1. Background
Spermidine is a natural polyamine with the molecular formula of C7H19N3 [1]. It can be found in cereals, legumes, soy derivatives [2], mushrooms, green peppers, and peas [3]. Instead of relying solely on oral uptake, spermidine can be generated by the human body by cellular pathways such as biosynthesis, and catabolism and by microorganisms like microbiota, and probiotics in the small intestine [4]. Studies suggested that the health benefits of spermidine include preserving mitochondrial function, exhibiting anti-inflammatory properties, and preventing stem cell senescence [5]. Looking behind the induced health effects, the primary cellular mechanism is the process of autophagy.
Cellular materials need to be recycled in response to conditions of stress, such as nutrient deprivation, viral infection, and genotoxic stress [6]. Autophagy is a fundamental process where cells eliminate and recycle the waste products by lysosome-derived degrading actions, and the waste products include nucleic acids, proteins, lipids, and organelles [7]. Autophagy plays a crucial role since it prevents the misfolds of proteins and clears the cells with injuries that might cause functional problems in the human body [8]. The process of autophagy could be induced by several pathways, such as the inhibition of TORC1, Ras/cAMP-dependent protein kinase A (PKA), Snf1, and mTOR, as well as the activation of AMPK [9]. Once spermidine is added, it inhibits the mTOR pathway and activates AMPK and FOXOs, and therefore, leads to the activation of the autophagy process, just as Figure 1 shows [10].
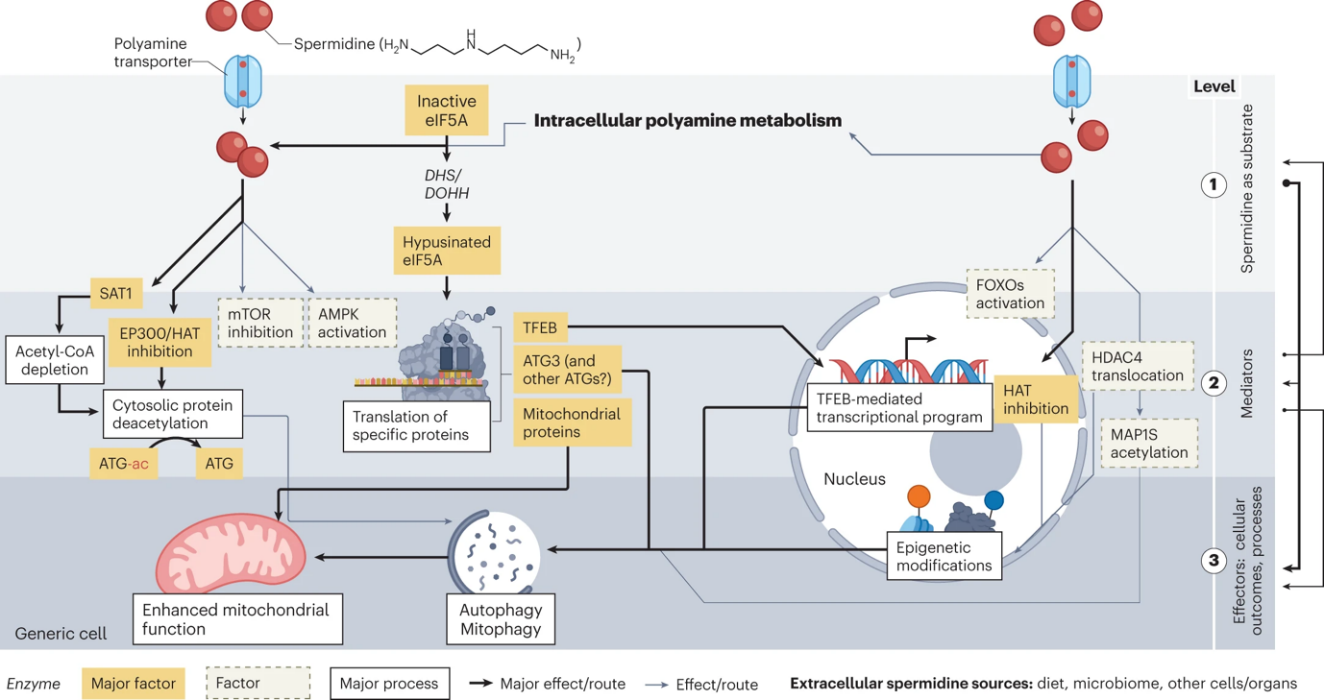
Figure 1. Activation of autophagy by spermidine at the cellular level [10]
While spermidine has been demonstrated to have effects on yeast, insects, and mammals, recent research aims to provide more insights into the effects of spermidine on the human body [11]. A study that focuses on the safety and tolerability of spermidine supplementation in mice and humans demonstrates that spermidine did not result in morbidities or changes in behavior by applying it to both mice for 28 days and to older adults for 3 months [12]. After the establishment of the safe intake on humans, more human-based research in combination with animal studies came out to investigate the health benefits of spermidines induced by autophagy. These benefits include inducing longevity [5], reducing the incidences of neurological disorders [13], cardiovascular diseases [14], and metabolic disorders [13]. This review summarises the present evidence (including both human and animal studies) on how autophagy brings the four listed advantages of autophagy after the ingestion of spermidine.
2. Study selection
2.1. Eligibility criteria
This review focused on studies investigating the effects of spermidine on longevity, neurological disorders, cardiovascular diseases, and metabolic disorders, specifically through the mechanism of autophagy. Inclusion criteria were limited to clinical studies, randomized controlled trials (RCTs), cohort studies, populational-based studies, and observational studies published in English that directly examined the role of spermidine in promoting longevity, neurological health, cardiovascular health, and metabolic health. Excluded were review articles, case studies, and research that did not specifically address the link between spermidine and the targeted health outcomes.
