45. https://doi.org/10.1016/j.tibtech.2013.09.007
[4]. Yang, R., He, Y., Lu, Z., et al. (2023). Progress in the study of natural polysaccharide-enhanced drug delivery systems for anti-tumor effects. Chinese Medicinal Materials, 46(3), 789-795.
[5]. Li, K., Liu, Y., Dai, Y., et al. (2022). Research progress on gold nanoparticles as anti-tumor drug carriers. Chinese Journal of Pharmaceutical Sciences, 4(004), 20.
[6]. Sun, T., Zhang, Y. S., Pang, B., et al. (2014). Engineered nanoparticles for drug delivery in cancer therapy. Angewandte Chemie International Edition. https://doi.org/10.1002/anie.201403036
[7]. Jha, S., Sheetal, K., et al. (2016). Liposomal drug delivery system for cancer therapy: Advancement and patents. Recent Patents on Drug Delivery & Formulation.
[8]. Noble, G. T., Stefanick, J. F., Ashley, J. D., et al. (2014). Ligand-targeted liposome design: Challenges and fundamental considerations. Trends in Biotechnology, 32(1), 32-45. https://doi.org/10.1016/j.tibtech.2013.09.007
[9]. Hafner, A., Lovrić, J., Perina Lakoš, G., et al. (2014). Nanotherapeutics in the EU: An overview on current state and future directions. International Journal of Nanomedicine, 9(1), 1005-1023. https://doi.org/10.2147/IJN.S55359
[10]. McClements, D. J., & Rao, J. (2011). Food-grade nanoemulsions: Formulation, fabrication, properties, performance, biological fate, and potential toxicity. Critical Reviews in Food Science & Nutrition, 51(4), 285-330. https://doi.org/10.1080/10408398.2011.559558
[11]. Chen, T., Gong, T., Zhao, T., et al. (2018). A comparison study between lycobetaine-loaded nanoemulsion and liposome using nRGD as a therapeutic adjuvant for lung cancer therapy. European Journal of Pharmaceutical Sciences. https://doi.org/10.1016/j.ejps.2017.09.041
[12]. Maja, L., Eljko, K., & Mateja, P. (2020). Sustainable technologies for liposome preparation. Journal of Supercritical Fluids, 165, 104984. https://doi.org/10.1016/j.supflu.2020.104984
[13]. Ong, S. G. M., Ming, L. C., Lee, K. S., et al. (2016). Influence of the encapsulation efficiency and size of liposome on the oral bioavailability of griseofulvin-loaded liposomes. Pharmaceutics, 8(3). https://doi.org/10.3390/pharmaceutics8030025
[14]. Wang, X., Dai, Y., Wang, D., et al. (2024). Research progress on the preparation methods and applications of liposomes. Chinese Journal of Pharmaceutical Sciences (Online Edition), (01).
[15]. Yip, A. G., Wang, Z., He, H., et al. (2023). Surface modification strategies for liposomes in anti-tumor drug delivery. West China Journal of Pharmacy, (02).
[16]. Yao, L. T., Liu, Y. T., Liu, Y. J., et al. (2019). Research progress of mesoporous silica in tumor treatment. Biotechnology Bulletin, 35(2), 10. https://doi.org/10.13560/j.cnki.biotech.bull.1985.2018-0404
[17]. Mao, Y. L., Zhu, Z., Wang, J. (2022). Nanomaterials promoting protein-loaded nanocarriers to escape endosomes/lysosomes: Current status and future directions. Chinese Tissue Engineering Research, 26(34), 9.
[18]. Xu, Y., Zheng, Y., Wu, L., et al. (2018). A novel solid lipid nanoparticle with endosomal escape function for oral delivery of insulin. ACS Applied Materials & Interfaces. https://doi.org/10.1021/acsami.8b00507
[19]. Zhao, H., Wang, J., Zhang, H., et al. (2009). Rapid preparation of hydrophobic drug nanoparticles using the ultracentrifugation anti-solvent precipitation method. Chinese Journal of Chemical Engineering (English Edition), 17(2), 318-323.
