1. Introduction
Traditional diagnostic methods for CRC include interventional endoscopy [5] and CT scans [6]. While widely used in clinical practice, each of these approaches has limitations. In recent years, imaging technology has played an increasingly important role in the detection, staging and treatment planning of rectal cancer [7]. Concurrently, the rapid advancement of artificial intelligence (AI) technology has opened new avenues for enhancing medical imaging analysis, significantly improving diagnostic accuracy and efficiency [8].
Magnetic resonance imaging (MRI) technology, in particular, has gained prominence due to its ability to provide high-resolution soft tissue contrast images. This capability potentially reduces the limitations of manual interpretation and the risk of missed diagnoses [9]. The integration of AI with MRI technology presents a promising frontier in CRC diagnosis, offering the potential to overcome the limitations of traditional methods and enhance overall diagnostic capabilities.
Our analysis will encompass three aspects. Firstly, it will include a review of traditional diagnostic techniques, including interventional endoscopy, CT scans and conventional MRI. Secondly, AI-enhanced MRI techniques will be explored, focusing on convolutional neural networks (CNN), generative adversarial networks (GAN) and transformer models. Finally, there will be a comparative analysis of these methods in terms of diagnostic accuracy, clinical applicability, cost-effectiveness and patient experience. By systematically examining these aspects, we aim to provide a comprehensive understanding of the current state and future potential of AI applications in CRC diagnosis, ultimately informing clinical practice and guiding future research directions in this rapidly evolving field.
2. Results
Among the traditional diagnostic methods for colorectal cancer, interventional endoscopy is an important technique, including colonoscopy, sigmoidoscopy and endoscopic ultrasonography [10]. Colonoscopy is an effective method to detect colorectal cancer; it allows doctors to directly observe the internal conditions of the whole colon and rectum and perform a biopsy or resection of small polyps when abnormalities are found [11]. Additionally, colonoscopies are extremely effective in reducing the incidence and mortality of colorectal cancer. However, they require good bowel preparation and the examination process may bring discomfort to patients [12].
Sigmoidoscopy is a relatively limited endoscopic examination, which can only detect the lower part of the colon, namely the sigmoid colon and rectum [13]. This method is suitable for the discovery of cancer or polyps in this area. It is a less invasive examination but because it cannot cover the whole colon, it may miss lesions in other parts [14]. Endoscopic ultrasonography (EUS) can image the colorectal wall and its surrounding tissues by installing an ultrasound probe at the top of the endoscope, which is helpful to evaluate the depth of the tumour, lymph node involvement and whether there is distant metastasis [15]. EUS has unique advantages in the preoperative staging of colorectal cancer, guiding the choice of treatment options and monitoring the prognosis of the disease, especially in evaluating the staging of rectal cancer [16].
CT is a medical imaging technique using X-rays to generate images of a patient’s body [17]. It plays an important role in the diagnosis, staging and treatment evaluation of colorectal cancer. It can provide images of the colon and help doctors identify the location, size, shape and relationship between the tumour and surrounding tissues [18]. However, CT use X-rays, and patients will be exposed to a dose of radiation during the examination process. Although the single dose of radiation is very low, multiple exposures to this radiation may be harmful to the health of the patients.
MRI is a type of imaging method that produces images of body tissues using magnetic fields and radio waves without the need for invasive procedures [9]. The special feature of MRI in the diagnosis of colorectal cancer is its high soft tissue resolution, which can clearly show the structure of each layer of the rectum and help to accurately evaluate the relationship between the depth of tumour invasion and surrounding tissues, especially in the evaluation of T stage and N stage of rectal cancer, which shows higher accuracy than CT. In addition, MRI can distinguish treatment-induced fibrosis from residual tumour tissue in the efficacy evaluation after neoadjuvant therapy and provide important information for clinical decision-making [19].