2.2. Information sources
A systematic literature search was conducted using databases such as PubMed, ScienceDirect, Web of Science, and Google Scholar. Key search terms included "spermidine," "autophagy," "longevity," "cardiovascular health," "cardiovascular diseases," "neuroprotection," "neurodegenerative diseases," "cognitive function," "metabolic disorders," "metabolic syndrome," "obesity," "diabetes."
2.3. Selection process
The selection of studies involved a two-stage process. Initially, two independent reviewers screened the titles and abstracts to identify studies that met the inclusion criteria. In the second stage, the full texts of potentially eligible studies were reviewed to confirm their relevance.
2.4. Data collection process
Data from the included studies were independently extracted by two reviewers using a pre-designed data extraction form. Information collected included study design, population characteristics, spermidine dosage, and administration, outcomes related to autophagy, and the health effects on longevity, neurological disorders, cardiovascular diseases, and metabolic disorders. Any disagreements in data extraction were resolved through consensus or with the involvement of a third reviewer. The data collection process was conducted manually without the use of automation tools.
3. Results
53 articles were identified to be relevant when keyed in the key words on searching engines as described above. 23 articles were removed due to duplication. 16 articles were selected as the other 14 did not have a clear abstract or data presented or did not fit the inclusion and exclusion criteria.
3.1. Effects of spermidine on aging and longevity
Mentioning the terms "anti-aging" and "longevity", autophagy could be one of the mechanisms behind them. Wilson et al. in their finding illustrated that the rate of autophagy decreases significantly with age, which could be suggested as an indicator of aging. Spermidine, which has the significant function of enhancing autophagy, has been suggested to have possible benefits in reducing cellular aging and promoting longevity.
A populational-based study by [15] found that spermidine intake is associated with a mortality rate. This study included 829 participants aged 45–84. The results show that, under all causes of death, spermidine intake is inversely proportional to the mortality rate in a general community. The author brought up the concept that nutrition rich in spermidine is linked to increased survival rates in humans. When discussing the highest possible mechanism regarding the results, the author specified that spermidine could restore or induce autophagy, which could further help to reduce cellular stress, lower blood pressure, reduce cancer risk, and assist the progression of lipid metabolism. The reduction of the main causes of death contributes to the extension of life span. A study published by [16] suggested that, as the body ages, erythrocyte mean cell volume (MCV) increases, this could be considered an indicator of aging. Surprisingly, the experiment data shows that MCV is inversely correlated with spermidine, which leads to the conclusion that spermidine is highly heritable in erythrocytes of people who have higher life expectancy. This might be due to the increased rate of erythrocyte autophagy promoted by spermidine. The direct relationship between spermidine concentration and MCV suggests that spermidine supplementation might have the effect of delaying aging. However, this conclusion requires more followed-up experimental designs and evidence to support it. Increased stiffness and endothelial dysfunction are the main signs of arterial aging, which is the main cause of cardiovascular diseases at later stages of life [17]. In the research [17], the authors used mice models to investigate the relationship between arterial aging and spermidine intake. The results supported their hypothesis that spermidine reduced arterial stiffness and improved arterial endothelial function in old mice. The authors suggested that spermidine has the effect of reversing large elastic artery stiffening by promoting arterial autophagy by modifying the transcription of autophagy-relevant proteins and de-acetylating histone H3. Furthermore, spermidine could restore NO-mediated endothelial function and act as an antioxidant to reduce oxidative stress.
3.2. Effects of spermidine on neurological disorders
When the effects of spermidine were just noticed to have beneficial effects on cognitive functions, Schroeder et al. have contributed a lot to this aspect. They first gave supplementation to fruit flies [18], and then, to mammals like mice and humans [19]. They have not only conducted clinical trials to investigate the relationship between spermidine intake and cognitive functions cross-species but also conducted a human epidemiological study to illustrate the effects of spermidine through different research angles [19]. The authors agreed that the fundamental mechanism behind the beneficial effects of spermidine on the brain is the ability to induce autophagy as the figure shows. The general process is that the elevated spermidine intake leads to elF5A hypusination, which activates the autophagy process to improve mitochondrial function, and finally results in cognitive improvement [19]. Figure 2 also demonstrates the pathways of how spermidine enhances neurological functions in fruit flies, mice, and humans according to the existing evidence from the research they have done so far.
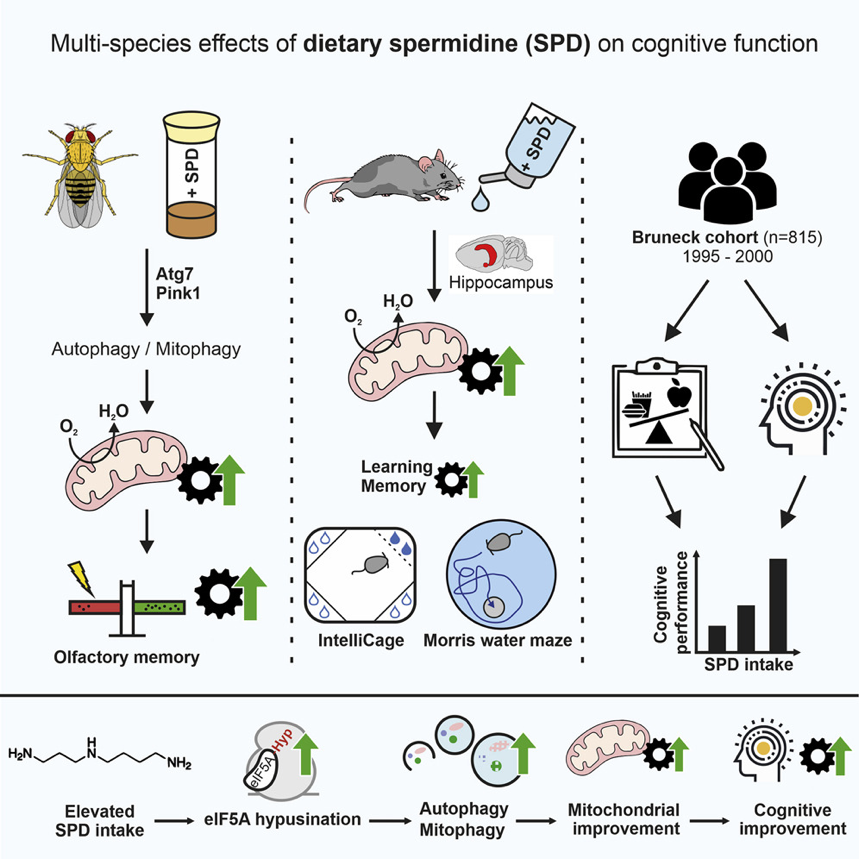
Figure 2. Multi-species effects of dietary spermidine on cognitive function [19]. Left: fruit flies, middle: mice, right: human. The general mechanism of spermidine to boost cognitive function is autophagy, which is induced by eIF5A hypusination empowered by elevated spermidine intake, which leads to mitochondrial improvement and thus, cognitive improvement.
