1. Introduction
According to the WHO 2022 report, breast cancer is the most frequently diagnosed cancer in women, with 2.3 million new cases and 670,000 deaths globally [1]. Breast cancer is broadly classified into two main categories: invasive and non-invasive. Invasive breast cancers make up about 70-80% of cases, while non-invasive types constitute around 20% [2, 3]. Invasive breast cancer is further subdivided into invasive ductal carcinoma (80%), invasive lobular carcinoma (10%), inflammatory breast cancer (5%), and triple-negative breast cancer (5%). These subtypes are typically categorized based on the immunohistochemical presence of the estrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor receptor 2 (HER2) [4].
Treatment options for stages I to III breast cancer usually include surgery and radiation therapy. For stage IV, systemic therapies such as hormone therapy, chemotherapy, immunotherapy, and targeted treatments are the primary methods. Around 67% of invasive breast cancers are driven by hormones like estrogen and progesterone, which stimulate cancer cell growth through hormone receptor binding. Hormone therapies work by blocking these hormones from interacting with their receptors, inhibiting further growth. However, these therapies can result in severe side effects such as blood clots, cataracts, and tumor flare [1-3].
Chemotherapy uses anti-cancer drugs to target and kill cancer cells throughout the body, but this approach often leads to side effects including changes in menstruation, fertility issues, and nerve damage [4, 5]. Immunotherapy, which strengthens the immune system's ability to target cancer cells by blocking PD-1 inhibitors, also carries risks such as infusion reactions and autoimmune responses [6]. Targeted drug therapy aims to disrupt cancer cell growth by focusing on specific proteins that regulate cell proliferation and survival [7]. Although these therapies have shown promise in clinical settings, drug resistance continues to pose a major challenge [8, 9].
In light of these developments, this review will evaluate the current state of clinical HER2-targeted drugs, explore their mechanisms of action, discuss resistance mechanisms, and consider possible future therapeutic advancements.
2. HER2-targeted drugs and mechanisms
2.1. Development of HER2
The ErbB family of tyrosine kinases is essential for signaling cells that regulate regular cell division and proliferation [1]. One of the primary causes of cancer growth is unchecked cell division brought on by interference with several signaling networks [2]. Tumor growth is stimulated by overexpression of the HER2 protein, which is expressed in 15–25% of cancer cases [3]. Treatment for invasive HER2 positive breast cancer has advanced significantly as a result of HER2 overexpression, making HER2 an extremely sensitive and treatable target [4]. As a result, numerous medications that specifically target the HER2 protein have been developed and extensively researched [5].
2.2. HER2-targeted drugs
Monoclonal antibodies (mAbs), tyrosine- kinase Inhibitors (TKIs) and antibody-drug conjugates (ADCs) are the three main groups into which HER2-targeted medicines can be divided [1].
2.2.1. Monoclonal antibodies. mAbs that targeted HER2 were the first group of anti-HER2 medications, marking a significant advancement in cancer targeted therapy in the 1990s [1]. These antibodies connect to the cancer cells' HER2 protein, which stops the cells from growing in a number of ways [2]. By inhibiting HER2 cleavage, suppressing angiogenesis, inducing G1 cell cycle arrest via the p27 tumor suppressor factor, and activating immune-mediated processes, the downregulation of HER2 protein limits the creation of heterodimers containing HER2 [3].
Treatment for HER2-positive breast cancer has been greatly altered by two important mAbs:
Trastuzumab: The first HER2-targeted mAbs, it was granted FDA approval in 1998. Both of the beginning and late breast cancers are treated with it; chemotherapy is frequently combined with it. It is used either before or after surgery for six months to a year in cases of early-stage breast cancer. Treatment for advanced cancer is ongoing as long as the medication is working [1-4].
Pertuzumab: Granted FDA approval in 2012, Pertuzumab is used in conjunction with Trastuzumab and chemotherapy to treat HER2-positive breast cancer that is metastatic or in the early stages, both prior to and following surgery [5].