Compared to invasive endoscopic examinations, MRI provides comprehensive internal images without damaging tissues, reducing patient discomfort and the risk of complications. Additionally, MRI, with excellent soft tissue contrast, no radiation risk, multi-plane imaging capability and functional imaging technology, provides a highly sensitive assessment of tumour invasion depth and surrounding structural invasion in the detection of colorectal cancer [20]. Colonoscopy, on the other hand, provides an effective means of early detection and treatment by directly observing the interior of the colon and conducting a biopsy, which is especially suitable for polypectomy and cancer screening [12].
Table 1. Accuracy of different diagnostic techniques for colorectal cancer.
Diagnostic method | Accuracy (%) | Source |
Endoscopy | 92 | Obstein and Valdastri 2013 |
Endoscopic ultrasound (EUS) | 87 | Wadhwa, Patel et al. 2023 |
CT scan | 85 | Kijima, Sasaki et al. 2014 |
MRI (traditional) | 90 | Kijima, Sasaki et al. 2014&Low 2002 |
The data in Table 1 shows that the diagnostic accuracy of MRI is higher than that of any technology except endoscopy, which is the gold standard. Although CT scanning can provide detailed structural images, its resolution for soft tissue is not as good as MRI [21]. Kijima et al. also believed that MRI can provide higher-resolution images [22]. Furthermore, Del Ciello presented convincing evidence that CT may lead to missed diagnosis when scanning small lesions, especially lesions with a diameter of less than 5 mm [23].
3. AI-Enhanced MRI Techniques
By comparing the data in Table 2, it is evident that the diagnostic accuracy of MRI combined with AI surpasses that of traditional MRI, CT scans and even CT scans augmented with AI. The combination of MRI and AI showed the highest overall accuracy (95%) among all methods reviewed. This clearly demonstrates that AI technology significantly enhances the diagnostic capabilities of MRI. The integration of AI likely aids in improving diagnosis through advanced image analysis, pattern recognition and other techniques, enabling physicians to make more precise diagnoses. At the current level of technology, MRI already outperforms CT in terms of soft tissue contrast and the addition of AI further strengthens its diagnostic power.
Table 2. Summary of diagnostic accuracy of different techniques combined with AI.
Diagnostic method | Accuracy (%) | Source |
MRI + AI | 95 | Wong, Fu et al. 2023 |
MRI (traditional) | 90 | Kijima, Sasaki et al. 2014&Low 2002 |
CT SCAN (traditional) | 85 | Kijima, Sasaki et al. 2014 |
CT scan + AI | 88 | Rompianesi, Pegoraro et al. 2022 |
The currently proposed deep learning algorithms and methods for colorectal cancer diagnosis include convolutional neural networks (CNN), generative adversarial networks (GAN) and transformer models. CNN assists in accurate diagnosis by automatically extracting image features. GAN can improve image quality by generating high-quality image data for image enhancement and reconstruction. Transformer models use a self-attention mechanism to capture long-distance dependencies in images and provide global context understanding. The comprehensive application of these models enhances the ability of doctors to recognise lesions and improves the accuracy and efficiency of diagnosis [24].
3.1. CNN and MRI for CRC Diagnosis
Convolutional neural networks are a type of deep learning model that are particularly suitable for image processing tasks. The core idea is to extract image features through multiple convolutional and pooling layers and then classify them through fully connected layers. CNNs have shown great potential in the diagnosis of colorectal cancer, especially through the analysis of colonoscopy images. This analysis consists of convolutional layers, activation functions, pooling layers, fully connected layers and output layers. The convolutional layer uses multiple convolutional kernels (filters) to locally scan the input image and extract local features. Each convolution kernel generates a feature map. Then, the activation function introduces nonlinearity using the ReLU (Rectified Linear Unit) function. Following this, the pooling layer reduces the size of the feature map and lowers computational complexity through downsampling. The fully connected layer expands the previous feature map into a one-dimensional vector and classifies it through multiple neurons. The final layer outputs the classification results through Softmax or Sigmoid functions.
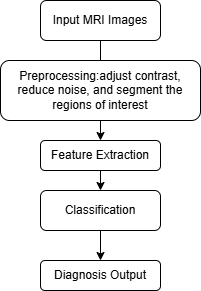
Figure 1. The workflow of CNN application in MRI.