Symptoms associated with cognitive decline are shown to be reduced with spermidine supplementation according to several studies. Dementia is a typical symptom that is usually found in the elderly with progressive cognitive loss, such as in people with Alzheimer's disease [20]. [21] conducted a double-blinded randomised controlled trial to give participants oral spermidine supplementation and performed a memory test on 85 subjects aged between 60 and 96 years. The results showed that the group that had a lower spermidine intake showed consistent or declining cognitive performance. Similarly in another 3-month randomise, placebo-controlled experiment conducted by (Wirth et al., 2018) [22], the result gave the same conclusion that memory performance was moderately enhanced in the spermidine group compared with placebo at the end of the intervention. Pekar et al. explained that the reduction of the symptoms is possibly due to the dissolving of amyloid-beta plaques by autophagy. However, there is not only just a single cause that results in the progression of Alzheimer's disease and dementia [20]. The possible mechanisms underlying these diseases are not fully understood. Therefore, the potential of spermidine in dementia treatment remains to be discussed.
Subsequently, spermidine may also have effects on emotional well-being. Studies by [23] and [24] showed that spermidine might help to reduce the incidences of cognitive and psychological diseases such as depression. [23] suggested that activation of the autophagic system can be established by the transcription factor EB (TFEB), which regulates both autophagosome formation and lysosomal biogenesis and function could be the main mechanism to improve the number and functionality of the brain cells. However, the link between the increased brain cells and improvement of cognition and emotional well-being is barely mentioned throughout the whole article. Qi et al. have found that, Among the 19,306 participants, people with more dietary spermidine intake have less depression rate. The prevalences of depression under all sources of spermidine (fruits, vegetables, cereals, nuts, eggs, and seafood) are all lying between the confidential intervals. Together with the large sample size, further gave the conclusion that the spermidine intake is significantly relevant to depression incidence.
Although the above evidence all pointed to the same conclusion that spermidine could have the potential to improve cognitive decline. However, a study done by [12] reported differently. Their study recruited one hundred participants who were randomly assigned (1:1 ratio) to 12 months of dietary supplementation with either a spermidine-rich dietary supplement or placebo. Surprisingly, the results showed that no significant changes were observed between the intervention and placebo group, which suggests that the effects of spermidine on cognitive decline still need more research to confirm.
3.3. Effects of spermidine on cardiovascular diseases
Starting from a recent search, [25] investigated the causal relationships between spermidine levels and CVD risk factors such as blood pressure, blood glucose, and lipid profiles using a bi-directional Mendelian randomisation analysis. The data source came from Genome-Wide Association Studies, the large sample size provided a strong base for the population study. By analysing the pattern of cardiovascular diseases and spermidine intake, researchers found that a high spermidine diet could manage elevated blood glucose levels, lower LDL, control the development of hypertension, and elevate HDL. Just as the authors stated in the article, spermine boosts autophagy, which eliminates damaged proteins and organelles within cells, however, although the authors mentioned autophagy is a process that spermidine could induce, how exactly autophagy-induced processes could act on cardiovascular sides where not clearly explained. Instead, the authors focused more on other mechanisms like facilitating RNA transcription and protein synthesis to prevent the formation of glycans and enhance insulin sensitivity.
According to [26] and [27], spermidine contributes to cardiovascular health mainly by changing the arterial properties, and hence, reducing hypertension. [27] suggested that the reduction of hypertension is by reducing arterial stiffness. This could be achieved through spermidine-induced mechanisms likely dependent on the downregulation of oxidative stress and stimulation of autophagy. The experimental results showed that there was a significant increase in autophagy, which brought benefit to arterial endothelial function, however, this evidence seemed not strong enough to bring a change in blood pressure that was statistically significant. [26] experiment gave the results that the spermidine intake significantly improved right ventricular function and pulmonary tissue morphology. The authors addressed that by inhibiting purine metabolism and polyamine synthesis-associated vascular remodelling. However, throughout the whole experiment which was on investigation of spermidine on reduction of hypertension, the outcome did not include the comparison of blood pressure before and after spermidine intake although all other outcomes and results indirectly pointed to the conclusion that spermidine could recuse pulmonary hypertension. Moreover, the authors did not consider or specify how autophagy was induced by spermidine in this case.
3.4. Effects of spermidine on metabolic disorders
Metabolic syndrome appears when the body has reduced ability to have normal metabolic functions, the symptoms include central obesity, insulin resistance, hypertension, and dyslipidemia, leading to an increased risk of atherosclerotic cardiovascular diseases and type II diabetes mellitus development [28]. From the previous section, research showed that spermidine and its benefits on arteries could induce an anti-hypertensive effect. According to the study done by [29] with the injection of spermidine nanoparticles inside mice bodies, there are significant improvements shown in the aspects of hypoglycaemic efficacy, Long-term glycaemic control and relief of excessive lipid metabolism and myocardial fibrosis and cardiac function, which could be considered as key factors in reducing metabolic diseases like hypertension and diabetes. Different from taking spermidine supplementation, this research brought a new concept by using a more direct method of subcutaneous injection, which was shown to have no big influence on organ functions of mice and reduced blood sugar in both short and long term [29]. However, although the authors demonstrated that spermidine is an effective agent contributing to metabolic health, the reason that led to the change, whether this was influenced by autophagy, was not clearly stated.