2.2.2. Tyrosine kinase inhibitors. HER2 is a glycoprotein that spans the membrane and has intrinsic tyrosine kinase activity. In order to impede HER2-mediated signaling pathways, HER2-specific TKIs compete with ATP for attachment to the HER2 catalytic kinase domain [1, 2]. The ability to traverse the blood-brain barrier, tiny molecular size, and excellent bioavailability through oral administration of TKIs make them noteworthy [3]. They have the ability to stop numerous kinases at once, which makes it possible to block various growth-promoting mechanisms at once, but this may also enhance toxicity. TKIs that are more specific for HER2 are therefore especially useful [4, 5]. Key TKIs include:
Lapatinib: a reversible TKI, was initially authorized by the US FDA in the year 2007. It is used to treat advanced breast cancer, frequently in conjunction with chemotherapy and trastuzumab. When combined with letrozole, it was authorized in 2010 as the initial line of treatment for HER2-positive aggressive breast carcinoma [1-3].
Tucatinib: A third-generation reversible TKI with good selectivity that is more than one thousand times more potent against HER2 than EGFR. When at least one previous anti-HER2 therapy fails, it is usually used in conjunction with trastuzumab and the chemotherapeutic medication capecitabine to treat advanced cancer. 2020 witnessed the FDA authorize Tucatinib for the treatment of positive for HER2-cancer with metastatic progression [4-6].
2.2.3. Antibody-drug conjugates. ADCs are a family of specifically targeted cancer treatments made up of cytotoxic medicines (payloads) and mAbs connected by cleavable or non-cleavable linkers [1]. When ADCs connect to cancer cells' surface antigens, the cytotoxic medication is internalized and released into the cell, which is how ADCs work as a treatment [2]. This focused strategy reduces systemic toxicity while increasing the drug's effectiveness [3]. Notable ADCs include:
Ado-Trastuzumab Emtansine (TDM-1): It is the 2nd generation ADC that outperforms Lapatinib and Capecitabine in terms of long-term survival for aggressive HER2-expressing breast carcinoma in the 2nd and third lines. TDM-1 was first licensed by the FDA in 2013 for HER2-positive breast cancer with metastatic disease, and its indication was expanded in 2019 to cover adjuvant treatment for the initial stages HER2-positive breast cancer with persistent invasive disease following neoadjuvant chemotherapy [1, 2].
Familiab Trastuzumab Deruxtecan-nxki (T-DXd): This third-generation ADC has a notable impact on bystanders and has proven to be more effective than TDM-1 in patients with HER2-positive breast cancer with metastatic disease who have already received treatment. T-DXd was first given authorization by the FDA in the year 2019 for this particular indication, and it has since been given permission for medicinal use of other cancer types [3, 4].
2.3. HER2-targeted drugs' mechanisms of action
2.3.1. Monoclonal antibodies. Trastuzumab: Trastuzumab suppresses tumor growth by attaching to the extracellular domain (ECD) of the HER2 expression receptors, thereby preventing ligand-independent dimerization of the receptor and inducing antibody-dependent cell-mediated cytotoxicity (ADCC). It also obstructs DNA repair mechanisms, promotes arrest of the cell cycle and apoptosis, and inhibits downstream signaling cascades and angiogenesis (Figure 1a) [1, 2].
Pertuzumab: By inhibiting the ligand-dependent HER3 signaling process, pertuzumab inhibits HER2/HER3 heterodimerization by targeting a distinct epitope on the ECD of HER2, hence reducing cell proliferation. In order to further inhibit tumor growth, pertuzumab and trastuzumab work together to disable a number of downstream signaling systems which includes the pathway known as PI3K/AKT/mTOR and RAS/RAF/MEK/ERK processes. (Figure 1a) [3,4].
2.3.2. Tyrosine kinase inhibitors. Lapatinib: Lapatinib is a small molecule TKI that inhibits HER2 signaling by blocking receptor autophosphorylation and the resulting activation of downstream pathways like PI3K/AKT and MAPK/ERK. It does this by interacting with ATP at the kinase domain (Figure 1d) [1, 2].
Pyrotinib: Pyrotinib inhibits tumor cell proliferation by binding to ATP-binding domains in HER1, HER2, and HER4 kinase areas, inhibiting the development of HER family homodimers and downstream signaling cascades [3].
2.3.3. Antibody-drug conjugates. T-DXd: T-DXd's monoclonal antibody component attaches itself specifically to the tumor surface's HER2. After attaching, the ADC is taken up by the tumor cells' lysosomal enzymes, which then sever the linker (Figure 1b). A topoisomerase I inhibitor that is released by this cleavage penetrates the nucleus and causes cell death. Moreover, the cytotoxic payload that has been released may permeate into nearby cells and cause a "bystander effect" that destroys nearby tumor cells independent of the degree of HER2 expression (Figure 1c) [1-3].