In this process, a series of MRI scans of the captured colorectal region were input first. These images serve as input to the neural network. Then pretreatment was performed. This step involves improving image quality by adjusting contrast, reducing noise and segmenting regions of interest (such as tissues or potential tumour regions). The next step is feature extraction. CNN recognises and learns spatial patterns and features in MRI images through the convolution layer, pooling layer and full connection layer. These patterns may include the typical texture, shape, and structure of benign or malignant tumours. The next step is classification: the extracted features pass through the fully connected layer and CNN predicts according to the learned data. The network classifies whether a tumour is benign or malignant. Finally, diagnostic output is carried out; this last output provides diagnostic decisions and confidence scores to help radiologists or clinicians decide the next action.
CNNs have many applications and can solve numerous problems in the diagnosis of colorectal cancer. CNNs can automatically extract complex image features from colonoscopy images (CT or MRI), such as tumour morphology, colour, texture, etc. [25, 24]. They also reduce the steps of manual feature extraction, improving efficiency and accuracy. This is compared to traditional methods which rely on manual feature extraction, where it is easy to miss or fail to capture subtle features. CNN automatically learns features through multi-layer convolution, greatly improving the quality of feature extraction. In addition, through a large amount of training data, CNNs can learn the characteristics of various types of colorectal cancer lesions.
Without the use of AI, there are consistency issues in doctors’ diagnoses due to the significant influence of experience and other subjective factors. However, CNN provides consistent diagnostic results through standardised algorithmic processes. This can greatly reduce the rate of misdiagnosis; especially in early cancer recognition, CNN plays an important role. Through the application of CNN, the diagnosis of colorectal cancer has become more automated, accurate and efficient, providing important support for early detection and treatment.
3.2. GAN and MRI for CRC Diagnosis
Generative adversarial networks are a type of deep learning model primarily used to generate new data that is similar to real data. They consist of two neural networks: a generator and a discriminator. The generator inputs random noise and generates realistic images through a series of deconvolution layers.
The goal is to deceive the discriminator into thinking that the generated image is real. The discriminator inputs real images and generated images, extracts features through convolutional layers and performs classification. The goal is to distinguish between real images and generated images. These two networks compete with each other during the training process and ultimately the generator is able to generate high-quality fake data that is difficult to distinguish from real data. The following is a schematic diagram of the basic structure of a GAN:
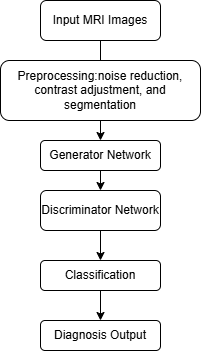
Figure 2. The workflow of GAN application in MRI.
In this process, an MRI scan of the colorectal region was input first. Then, pretreatment was performed. The image is pre-processed by noise reduction, contrast adjustment, segmentation and other standards to prepare for GAN analysis. The next step is the generator network; the generator is a neural network that can create synthetic MRI images similar to real images of healthy colorectal tissue. This helps the GAN system learn the differences between normal and abnormal tissues. The data is then transmitted to the discriminator network. The discriminator compares the real MRI image with the synthetic image generated by the generator. It attempts to detect abnormalities by distinguishing between normal and cancerous regions. Following this is the classifying stage. After the discriminator recognises the differential pattern, the system determines whether the MRI image contains benign or malignant features. Finally, the diagnostic output is carried out. The final output is a likelihood score or classification (benign versus malignant) based on discriminator analysis.
GAN has many applications in the diagnosis of colorectal cancer and has solved many problems. Medical image data acquisition has high costs and limited samples, especially for rare diseases. GAN can generate a large number of fake images that are similar to real data, enriching the training dataset. It can enhance the generalisation ability and robustness of the model through data augmentation. The actual medical images obtained may have issues such as noise, blurring, or incompleteness. However, GAN can perform image restoration or completion in low-quality or partially missing biological images, improve image quality, provide clearer images of lesion sites and assist doctors in diagnosis.