Additionally, more evidence suggests that spermidine also contributes to other sides of metabolism, especially lipid metabolism-related problems. By comparing the lipid accumulation and formation of atherosclerosis plaque in over-feeding mice, [30] found that mice with 5 mM spermidine intake per day for 20 weeks have reduced necrotic core formation (p=0.0008), lipid accumulation inside the plaque (p = 0.017), and cholesterol efflux (p = 0.0118). However, the authors stated that spermidine might not have the effects in reducing the advanced plaques (Michiels et al., 2016). The results from a study done by [31] suggested that spermidine administration did not make any difference in lean mice but increased the activation of adipose tissue thermogenesis through autophagy. All two studies mentioned that the effects of lipid oxidation were autophagy-driven, mainly by its functions of against oxidative stress, hypoxia, and metabolic stress.
4. Discussion
This review aggregates current research articles regarding spermidine and its health benefits. Autophagy is the main strategy that leads to these changes induced by spermidine administration as discussed above. However, several aspects remain to be questionable.
Firstly, initial observations indicate that spermidine and its effects on human health constitute a relatively nascent research area, as a significant portion of the studies examining this relationship have emerged within the past four years. Therefore, the number of current research is still not enough to support the hypothesis that spermidine could induce significant health benefits. Moreover, there is a limited number of randomised controlled trials, and the current evidence is more at the stage of mice experiments. More research regarding the administration safety and recommended daily intake of spermidine is the major focus of proceeding to the next stage of human trials.
In terms of the health benefits associated with spermidine, it is important to recognize that the underlying mechanisms extend beyond autophagy. Recent findings suggest that spermidine plays a crucial role in maintaining mitochondrial function through the posttranslational modification of mitochondrial mRNA [5]. Moreover, its anti-inflammatory properties and potential for preventing stem cell senescence have been identified as additional mechanisms that may contribute to improved health outcomes [14]. Furthermore, though most studies although they mentioned autophagy as the main mechanism that led to the changes, the exact pathways of how autophagy could induce the benefits at the cellular level in each case were not fully explained in detail.
To conclude, the suture research of spermidine could focus more on human administration and safety. The mechanisms of autophagy-induced benefit could be investigated and explained at the cellular level, and more importantly, identifying mechanisms other than autophagy could further help the applications of spermidine in various cases.
5. Conclusion
Overall, spermidine is demonstrated to have multiple health benefits shown in most of the studies discussed above, including longevity, cardiovascular, neurological, and metabolic health. Among these studies, spermidine was not only applied to animal models but also to humans. The main mechanism that leads to the beneficial effects is the process of autophagy. Apart from autophagy, spermidine could also induce various benefits by different pathways, which will be discussed further in the future.
Acknowledgments
I would like to express my deepest gratitude to the professors in charge of the course Nutrition and Medical Sciences at University College London Medical School. The dedication and expertise in teaching have provided me with the fundamental knowledge essential to understanding food science and human nutrition.
A special thanks to the Associate Professor of Biology, Gerwald Jogl at Brown University for his invaluable discussions during the summer camp workshop, which greatly enhanced my understanding of the topics related to this research. The insights and guidance have been instrumental in shaping the direction of this work.
References
[1]. Zou, D., Zhao, Z., Li, L., Yu Yu Min, Zhang, D., Ji, A., Jiang, C., Wei, X. and Wu, X. (2022). A comprehensive review of spermidine: Safety, health effects, absorption and metabolism, food materials evaluation, physical and chemical processing, and bioprocessing. Comprehensive Reviews in Food Science and Food Safety, 21(3), pp.2820–2842. doi:https://doi.org/10.1111/1541-4337.12963.
[2]. Muñoz-Esparza, N.C., Latorre-Moratalla, M.L., Comas-Basté, O., Toro-Funes, N., Veciana-Nogués, M.T. and Vidal-Carou, M.C. (2019). Polyamines in Food. Frontiers in Nutrition, [online] 6. doi:https://doi.org/10.3389/fnut.2019.00108.
[3]. Muñoz-Esparza, N.C., Costa-Catala, J., Comas-Basté, O., Toro-Funes, N., Latorre-Moratalla, M.L., Veciana-Nogués, M.T. and Vidal-Carou, M.C. (2021). Occurrence of Polyamines in Foods and the Influence of Cooking Processes. Foods, 10(8), p.1752. doi:https://doi.org/10.3390/foods10081752.
[4]. Hofer, S.J., Simon, A.K., Bergmann, M., Eisenberg, T., Kroemer, G. and Madeo, F. (2022). Mechanisms of spermidine-induced autophagy and geroprotection. Nature Aging, [online] 2(12), pp.1112–1129. doi:https://doi.org/10.1038/s43587-022-00322-9.
[5]. Madeo, F., Bauer, M.A., Carmona-Gutierrez, D. and Kroemer, G. (2018a). Spermidine: a physiological autophagy inducer acting as an anti-aging vitamin in humans? Autophagy, [online] 15(1), pp.165–168. doi:https://doi.org/10.1080/15548627.2018.1530929.
[6]. Filomeni, G., De Zio, D. and Cecconi, F. (2015). Oxidative stress and autophagy: the clash between damage and metabolic needs. Cell Death & Differentiation, [online] 22(3), pp.377–388. doi:https://doi.org/10.1038/cdd.2014.150.