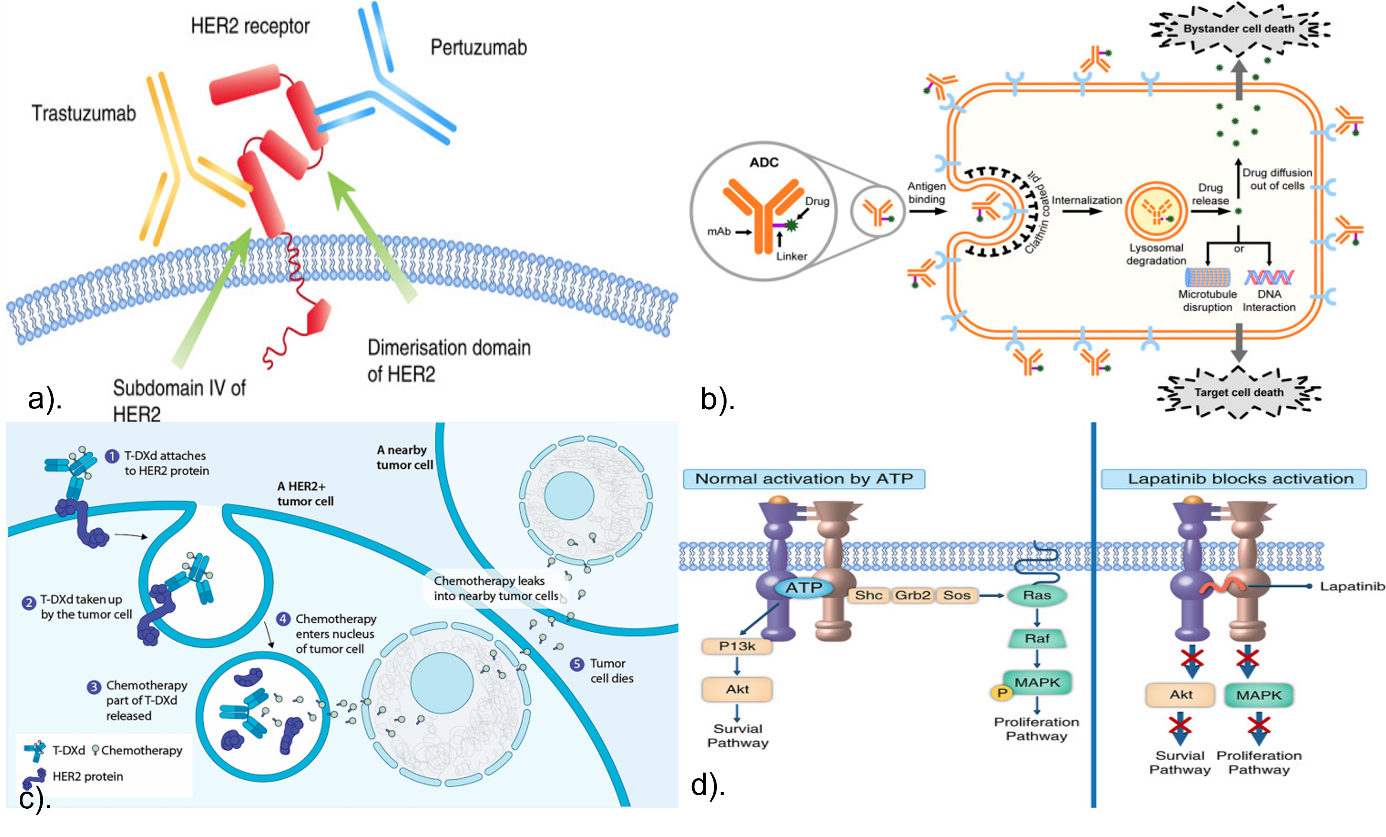
Figure 1. HER2-Targeted Drugs' Mechanisms of Action. a). Trastuzumab and pertuzumab bind in different regions of HER2 [1]. b) The structure and mechanism of ADC [2]. c) Mechanism of action of T-DXd [3]. d) Mechanism of action of lapatinib [4].