GAN can also achieve image conversion between different imaging modes (such as CT, MRI), enabling data exchange between different modalities and improving the comprehensive utilisation efficiency of multimodal data. It can also assist doctors in analysing lesions from different perspectives to improve diagnostic accuracy. The application of GAN in the diagnosis of colorectal cancer has greatly enhanced medical image processing, providing important support for early detection and precise treatment.
3.3. Transformer and MRI for CRC Diagnosis
A transformer model is a deep learning model, originally designed for natural language processing (NLP) tasks. They have many applications in the diagnosis of colorectal cancer and have solved many problems. A traditional convolutional neural network (CNN) has a limited ability to extract local features when processing images. However, the self-attention mechanism of transformer models can capture the long-distance relationship between different regions in the image, so as to more accurately extract and understand the global features of the image. Furthermore, through the multi-head attention mechanism, a transformer model can process different scales of information, which helps to identify different details of the tumour region, thereby improving the accuracy of diagnosis.
In addition, in biological images, location information is very important for feature analysis. Location coding can make up for the lack of spatial information of a transformer model and make the model better understand the spatial distribution in the image. Moreover, because a transformer can effectively learn long-distance dependencies, it can effectively reduce the dependence on large-scale annotation data.
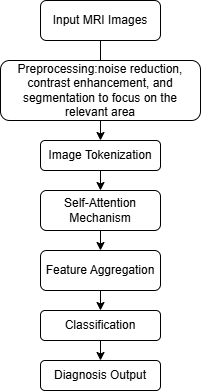
Figure 3. The workflow of Transformer application in MRI.
In this process, the MRI image of the colorectal region was input first. Then pretreatment was performed. The image was pre-processed by noise reduction, contrast enhancement and segmentation to focus on the relevant region. Then image tokenisation was performed. In the transformer model, the MRI image was decomposed into markers. Each marker represents a part of the image and the model processes these markers separately. Subsequently, the data is transferred to the self-attention mechanism. During this period, the converter uses self-attention to compare the importance of each token relative to other tokens. This enables the model to understand spatial relationships and patterns in the image (e.g., areas that may indicate cancer cell growth). The converter then integrates the features learned from the attention mechanism, providing a high-level understanding of MRI images and their tumour characteristics. Following this is the classifying stage. Similar to CNN, a transformer model classifies tumours as benign or malignant according to the characteristics of aggregation. Finally, diagnostic output is carried out. The output is a diagnostic judgment and a confidence level, which provides insights into the possibility of tumour carcinogenesis. These help reduce errors caused by judgment and facilitate detection and treatment [26, 19].
However, there are drawbacks to combining MRI with AI technology. Firstly, MRI exams can be costly [27]. Secondly, MRI scans are time-consuming, requiring patients to remain motionless in the scanner, which may not be suitable for the old or those with limited mobility. Moreover, developing AI models necessitates large amounts of high-quality data. Lastly, rigorous clinical validation of AI algorithms is essential to ensure their safety and efficacy before obtaining approval from official bodies [28].
In the MRI diagnosis of colorectal cancer (CRC), the applications of convolutional neural network (CNN) and generative adversary network (GAN) are constantly progressing. CNN is mainly used for automatic feature extraction and image classification in MRI image analysis. For example, a study analysed MRI images of rectal cancer through CNN, which can automatically extract image features, thus improving the diagnostic accuracy of rectal cancer [29]. In addition, CNN has also been used as an automatic diagnosis platform for TN staging of rectal cancer. Target detection through the faster R-CNN model provides a new possibility for rectal cancer staging [30].
On the other hand, GANs show potential in medical image enhancement, especially in MRI image quality improvement. GANs can generate higher quality image data through the generation of the confrontation process, which is particularly important for the improvement of MRI image details and contrast. In the diagnosis of rectal cancer, GANs can help generate clearer images to assist doctors in making more accurate diagnosis [31] while transformer models are computationally intensive and may require more resources to implement effectively.
These studies show that deep learning technology has significant application value in the MRI diagnosis of colorectal cancer, which can not only improve the accuracy of diagnosis but also improve the quality of MRI images through image enhancement technology, providing more powerful diagnostic support for clinicians. With the continuous progress of technology, it is expected that these methods will play a greater role in future clinical practice.