[7]. Aman, Y., Schmauck-Medina, T., Hansen, M., Morimoto, R.I., Simon, A.K., Bjedov, I., Palikaras, K., Simonsen, A., Johansen, T., Tavernarakis, N., Rubinsztein, D.C., Partridge, L., Kroemer, G., Labbadia, J. and Fang, E.F. (2021). Autophagy in healthy aging and disease. Nature Aging, 1(8), pp.634–650. doi:https://doi.org/10.1038/s43587-021-00098-4.
[8]. Shabkhizan, R., Haiaty, S., Moslehian, M.S., Bazmani, A., Sadeghsoltani, F., Saghaei Bagheri, H., Rahbarghazi, R. and Sakhinia, E. (2023). The Beneficial and Adverse Effects of Autophagic Response to Caloric Restriction and Fasting. Advances in Nutrition, [online] 14(5), pp.1211–1225. doi:https://doi.org/10.1016/j.advnut.2023.07.006.
[9]. Yang, Z. and Klionsky, D.J. (2009). An Overview of the Molecular Mechanism of Autophagy. Current Topics in Microbiology and Immunology, 335, pp.1–32. doi:https://doi.org/10.1007/978-3-642-00302-8_1.
[10]. Hofer, S.J., Simon, A.K., Bergmann, M., Eisenberg, T., Kroemer, G. and Madeo, F. (2022). Mechanisms of spermidine-induced autophagy and geroprotection. Nature Aging, [online] 2(12), pp.1112–1129. doi:https://doi.org/10.1038/s43587-022-00322-9.
[11]. Hofer, S.J., Ioanna Daskalaki, Bergmann, M., Jasna Friščić, Zimmermann, A., Mueller, M.I., Abdellatif, M., Nicastro, R., Masser, S., Durand, S., Nartey, A., Waltenstorfer, M., Enzenhofer, S., Faimann, I., Gschiel, V., Bajaj, T., Niemeyer, C., Ilias Gkikas, Pein, L. and Cerrato, G. (2024). Spermidine is essential for fasting-mediated autophagy and longevity. Nature Cell Biology. [online] doi:https://doi.org/10.1038/s41556-024-01468-x.
[12]. Schwarz, C., Stekovic, S., Wirth, M., Benson, G., Royer, P., Sigrist, S.J., Pieber, T., Dammbrueck, C., Magnes, C., Eisenberg, T., Pendl, T., Bohlken, J., Köbe, T., Madeo, F. and Flöel, A. (2018). Safety and tolerability of spermidine supplementation in mice and older adults with subjective cognitive decline. Aging, 10(1), pp.19–33. doi:https://doi.org/10.18632/aging.101354.
[13]. Ni, Y.-Q. and Liu, Y.-S. (2021). New Insights into the Roles and Mechanisms of Spermidine in Aging and Age-Related Diseases. Aging and disease, 12(8), p.1948. doi:https://doi.org/10.14336/ad.2021.0603.
[14]. Madeo, F., Eisenberg, T., Pietrocola, F. and Kroemer, G. (2018b). Spermidine in health and disease. Science, 359(6374), p.eaan2788. doi:https://doi.org/10.1126/science.aan2788.
[15]. Kiechl, S., Pechlaner, R., Willeit, P., Notdurfter, M., Paulweber, B., Willeit, K., Werner, P., Ruckenstuhl, C., Iglseder, B., Weger, S., Mairhofer, B., Gartner, M., Kedenko, L., Chmelikova, M., Stekovic, S., Stuppner, H., Oberhollenzer, F., Kroemer, G., Mayr, M. and Eisenberg, T. (2018). Higher spermidine intake is linked to lower mortality: a prospective population-based study. The American Journal of Clinical Nutrition, 108(2), pp.371–380. doi:https://doi.org/10.1093/ajcn/nqy102.
[16]. Raife, T., Mill, J., Hess, A.S. and Haj, A. (2022). The Longevity Factor Spermidine Is Highly Heritable in Human Erythrocytes and Is Part of a Complex Phenotype Associated with Human Longevity. Blood, 140(Supplement 1), pp.5311–5312. doi:https://doi.org/10.1182/blood-2022-158274.
[17]. LaRocca, T.J., Gioscia-Ryan, R.A., Hearon, C.M. and Seals, D.R. (2013). The autophagy enhancer spermidine reverses arterial aging. Mechanisms of Ageing and Development, 134(7-8), pp.314–320. doi:https://doi.org/10.1016/j.mad.2013.04.004.
[18]. Büttner, S., Broeskamp, F., Sommer, C., Markaki, M., Habernig, L., Alavian-Ghavanini, A., Carmona-Gutierrez, D., Eisenberg, T., Michael, E., Kroemer, G., Tavernarakis, N., Sigrist, S.J. and Madeo, F. (2014). Spermidine protects against α-synuclein neurotoxicity. Cell Cycle, 13(24), pp.3903–3908. doi:https://doi.org/10.4161/15384101.2014.973309.
[19]. Schroeder, S., Hofer, S.J., Zimmermann, A., Pechlaner, R., Dammbrueck, C., Pendl, T., Marcello, G.M., Pogatschnigg, V., Bergmann, M., Müller, M., Gschiel, V., Ristic, S., Tadic, J., Iwata, K., Richter, G., Farzi, A., Üçal, M., Schäfer, U., Poglitsch, M. and Royer, P. (2021). Dietary spermidine improves cognitive function. Cell Reports, [online] 35(2), p.108985. doi:https://doi.org/10.1016/j.celrep.2021.108985.
[20]. National Institute on Aging (2022). What Is Dementia? Symptoms, Types, and Diagnosis. [online] What Is Dementia? Symptoms, Types, and Diagnosis. Available at: https://www.nia.nih.gov/health/alzheimers-and-dementia/what-dementia-symptoms-types-and-diagnosis.