2.4. Summary
Treatment for HER2-positive breast tumors has come a long way because to the introduction of HER2-targeted medicines such as mAbs, TKIs, and ADCs [1]. These treatments were first created exclusively for cancer and later extended to other solid tumors as medical technology evolved [2]. These therapies do not, however, come without adverse effects. Congestive heart failure may result from heart damage brought on by mAbs and ADCs during or after therapy. Severe diarrhea can also be brought on by Lapatinib, Tucatinib, and Pertuzumab plus Trastuzumab. Hand-foot syndrome (sore, red, blistering, peeling hands and feet) is another side effect of lapatinib with tucacine. Tucatinib, and Lapatinib may cause hepatic toxicity [3-6]. Therefore, in order to overcome issues like drug resistance and maximize their use in a variety of cancer types, it is crucial to improve these medicines through more thorough study [7]. Targeted therapy and HER2 signaling will probably become more significant in the field of oncology in the future as our understanding of their mechanisms deepens [8].
3. Pathways and mechanisms of HER2-targeted resistance
3.1. Targeting the HER2 intracellular signaling pathway
The control of angiogenesis, growth, and the progression of cell cycles is largely dependent on the signaling pathway consisting of PI3K/AKT/mTOR. This pathway is also important to target in order to overcome endocrine resistance as well as HER2-targeted resistance because it affects the expression of HER2 and estrogen receptors (ER) [1, 2]. Even when HER2 is inhibited, inappropriate stimulation of the PI3K/AKT/mTOR cascade frequently continues, which adds to resistance mechanisms [3]. Using certain inhibitors to target this pathway, either by themselves or in conjunction with other treatments, offers a viable option to improve the therapeutic impact and combat resistance [4].
3.2. Mechanisms of HER2-targeted resistance
Many mechanisms of opposition to HER2-targeted therapy have evolved despite considerable advancements in the treatment of HER2 receptor positive breast carcinoma, which have improved the outcome of patients and resulted in an increasing number of authorized medications [1]. By avoiding HER2 suppression, cancer cells are able to proliferate and spread their tumors (Figure 2) [2].
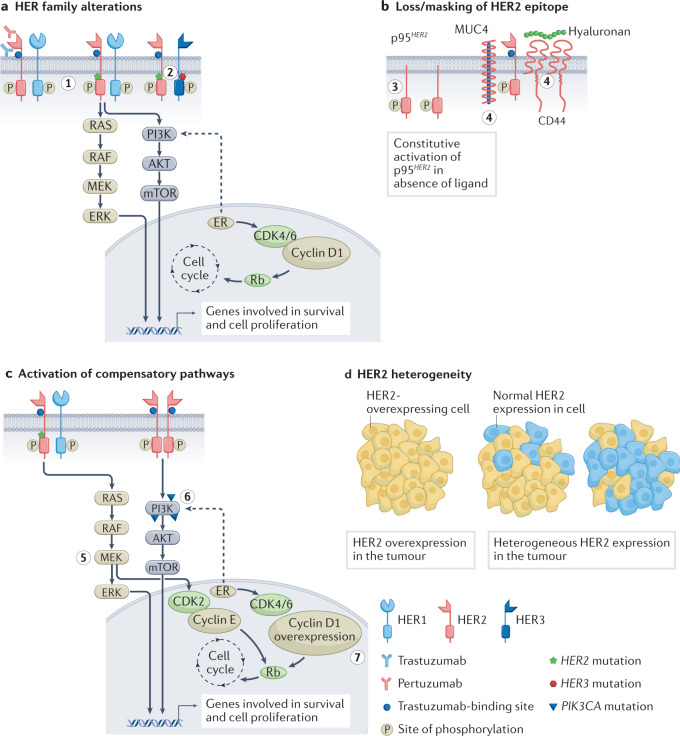
Figure 2. Targeted Resistance Mechanisms for HER2. (a) Stimulation of subsequent signaling systems is the outcome of mutations or changes in the HER receptor families [1]: (1) The RAS-MAPK and P13K-AKT pathways are activated by HER2 mutations; (2) The PI3K-AKT pathway is activated when HER2 and HER3 mutations occur together [2, 3]. (b) In cells that overexpress the p95HER2 receptor, the extracellular domain of HER2 is deleted. Trastuzumab binding sites on HER2 can be obscured by overexpressing CD44 polymer hyaluronic acid complexes and mucin 4 (MUC4) [4, 5]: (3) Overexpression of p95HER2 [6]; (4) Overexpression of the hyaluronic acid complex-containing CD44 polymer and MUC4 [7]. (c) Compensatory pathways that are active [8]: (5) CDK2 kinase is activated and MEK-ERK signaling is promoted by HER2 mutation [9]; (6) The PIK3CA mutation triggers the P13K-AKT pathway [10]; (7) Resistance to HER2 treatment is caused by cyclin D1 gene overexpression. (d) HER2-targeted therapies rely on decreased sensitivity to HER2 overexpression because of the variable expression of HER2 receptors in malignancies.