4. Discussion
In conclusion, MRI combined with AI has shown significant advantages in the diagnosis of colorectal cancer. In contrast, although traditional endoscopy, sigmoid endoscopy, endoscopic ultrasonography and CT scans have the same important position in clinical practice, they have notable limitations. The invasiveness of traditional endoscopy may lead to incomplete data and complications and the technical requirements for operators are high. Although endoscopic ultrasonography can affect the performance of local lesions, its operation is complex and limited. CT scans can provide a comprehensive image but its resolution of soft tissue is not as good as MRI and there is a risk of radiation. Moreover, MRI scans are costly and resource-intensive. Consequently, it is important to develop the technology and reduce its cost.
With this in mind, the combination of MRI with AI technology in the diagnosis of colorectal cancer is increasingly obvious. Although traditional methods still have value in some specific cases, considering all factors, MRI+AI is undoubtedly more powerful and will continue to advance with the continuous progress of technology. In the future, MRI+AI is expected to become the mainstream method of colorectal cancer diagnosis in the future, further improving the survival rate and quality of life of patients. In the future, the combination of MRI+AI and traditional endoscopy may provide comprehensive information for the diagnosis and treatment of colorectal cancer.
This research did not conduct any experiments and the data and results used are all from external sources. Therefore, the data and results used are not the most current and the conclusions drawn from the data may not be applicable to the present. Despite these limitations, the study certainly adds to our understanding of MRI combined with AI. However, considerably more work will need to be done to determine this result.
References
[1]. Sawicki et al., 2021
[2]. Mármol, I., Sánchez-de-Diego, C., Pradilla Dieste, A., Cerrada, E. and Rodriguez-Yoldi, M. J. (2017) Colorectal carcinoma: a general overview and future perspectives in colorectal cancer. International Journal of Molecular Sciences, 18(1), 197.
[3]. Granados-Romero, J. J., Valderrama-Treviño, A. I., Contreras-Flores, E. H., Barrera-Mera, B., Herrera Enríquez, M., Uriarte-Ruíz, K., Ceballos-Villalba, J. C., Estrada-Mata, A. G., Alvarado Rodríguez, C. and Arauz-Peña, G. (2017) Colorectal cancer: a review. International Journal of Research in Medical Sciences, 5(11), 4667.
[4]. Cappell, M. S. (2008) Pathophysiology, clinical presentation, and management of colon cancer. Gastroenterology Clinics of North America, 37(1), 1-24.
[5]. Obstein, K. L. and Valdastri, P. (2013) Advanced endoscopic technologies for colorectal cancer screening. World Journal of Gastroenterology, 19(4), 431.
[6]. Rocca, A., Brunese, M. C., Santone, A., Avella, P., Bianco, P., Scacchi, A., Scaglione, M., Bellifemine, F., Danzi, R. and Varriano, G. (2021) Early diagnosis of liver metastases from colorectal cancer through CT radiomics and formal methods: a pilot study. Journal of Clinical Medicine, 11(1), 31.
[7]. Bates, D. D. B., Homsi, M. E., Chang, K. J., Lalwani, N., Horvat, N. and Sheedy, S. P. (2022) MRI for Rectal Cancer: Staging, mrCRM, EMVI, Lymph Node Staging and Post-Treatment Response. Clinical Colorectal Cancer, 21(1), 10-18.
[8]. Mitsala, A., Tsalikidis, C., Pitiakoudis, M., Simopoulos, C. and Tsaroucha, A. K. (2021) Artificial intelligence in colorectal cancer screening, diagnosis and treatment. A new era. Current Oncology 28(3), 1581-1607.
[9]. Hashemi, R. H., Bradley, W. G. and Lisanti, C. J. (2012) MRI: The Basics, Lippincott Williams & Wilkins.