[21]. Pekar, T., Bruckner, K., Pauschenwein-Frantsich, S., Gschaider, A., Oppliger, M., Willesberger, J., Ungersbäck, P., Wendzel, A., Kremer, A., Flak, W., Wantke, F. and Jarisch, R. (2021). The positive effect of spermidine in older adults suffering from dementia. Wiener Klinische Wochenschrift, [online] 133(9), pp.484–491. doi:https://doi.org/10.1007/s00508-020-01758-y.
[22]. Wirth, M., Benson, G., Schwarz, C., Köbe, T., Grittner, U., Schmitz, D., Sigrist, S.J., Bohlken, J., Stekovic, S., Madeo, F. and Flöel, A. (2018). The effect of spermidine on memory performance in older adults at risk for dementia: A randomized controlled trial. Cortex, 109, pp.181–188. doi:https://doi.org/10.1016/j.cortex.2018.09.014.
[23]. Trovò, L., Angélique Vaucher, Pan, Y., Steiner, P., Flunkert, S., Fleming, S.A. and Preitner, N. (2023). In vitro assessment of dietary bioactives for TFEB activation as a possible target to support cognitive and emotional wellbeing. Journal of Functional Foods, 111, pp.105855–105855. doi:https://doi.org/10.1016/j.jff.2023.105855.
[24]. Qi, G., Wang, J., Chen, Y., Wei, W. and Sun, C. (2024). Association between dietary spermidine intake and depressive symptoms among US adults: National Health and Nutrition Examination Survey (NHANES) 2005–2014. Journal of Affective Disorders, 359, pp.125–132. doi:https://doi.org/10.1016/j.jad.2024.05.041.
[25]. Wang, T., Li, N. and Zeng, Y. (2024). Protective effects of spermidine levels against cardiovascular risk factors: an exploration of causality based on a bi-directional Mendelian randomization analysis. Nutrition, pp.112549–112549. doi:https://doi.org/10.1016/j.nut.2024.112549.
[26]. Chen, Y., Li, H., Zhao, F., Yu, M., Pan, S., Sun, W., Yin, Y. and Zhu, T. (2024). Spermidine attenuates monocrotaline-induced pulmonary arterial hypertension in rats by inhibiting purine metabolism and polyamine synthesis-associated vascular remodeling. International Immunopharmacology, 132, pp.111946–111946. doi:https://doi.org/10.1016/j.intimp.2024.111946.
[27]. Tocci, G., Biondi-Zoccai, G., Forte, M., Gallo, G., Giulia Nardoianni, Fiori, E., Luca D'Ambrosio, Riccardo Di Pietro, Stefanini, G., Cannata, F., Rocco, E., Simeone, B., Sarto, G., Schirone, L., D’Amico, A., Peruzzi, M., Nocella, C., Volpe, M. and Speranza Rubattu (2023). Effects of two-month treatment with a mixture of natural activators of autophagy on oxidative stress and arterial stiffness in patients with essential hypertension: A pilot study. Nutrition Metabolism and Cardiovascular Diseases, 33(11), pp.2287–2293. doi:https://doi.org/10.1016/j.numecd.2023.07.026.
[28]. Swarup, S. and Zeltser, R. (2024). Metabolic Syndrome. [online] Nih.gov. Available at: https://www.ncbi.nlm.nih.gov/books/NBK459248/.
[29]. Nie, T., Fang, Z., Liu, H., Zhang, X., Ying, F., Xu, X., Huang, H. and Wu, J. (2022). Bioactive spermidine nanoparticles for effective cardiovascular recovery and diabetic therapy. Chemical Engineering Journal, 446, pp.137353–137353. doi:https://doi.org/10.1016/j.cej.2022.137353.
[30]. Michiels, C.F., Kurdi, A., Timmermans, J.-P., De Meyer, G.R.Y. and Martinet, W. (2016). Spermidine reduces lipid accumulation and necrotic core formation in atherosclerotic plaques via induction of autophagy. Atherosclerosis, 251, pp.319–327. doi:https://doi.org/10.1016/j.atherosclerosis.2016.07.899.
[31]. Ni, Y., Zheng, L., Zhang, L., Li, J., Pan, Y., Du, H., Wang, Z. and Fu, Z. (2024). Spermidine activates adipose tissue thermogenesis through autophagy and fibroblast growth factor 21. The Journal of Nutritional Biochemistry, 125, pp.109569–109569. doi:https://doi.org/10.1016/j.jnutbio.2024.109569.
Cite this article
Gai,S. (2025). The beneficial effects of spermidine via autophagy: a systematic review. Theoretical and Natural Science,73,309-317.
Data availability
The datasets used and/or analyzed during the current study will be available from the authors upon reasonable request.
Disclaimer/Publisher's Note
The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of EWA Publishing and/or the editor(s). EWA Publishing and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
About volume
Volume title: Proceedings of the 4th International Conference on Biological Engineering and Medical Science
© 2024 by the author(s). Licensee EWA Publishing, Oxford, UK. This article is an open access article distributed under the terms and
conditions of the Creative Commons Attribution (CC BY) license. Authors who
publish this series agree to the following terms:
1. Authors retain copyright and grant the series right of first publication with the work simultaneously licensed under a Creative Commons
Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this
series.
2. Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the series's published
version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial
publication in this series.
3. Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and
during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See
Open access policy for details).
References
[1]. Zou, D., Zhao, Z., Li, L., Yu Yu Min, Zhang, D., Ji, A., Jiang, C., Wei, X. and Wu, X. (2022). A comprehensive review of spermidine: Safety, health effects, absorption and metabolism, food materials evaluation, physical and chemical processing, and bioprocessing. Comprehensive Reviews in Food Science and Food Safety, 21(3), pp.2820–2842. doi:https://doi.org/10.1111/1541-4337.12963.
[2]. Muñoz-Esparza, N.C., Latorre-Moratalla, M.L., Comas-Basté, O., Toro-Funes, N., Veciana-Nogués, M.T. and Vidal-Carou, M.C. (2019). Polyamines in Food. Frontiers in Nutrition, [online] 6. doi:https://doi.org/10.3389/fnut.2019.00108.