3.3. HER2 heterogeneity
HER2-targeted medicines have a great deal of difficulty due to HER2 heterogeneity within tumors. Differential responses to treatment and resistance can result from variable HER2 expression [1].With a drug-to-antibody ratio (DAR) of 7-8, T-DXd, a new HER2-targeted ADC, enables effective transport of cytotoxic payloads to HER2-expressing tumours. Following T-DXd's attachment to HER2, it internalizes and the payload is released into the cell by lysosomal enzymes cleaving the cleavable peptide-based linker. Through a bystander effect, this payload can also diffuse into nearby cells and produce lethal effects independent of HER2 expression [2-4]. According to recent research, patients treated with T-DXd who have low HER2 expression have much longer survival without progression and overall survival than patients getting chemotherapy of their doctor's choosing [5]. This suggests that T-DXd's high DAR and bystander impact may be the cause of its anticancer effectiveness in heterogeneity or low-HER2 expressing malignancies [6]. To fully comprehend the effects of HER2-targeted therapy on tumors exhibiting HER2 heterogeneity, more research is required, especially when combined with non-targeted medications. Such studies are necessary to overcome the constraints imposed by HER2 expression fluctuation and to design more efficacious treatment plans [7].
4. Future functions
Patients with HER2-positive breast cancer now have a much better prognosis thanks to HER2-targeted treatment. A range of drugs have been approved or are in late-stage clinical development, contributing to this improvement [1, 2]. However, there are still some side effects and challenges in current HER2-targeted therapy [3]. Thus, ongoing efforts are being undertaken to create novel therapeutic options in the era of precision medicine with the goal of improving patient prognosis even more [4].
4.1. Clinical Treatment
The specificity of mAbs is used by ADCs, a potential category of biologic therapeutics, to deliver highly lethal small molecules to specific cancer cells [1]. With less off-target toxicity and more anti-tumor effectiveness, this strategy seeks to raise the therapeutic index [2]. As a result, ADCs have a lot of potential for treating breast cancer with HER2-positive mutations. New ADC medications are constantly being developed with this indication in mind, focusing on various phases of clinical development [3, 4].
4.1.1. A166. A166 has a trastuzumab site that binds selectively to the payload duostatin-5, a new and potent anti-microtubule auristatin produced from monomethyl auristatin F, through a stable protease-cleavable VC junction [1]. Following the internalization of the antigen/ADC and the binding of A166 to HER2, the enzyme-facilitated connection in tumor cells is broken, releasing a free payload that attaches to tubulin and prevents polymerization, which in turn triggers death in tumor cells [2]. Currently, A166 is being studied in a number of clinical trials to treat breast cancer with HER2-positive mutations. In these trials, A166 has shown high tolerability, a satisfactory safety profile, and promising anti-tumor activity [3, 4].
4.1.2. Trastuzumab duocarmazine. An individualized anti-HER2 IgG1 mAb that shares the same amino acid structure as trastuzumab makes up trastuzumab domacrimine. It attaches itself to a highly effective DNA alkylation payload via a vc junction that is cleavable by proteinase [1]. A protease binds dicarbicamycin to a cleavable vc connector. Following the antigen/ADC internalization, the connector is broken by proteolytic hydrolysis inside the lysosome, releasing the payload into the cell and causing DNA damage that causes cell death in both separating and non-separating cells because the alkylated DNA becomes irreversible during hydrolysis [2, 3]. Preclinical research has shown that trastuzumab docarmazine has good anticancer effect in PDX models of HER2-positive metastatic breast cancer and is stable in vitro in humans and several experimental animals [4]. Trastuzumab docarmazine is presently undertaking a number of stage I, II, and III clinical studies [5]. When compared to other late-stage treatment options, it dramatically improves progression-free survival and may have anticancer benefits in positive for HER2 breast carcinoma [6]. Therefore, patients with locally progressed positive for HER2 breast carcinoma may benefit from trastuzumab dokamazine as a therapy strategy [7].