[10]. Serracant, A., Consola, B., Ballesteros, E., Sola, M., Novell, F., Montes, N. and Serra-Aracil X. (2024) How to Study the Location and Size of Rectal Tumors That Are Candidates for Local Surgery: Rigid Rectoscopy, Magnetic Resonance, Endorectal Ultrasound or Colonoscopy? An Interobservational Study. Diagnostics (Basel), 14(3), 315.
[11]. Williams and Teague 1973
[12]. Fisher, Maple et al. 2011
[13]. Brenner, Stock et al. 2014
[14]. Waye, Kahn et al. 1996
[15]. Gao, Hu et al. 2020
[16]. Bratanic, Bozic et al. 2021
[17]. Goldman, L. W. (2007) Principles of CT and CT technology. Journal of Nuclear Medicine Technology, 35(3), 115-128.
[18]. Halligan, S., Wooldrage, K., Dadswell, E., Kralj-Hans, I., Von Wagner, C., Edwards, R., Yao, G., Kay, C., Burling, D. and Faiz, O. (2013) Computed tomographic colonography versus barium enema for diagnosis of colorectal cancer or large polyps in symptomatic patients (SIGGAR): a multicentre randomised trial. The Lancet, 381(9873), 1185-1193.
[19]. Zhu et al., 2021) / Zhu, Xu et al. 2021
[20]. Tsili, Alexiou et al. 2021
[21]. Guimarães et al., 2024
[22]. Kijima et al. 2023
[23]. Del Ciello, A., Franchi, P., Contegiacomo, A., Cicchetti, G., Bonomo, L. and Larici, A. R. (2017) Missed lung cancer: when, where, and why? Diagnostic and Interventional Radiology, 23(2), 118.
[24]. Jiang, Zhao et al. 2023
[25]. Rompianesi, Pegoraro et al. 2022
[26]. Holzinger, Langs et al. 2019
[27]. Hilabi, B. S., Alghamdi, S. A. and Almanaa, M. (2023) Impact of magnetic resonance imaging on healthcare in low-and middle-income countries. Cureus, 15(4).
[28]. Chen, Y., Schönlieb, C.-B., Liò, P., Leiner, T., Dragotti, P. L., Wang, G., Rueckert, D., Firmin, D. and Yang, G. (2022) AI-based reconstruction for fast MRI - A systematic review and meta-analysis. Proceedings of the IEEE, 110(2), 224-245.
[29]. Wan, Hu et al. 2023
[30]. Zhou, Greenspan et al. 2021
[31]. Shende, Pawar et al. 2019
Cite this article
Wang,J. (2025). Application of AI Deep Learning in Colorectal Cancer. Theoretical and Natural Science,76,20-27.
Data availability
The datasets used and/or analyzed during the current study will be available from the authors upon reasonable request.
Disclaimer/Publisher's Note
The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of EWA Publishing and/or the editor(s). EWA Publishing and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
About volume
Volume title: Proceedings of the 4th International Conference on Biological Engineering and Medical Science
© 2024 by the author(s). Licensee EWA Publishing, Oxford, UK. This article is an open access article distributed under the terms and
conditions of the Creative Commons Attribution (CC BY) license. Authors who
publish this series agree to the following terms:
1. Authors retain copyright and grant the series right of first publication with the work simultaneously licensed under a Creative Commons
Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this
series.
2. Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the series's published
version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial
publication in this series.
3. Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and
during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See
Open access policy for details).
References
[1]. Sawicki et al., 2021
[2]. Mármol, I., Sánchez-de-Diego, C., Pradilla Dieste, A., Cerrada, E. and Rodriguez-Yoldi, M. J. (2017) Colorectal carcinoma: a general overview and future perspectives in colorectal cancer. International Journal of Molecular Sciences, 18(1), 197.
[3]. Granados-Romero, J. J., Valderrama-Treviño, A. I., Contreras-Flores, E. H., Barrera-Mera, B., Herrera Enríquez, M., Uriarte-Ruíz, K., Ceballos-Villalba, J. C., Estrada-Mata, A. G., Alvarado Rodríguez, C. and Arauz-Peña, G. (2017) Colorectal cancer: a review. International Journal of Research in Medical Sciences, 5(11), 4667.