[3]. Muñoz-Esparza, N.C., Costa-Catala, J., Comas-Basté, O., Toro-Funes, N., Latorre-Moratalla, M.L., Veciana-Nogués, M.T. and Vidal-Carou, M.C. (2021). Occurrence of Polyamines in Foods and the Influence of Cooking Processes. Foods, 10(8), p.1752. doi:https://doi.org/10.3390/foods10081752.
[4]. Hofer, S.J., Simon, A.K., Bergmann, M., Eisenberg, T., Kroemer, G. and Madeo, F. (2022). Mechanisms of spermidine-induced autophagy and geroprotection. Nature Aging, [online] 2(12), pp.1112–1129. doi:https://doi.org/10.1038/s43587-022-00322-9.
[5]. Madeo, F., Bauer, M.A., Carmona-Gutierrez, D. and Kroemer, G. (2018a). Spermidine: a physiological autophagy inducer acting as an anti-aging vitamin in humans? Autophagy, [online] 15(1), pp.165–168. doi:https://doi.org/10.1080/15548627.2018.1530929.
[6]. Filomeni, G., De Zio, D. and Cecconi, F. (2015). Oxidative stress and autophagy: the clash between damage and metabolic needs. Cell Death & Differentiation, [online] 22(3), pp.377–388. doi:https://doi.org/10.1038/cdd.2014.150.
[7]. Aman, Y., Schmauck-Medina, T., Hansen, M., Morimoto, R.I., Simon, A.K., Bjedov, I., Palikaras, K., Simonsen, A., Johansen, T., Tavernarakis, N., Rubinsztein, D.C., Partridge, L., Kroemer, G., Labbadia, J. and Fang, E.F. (2021). Autophagy in healthy aging and disease. Nature Aging, 1(8), pp.634–650. doi:https://doi.org/10.1038/s43587-021-00098-4.
[8]. Shabkhizan, R., Haiaty, S., Moslehian, M.S., Bazmani, A., Sadeghsoltani, F., Saghaei Bagheri, H., Rahbarghazi, R. and Sakhinia, E. (2023). The Beneficial and Adverse Effects of Autophagic Response to Caloric Restriction and Fasting. Advances in Nutrition, [online] 14(5), pp.1211–1225. doi:https://doi.org/10.1016/j.advnut.2023.07.006.
[9]. Yang, Z. and Klionsky, D.J. (2009). An Overview of the Molecular Mechanism of Autophagy. Current Topics in Microbiology and Immunology, 335, pp.1–32. doi:https://doi.org/10.1007/978-3-642-00302-8_1.
[10]. Hofer, S.J., Simon, A.K., Bergmann, M., Eisenberg, T., Kroemer, G. and Madeo, F. (2022). Mechanisms of spermidine-induced autophagy and geroprotection. Nature Aging, [online] 2(12), pp.1112–1129. doi:https://doi.org/10.1038/s43587-022-00322-9.
[11]. Hofer, S.J., Ioanna Daskalaki, Bergmann, M., Jasna Friščić, Zimmermann, A., Mueller, M.I., Abdellatif, M., Nicastro, R., Masser, S., Durand, S., Nartey, A., Waltenstorfer, M., Enzenhofer, S., Faimann, I., Gschiel, V., Bajaj, T., Niemeyer, C., Ilias Gkikas, Pein, L. and Cerrato, G. (2024). Spermidine is essential for fasting-mediated autophagy and longevity. Nature Cell Biology. [online] doi:https://doi.org/10.1038/s41556-024-01468-x.
[12]. Schwarz, C., Stekovic, S., Wirth, M., Benson, G., Royer, P., Sigrist, S.J., Pieber, T., Dammbrueck, C., Magnes, C., Eisenberg, T., Pendl, T., Bohlken, J., Köbe, T., Madeo, F. and Flöel, A. (2018). Safety and tolerability of spermidine supplementation in mice and older adults with subjective cognitive decline. Aging, 10(1), pp.19–33. doi:https://doi.org/10.18632/aging.101354.
[13]. Ni, Y.-Q. and Liu, Y.-S. (2021). New Insights into the Roles and Mechanisms of Spermidine in Aging and Age-Related Diseases. Aging and disease, 12(8), p.1948. doi:https://doi.org/10.14336/ad.2021.0603.
[14]. Madeo, F., Eisenberg, T., Pietrocola, F. and Kroemer, G. (2018b). Spermidine in health and disease. Science, 359(6374), p.eaan2788. doi:https://doi.org/10.1126/science.aan2788.
[15]. Kiechl, S., Pechlaner, R., Willeit, P., Notdurfter, M., Paulweber, B., Willeit, K., Werner, P., Ruckenstuhl, C., Iglseder, B., Weger, S., Mairhofer, B., Gartner, M., Kedenko, L., Chmelikova, M., Stekovic, S., Stuppner, H., Oberhollenzer, F., Kroemer, G., Mayr, M. and Eisenberg, T. (2018). Higher spermidine intake is linked to lower mortality: a prospective population-based study. The American Journal of Clinical Nutrition, 108(2), pp.371–380. doi:https://doi.org/10.1093/ajcn/nqy102.
[16]. Raife, T., Mill, J., Hess, A.S. and Haj, A. (2022). The Longevity Factor Spermidine Is Highly Heritable in Human Erythrocytes and Is Part of a Complex Phenotype Associated with Human Longevity. Blood, 140(Supplement 1), pp.5311–5312. doi:https://doi.org/10.1182/blood-2022-158274.
[17]. LaRocca, T.J., Gioscia-Ryan, R.A., Hearon, C.M. and Seals, D.R. (2013). The autophagy enhancer spermidine reverses arterial aging. Mechanisms of Ageing and Development, 134(7-8), pp.314–320. doi:https://doi.org/10.1016/j.mad.2013.04.004.