4.2. Cancer vaccinations that target HER2
In order to identify and eliminate tumor cells, cancer vaccinations work to activate the patient's own immune system by enhancing the response of CD8+ and CD4+T cells to antigens unique to tumors [1]. However, the therapy setting is an important consideration for cancer vaccinations. It is commonly known that T cell activity in TME may be restricted as a result of the immunosuppressive effects of metastatic illnesses and the disease burden [2, 3]. Vaccines are therefore more likely to be successful in settings where the disease burden is very low [4]. Additionally, combining chemotherapy or targeted medications with cancer vaccines may increase their efficacy in adjuvant settings and aid in overcoming the inhibitory TME in late-stage disease environments [5].
4.2.1. Peptide vaccines. Peptide vaccines provide a number of advantages over other vaccination kinds, including ease of production, cost-effectiveness, ease of administration, and comparatively low side effects. They may be the most widely used vaccine design. [1, 2] Peptide vaccines containing various HER2 protein domains were used in the trial to assess patients with breast cancer. Encoded from the extracellular domain of HER2, E75 is an immunogenic peptide produced in 60-75% of patients, which is composed of 9 amino acids [3, 4]. The MHC stage I vaccination Nelipiput-S/NeuVax is made up of the immunological adjuvant GM-CSF192 and E75 [5]. Their drawback, though, is that because of their selectivity, the patient's T cell immune response might be more restricted in the variety of cell types and might not be able to fully activate B and T helper cells to strengthen the patient's immune system [6].
4.2.2. Dendritic cells. DC is a specialized APC and a strong immunological response regulator. It has the ability to both produce memory T cell lymphocytes and activate T cells. [1, 2] Rather than employing small molecule inhibitors or antibodies to target a single epitope, the DC vaccine platform enables patients to experience the processing of antigen through their own systems of immunity, eliciting immune responses against many epitopes on the target. [3] Patients with metastatic breast carcinoma have received the DC vaccine as part of a preliminary research. DC vaccines represent a potentially tailored approach, but in order to make vaccine production easier, the complicated manufacturing process must be streamlined [4, 5]. Therefore, research into allogeneic or synthetic APCs is necessary to get over this restriction [6].
4.3. CAR-M therapies
One important type of cellular immunotherapies is represented by CARs, which are designed molecules that combine a particular type of antibodies with T cell signaling downstream. [1] Although CAR-T treatments with FDA authorization are accessible for hematological cancers, their application in solid tumors is restricted due to the requirement of actively transferring and penetrating T cells into the TME [2]. Consequently, immune cells have been replaced by genetically modified human macrophages that use CAR-M to change their phagocytic function against solid tumors (Figure 3). A single injection of these CAR-Ms increased overall survival (OS) and reduced tumor size in experimental mice [3, 4]. Furthermore, CAR-Ms converted immunosuppressive bystander M2 macrophages into pro-inflammatory M1 macrophages, which resulted in the upregulation of the APC pathway. By stimulating immature dendritic cell proliferation and gathering activated CD8+T cells near the tumor site, the TME was altered to improve the anti-tumor response [5, 6].
Figure 3. CAR M cell.
4.4. Bispecific engagers
In breast cancer, the immunosuppressive TME following chemotherapy can lead to poor immunotherapy activity. This might be explained by the absence of MHC Class I receptors on tumors with metastatic spread, which would slow down or prevent certain antigen-T cell clones intended to target breast cancer from recovering [1, 2]. Nevertheless, new bispecific antibodies (BsAbs) may be able to get over this obstacle by attaching to immune cells and tumor-specific antigens at the same time, which will kill tumor cells. HER2 is one target that these BsAbs frequently hit [3, 4]. Using BsAbs binding CD3 and HER2, the bispecific T cell adapter (BiTE) guides T cells to tumor cells that exhibit HER2. The benefit of the BiTE strategy is its capacity to activate T cells without regard to antigen specificity, resulting in the activation of the majority of T cells [5, 6]. Furthermore, HER2-expressing cancer cells are successfully eliminated by bispecific killer cell engagers (BiKEs), which attach to natural killer (NK) cells, CD16 on monocytes, and HER2 on cancer cells [7].