[20]. Yin, S. W., Cao, X. X., Huang, X. N., et al. (2024). Antibacterial nanoparticles and their preparation methods and applications. CN202210008131.9, CN114451511B.
[21]. Wang, W. G., Wang, Z. P., Bian, X. W. (2024). Preparation of double disulfiram-loaded nanoparticles by solvent evaporation method: CN202210031136.3, CN202210031136.3.
[22]. Saxena, N., Kumar, A., & Mandal, A. (2022). Basic aspects of emulsion and nano-emulsion. In Advances in Emulsions and Nanomaterials (pp. 1-21). https://doi.org/10.1007/978-3-031-06689-4_1
[23]. Shi, F. F., Cao, J. H., Yu, S. L., et al. (2021). Preparation and properties of W/O/W-type Codonopsis pilosula polysaccharide nanoemulsions as immuno-enhancers. China Agricultural Science and Technology Bulletin, 23(8), 106-113. https://doi.org/10.13304/j.nykjdb.2020.0801
[24]. Wang, L., Cheng, X., Zhang, S., et al. (2023). The rheological/interfacial behavior and stability properties of nanoemulsions prepared using whey protein-carboxymethyl chitosan conjugates. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 662, 130924. https://doi.org/10.1016/j.colsurfa.2023.130924
[25]. Graves, S. M. (2008). The formation, optical properties, and structure of nanoemulsions. Dissertations & Theses - Gradworks.
[26]. McClements, D. J. (2011). Edible nanoemulsions: Fabrication, properties, and functional performance. Soft Matter, 7(6), 2297–2316. https://doi.org/10.1039/C0SM00549E
[27]. Gayoso, L., Ansorena, D., & Astiasarán, I. (2019). DHA-rich algae oil delivered by O/W or gelled emulsions: Strategies to increase its bioaccessibility. Journal of the Science of Food and Agriculture, 99(5), 2251–2258. https://doi.org/10.1002/jsfa.9420
[28]. Montes de Oca-Ávalos, J. M., Candal, R. J., & Herrera, M. L. (2017). Nanoemulsions: Stability and physical properties. Current Opinion in Food Science, 16, 1–6. https://doi.org/10.1016/j.cofs.2017.06.003
[29]. Thapa, R., Sai, K., Saha, D., et al. (2021). Synthesis and characterization of a nanoemulsion system for solubility enhancement of poorly water-soluble non-steroidal anti-inflammatory drugs. Journal of Molecular Liquids, Pt A, 115998. https://doi.org/10.1016/j.molliq.2021.115998
[30]. Luo, Y., Xiu, W., Wang, Z., et al. (2023). The preparation methods of nanoemulsions and their application research progress. Grain and Oil, 36(7), 5–9. https://doi.org/10.3969/j.issn.1008-9578.2023.07.002
[31]. Gazolu-Rusanova, D., Lesov, I., Tcholakova, S., et al. (2020). Food grade nanoemulsions preparation by rotor-stator homogenization. Food Hydrocolloids. https://doi.org/10.1016/J.FOODHYD.2019.105579
[32]. Chengxi, W., Rishuo, G., Quliang, G., et al. (2018). The prevention effects of cryptotanshinone nanoemulsion on postoperative peritoneal adhesions. Drug Development & Research, 1–24. https://doi.org/10.1080/03639045.2018.1529788
[33]. Li, X., Wang, L., & Dong, J. (2024). The low-energy emulsification preparation method and its application of nanoemulsions. In The 26th Academic Annual Meeting of the Chinese Chemical Society, Colloid and Interface Chemistry Session (pp. 5–9). https://doi.org/ConferenceArticle/5aa0b976c095d722207e01a3
[34]. Zheng, X. (2020). Preparation of sunscreen nanoparticles using phase transition component-based nanoemulsions as templates. (Master’s thesis, Shandong University).