[18]. Büttner, S., Broeskamp, F., Sommer, C., Markaki, M., Habernig, L., Alavian-Ghavanini, A., Carmona-Gutierrez, D., Eisenberg, T., Michael, E., Kroemer, G., Tavernarakis, N., Sigrist, S.J. and Madeo, F. (2014). Spermidine protects against α-synuclein neurotoxicity. Cell Cycle, 13(24), pp.3903–3908. doi:https://doi.org/10.4161/15384101.2014.973309.
[19]. Schroeder, S., Hofer, S.J., Zimmermann, A., Pechlaner, R., Dammbrueck, C., Pendl, T., Marcello, G.M., Pogatschnigg, V., Bergmann, M., Müller, M., Gschiel, V., Ristic, S., Tadic, J., Iwata, K., Richter, G., Farzi, A., Üçal, M., Schäfer, U., Poglitsch, M. and Royer, P. (2021). Dietary spermidine improves cognitive function. Cell Reports, [online] 35(2), p.108985. doi:https://doi.org/10.1016/j.celrep.2021.108985.
[20]. National Institute on Aging (2022). What Is Dementia? Symptoms, Types, and Diagnosis. [online] What Is Dementia? Symptoms, Types, and Diagnosis. Available at: https://www.nia.nih.gov/health/alzheimers-and-dementia/what-dementia-symptoms-types-and-diagnosis.
[21]. Pekar, T., Bruckner, K., Pauschenwein-Frantsich, S., Gschaider, A., Oppliger, M., Willesberger, J., Ungersbäck, P., Wendzel, A., Kremer, A., Flak, W., Wantke, F. and Jarisch, R. (2021). The positive effect of spermidine in older adults suffering from dementia. Wiener Klinische Wochenschrift, [online] 133(9), pp.484–491. doi:https://doi.org/10.1007/s00508-020-01758-y.
[22]. Wirth, M., Benson, G., Schwarz, C., Köbe, T., Grittner, U., Schmitz, D., Sigrist, S.J., Bohlken, J., Stekovic, S., Madeo, F. and Flöel, A. (2018). The effect of spermidine on memory performance in older adults at risk for dementia: A randomized controlled trial. Cortex, 109, pp.181–188. doi:https://doi.org/10.1016/j.cortex.2018.09.014.
[23]. Trovò, L., Angélique Vaucher, Pan, Y., Steiner, P., Flunkert, S., Fleming, S.A. and Preitner, N. (2023). In vitro assessment of dietary bioactives for TFEB activation as a possible target to support cognitive and emotional wellbeing. Journal of Functional Foods, 111, pp.105855–105855. doi:https://doi.org/10.1016/j.jff.2023.105855.
[24]. Qi, G., Wang, J., Chen, Y., Wei, W. and Sun, C. (2024). Association between dietary spermidine intake and depressive symptoms among US adults: National Health and Nutrition Examination Survey (NHANES) 2005–2014. Journal of Affective Disorders, 359, pp.125–132. doi:https://doi.org/10.1016/j.jad.2024.05.041.
[25]. Wang, T., Li, N. and Zeng, Y. (2024). Protective effects of spermidine levels against cardiovascular risk factors: an exploration of causality based on a bi-directional Mendelian randomization analysis. Nutrition, pp.112549–112549. doi:https://doi.org/10.1016/j.nut.2024.112549.
[26]. Chen, Y., Li, H., Zhao, F., Yu, M., Pan, S., Sun, W., Yin, Y. and Zhu, T. (2024). Spermidine attenuates monocrotaline-induced pulmonary arterial hypertension in rats by inhibiting purine metabolism and polyamine synthesis-associated vascular remodeling. International Immunopharmacology, 132, pp.111946–111946. doi:https://doi.org/10.1016/j.intimp.2024.111946.
[27]. Tocci, G., Biondi-Zoccai, G., Forte, M., Gallo, G., Giulia Nardoianni, Fiori, E., Luca D'Ambrosio, Riccardo Di Pietro, Stefanini, G., Cannata, F., Rocco, E., Simeone, B., Sarto, G., Schirone, L., D’Amico, A., Peruzzi, M., Nocella, C., Volpe, M. and Speranza Rubattu (2023). Effects of two-month treatment with a mixture of natural activators of autophagy on oxidative stress and arterial stiffness in patients with essential hypertension: A pilot study. Nutrition Metabolism and Cardiovascular Diseases, 33(11), pp.2287–2293. doi:https://doi.org/10.1016/j.numecd.2023.07.026.
[28]. Swarup, S. and Zeltser, R. (2024). Metabolic Syndrome. [online] Nih.gov. Available at: https://www.ncbi.nlm.nih.gov/books/NBK459248/.
[29]. Nie, T., Fang, Z., Liu, H., Zhang, X., Ying, F., Xu, X., Huang, H. and Wu, J. (2022). Bioactive spermidine nanoparticles for effective cardiovascular recovery and diabetic therapy. Chemical Engineering Journal, 446, pp.137353–137353. doi:https://doi.org/10.1016/j.cej.2022.137353.
[30]. Michiels, C.F., Kurdi, A., Timmermans, J.-P., De Meyer, G.R.Y. and Martinet, W. (2016). Spermidine reduces lipid accumulation and necrotic core formation in atherosclerotic plaques via induction of autophagy. Atherosclerosis, 251, pp.319–327. doi:https://doi.org/10.1016/j.atherosclerosis.2016.07.899.
[31]. Ni, Y., Zheng, L., Zhang, L., Li, J., Pan, Y., Du, H., Wang, Z. and Fu, Z. (2024). Spermidine activates adipose tissue thermogenesis through autophagy and fibroblast growth factor 21. The Journal of Nutritional Biochemistry, 125, pp.109569–109569. doi:https://doi.org/10.1016/j.jnutbio.2024.109569.