[4]. Cappell, M. S. (2008) Pathophysiology, clinical presentation, and management of colon cancer. Gastroenterology Clinics of North America, 37(1), 1-24.
[5]. Obstein, K. L. and Valdastri, P. (2013) Advanced endoscopic technologies for colorectal cancer screening. World Journal of Gastroenterology, 19(4), 431.
[6]. Rocca, A., Brunese, M. C., Santone, A., Avella, P., Bianco, P., Scacchi, A., Scaglione, M., Bellifemine, F., Danzi, R. and Varriano, G. (2021) Early diagnosis of liver metastases from colorectal cancer through CT radiomics and formal methods: a pilot study. Journal of Clinical Medicine, 11(1), 31.
[7]. Bates, D. D. B., Homsi, M. E., Chang, K. J., Lalwani, N., Horvat, N. and Sheedy, S. P. (2022) MRI for Rectal Cancer: Staging, mrCRM, EMVI, Lymph Node Staging and Post-Treatment Response. Clinical Colorectal Cancer, 21(1), 10-18.
[8]. Mitsala, A., Tsalikidis, C., Pitiakoudis, M., Simopoulos, C. and Tsaroucha, A. K. (2021) Artificial intelligence in colorectal cancer screening, diagnosis and treatment. A new era. Current Oncology 28(3), 1581-1607.
[9]. Hashemi, R. H., Bradley, W. G. and Lisanti, C. J. (2012) MRI: The Basics, Lippincott Williams & Wilkins.
[10]. Serracant, A., Consola, B., Ballesteros, E., Sola, M., Novell, F., Montes, N. and Serra-Aracil X. (2024) How to Study the Location and Size of Rectal Tumors That Are Candidates for Local Surgery: Rigid Rectoscopy, Magnetic Resonance, Endorectal Ultrasound or Colonoscopy? An Interobservational Study. Diagnostics (Basel), 14(3), 315.
[11]. Williams and Teague 1973
[12]. Fisher, Maple et al. 2011
[13]. Brenner, Stock et al. 2014
[14]. Waye, Kahn et al. 1996
[15]. Gao, Hu et al. 2020
[16]. Bratanic, Bozic et al. 2021
[17]. Goldman, L. W. (2007) Principles of CT and CT technology. Journal of Nuclear Medicine Technology, 35(3), 115-128.
[18]. Halligan, S., Wooldrage, K., Dadswell, E., Kralj-Hans, I., Von Wagner, C., Edwards, R., Yao, G., Kay, C., Burling, D. and Faiz, O. (2013) Computed tomographic colonography versus barium enema for diagnosis of colorectal cancer or large polyps in symptomatic patients (SIGGAR): a multicentre randomised trial. The Lancet, 381(9873), 1185-1193.
[19]. Zhu et al., 2021) / Zhu, Xu et al. 2021
[20]. Tsili, Alexiou et al. 2021
[21]. Guimarães et al., 2024
[22]. Kijima et al. 2023
[23]. Del Ciello, A., Franchi, P., Contegiacomo, A., Cicchetti, G., Bonomo, L. and Larici, A. R. (2017) Missed lung cancer: when, where, and why? Diagnostic and Interventional Radiology, 23(2), 118.
[24]. Jiang, Zhao et al. 2023
[25]. Rompianesi, Pegoraro et al. 2022
[26]. Holzinger, Langs et al. 2019
[27]. Hilabi, B. S., Alghamdi, S. A. and Almanaa, M. (2023) Impact of magnetic resonance imaging on healthcare in low-and middle-income countries. Cureus, 15(4).
[28]. Chen, Y., Schönlieb, C.-B., Liò, P., Leiner, T., Dragotti, P. L., Wang, G., Rueckert, D., Firmin, D. and Yang, G. (2022) AI-based reconstruction for fast MRI - A systematic review and meta-analysis. Proceedings of the IEEE, 110(2), 224-245.
[29]. Wan, Hu et al. 2023
[30]. Zhou, Greenspan et al. 2021
[31]. Shende, Pawar et al. 2019