[35]. Wu, J., Li, J., Zhou, X., et al. (2023). Research progress on silk fibroin nanoparticle-loaded anticancer drug delivery systems. Silk, 60(2), 24–34.
[36]. Rajan, S. S., Pandian, A., & Palaniappan, T. (2016). Curcumin loaded in bovine serum albumin–chitosan derived nanoparticles for targeted drug delivery. Bulletin of Materials Science, 39(3), 811–817. https://doi.org/10.1007/s12034-016-1213-z
[37]. Zhang, X., Yang, X., Ji, J., et al. (2016). Tumor targeting strategies for chitosan-based nanoparticles. Colloids and Surfaces B: Biointerfaces, 148, 460–473. https://doi.org/10.1016/j.colsurfb.2016.09.020
[38]. Yu, H., Wang, D., Yang, X., et al. (2018). Application of nanotechnology in tumor-targeted drug delivery systems. Science & Technology Guide, 36(22), 10. https://doi.org/CNKI:SUN:KJDB.0.2018-22-011
[39]. Wang, X., Li, Y., Li, Q., et al. (2017). Hyaluronic acid modification of RNase A and its intracellular delivery using lipid-like nanoparticles. Journal of Controlled Release, 39–45. https://doi.org/10.1016/j.jconrel.2017.01.037
[40]. Wu, H., Yan, H., & Xia, X. (2023). Research progress on gene delivery nanocarriers based on tumor microenvironment. Chinese Journal of Tumor Biological Therapy, 30(7), 639–644.
[41]. Fang, X. (2017). Functionalized nano-selenium drug delivery system preparation and anticancer activity research. (Master’s thesis, Jinan University).
[42]. Foldbjerg, R., Dang, D. A., & Autrup, H. (2011). Cytotoxicity and genotoxicity of silver nanoparticles in the human lung cancer cell line, A549. Archives of Toxicology, 85(7), 743–750. https://doi.org/10.1007/s00204-010-0545-5
[43]. Lee, Y., Jeong, M., Park, J., et al. (2023). Immunogenicity of lipid nanoparticles and its impact on the efficacy of mRNA vaccines and therapeutics. Experimental & Molecular Medicine, 55(10), 2085–2096. https://doi.org/10.1038/s12276-023-01086-x
[44]. Khlebtsov, N., & Dykman, L. (2011). Biodistribution and toxicity of engineered gold nanoparticles: A review of in vitro and in vivo studies. Cheminform, 42(3), 1647–1671. https://doi.org/10.1002/chin.201127274
[45]. Kapoor, D. N., Bhatia, A., Kaur, R., et al. (2015). PLGA: A unique polymer for drug delivery. Therapeutic Delivery, 6(1), 41–58. https://doi.org/10.4155/tde.14.91
[46]. Maeda, H. (2013). The enhanced permeability and retention (EPR) effect in tumor vasculature: The key role of tumor-selective macromolecular drug targeting.
[47]. Yin, H., Song, C. Q., Dorkin, J. R., et al. (2016). Therapeutic genome editing by combined viral and non-viral delivery of CRISPR system components in vivo. Nature Biotechnology, 34(3), 328–333. https://doi.org/10.1038/nbt.3471
[48]. Zhang, N. N., Li, X. F., Deng, Y. Q., et al. (2020). A thermostable mRNA vaccine against COVID-19. Cell. https://doi.org/10.1016/j.cell.2020.07.024
[49]. Fadeel, B., Bussy, C., Merino, S., et al. (2018). Safety assessment of graphene-based materials: Focus on human health and the environment. ACS Nano, 12. https://doi.org/10.1021/acsnano.8b04758
[50]. Lee, Y. K., Choi, E. J., Webster, T. J., et al. (2015). Effect of the protein corona on nanoparticles for modulating cytotoxicity and immunotoxicity. International Journal of Nanomedicine, 10, 97–113. https://doi.org/10.2147/IJN.S72998.