4.5. The possible role for AI in the of HER2 ITH
Significant variations were found in the evaluation of HER2 ITH and HER2 limited expression condition in cancer, according to the study [1]. Alternative techniques including molecular analysis or computerized pathology have been suggested as accurate ways to determine HER2 status [2]. Digital image analysis (DIA) has become a speedy, affordable, unbiased, and repeatable assessment tool for HER2 function through IHC and anti-HER2 therapies since full-slide imaging (WSI) has become widely used. In addition, several AI systems have been developed to distinguish between HER2-positive and HER2-negative cases [3, 4]. Even so, implementing this technique remains challenging, especially in groups with low to moderate HER2 expression [5]. While AI is rather good at displaying heterogeneous staining patterns, some studies have demonstrated that it can minimize suspected cases of HER2 IHC and enhance accuracy and inter-observer consistency. At the same time, the newly developed AI, by combining with pathologists, can help pathologists give feedback and adjust the AI results in real time. As a result, the accuracy of AI results will be greatly improved [6-8]. In summary, although AI can assess cases of low HER2 expression, its application and reliability, especially in HER2 ITH cases, have not yet been determined. [9]
4.6. Summary
HER2 has made unprecedented progress on oncogenes, biomarkers, and very aggressive BC targets, and patient survival has been greatly improved. This success is due to the precise development of HER2 receptors for HER2-targeted therapy [1, 2]. To significantly improve the quality of life of patients, a large number of basic and clinical experts are working to further create and research medications [3]. Yet, a crucial component in making this happen is the scientists who go from academic to industrial drug discovery. Although several drug pathways have been successfully targeted, resistance mechanisms will continue to be explored to potentially cure various cancers, such as breast cancer [4, 5]. Today, numerous well-known research fields are being investigated. In addition, a plethora of instruments, including AI, space proteomics, and therapeutic diagnostics tools, have been found and implemented to aid in the development of new drugs. Meanwhile, novel drug architectures like ADCs and soon-to-be bi-specificity will further enhance the security of targeting HER2+ cells and lower the risk of side effects [6-8]. Therefore, based on the great achievements of HER2-targeted therapy in the past, the research of these new methods and the development of new drugs are promising [9].
5. Conclusions
Not only is HER2 crucial for the treatment of diseases like breast cancer, but it also plays a major part in the growth and multiplication of cancer cells. As a result, we continue to investigate it in different cancer regions and examine it as a potential therapy for breast carcinoma. Since then, important progress has been made in the development and mechanism of mAbs, TKI, ADCs targeted by HER2, major breakthroughs have been made in the study of drug resistance, and new anti-HER2 therapies have been continuously innovated in the future. At present, many developmental studies are underway to change the landscape of HER2 overexpression or proliferation affecting cancer treatment. Despite its remarkable achievements in recent decades, it has inevitably had some side effects on patients, such as severe diarrhea, heart disease and other symptoms. As a result, in order to get past these obstacles, we must comprehend the extensive history and evolution of HER2 and evaluate the breadth of current research, both of which are crucial for both comprehending the future course of cancer research and its treatment in the present.
References
[1]. Gámez-Chiachio, M., Sarrió, D., & Moreno-Bueno, G. (2022). Novel Therapies and Strategies to Overcome Resistance to Anti-HER2-Targeted Drugs. Cancers, 14(18), 4543. https://doi.org/10.3390/cancers14184543
[2]. Yu, J., Fang, T., Yun, C., Liu, X., & Cai, X. (2022). Antibody-Drug Conjugates Targeting the Human Epidermal Growth Factor Receptor Family in Cancers. Frontiers in molecular biosciences, 9, 847835. https://doi.org/10.3389/fmolb.2022.847835
[3]. Goutsouliak, K., Veeraraghavan, J., Sethunath, V., De Angelis, C., Osborne, C. K., Rimawi, M. F., & Schiff, R. (2020). Towards personalized treatment for early stage HER2-positive breast cancer. Nature reviews. Clinical oncology, 17(4), 233–250. https://doi.org/10.1038/s41571-019-0299-9
[4]. Rubin, E., Shan, K. S., Dalal, S., Vu, D. U. D., Milillo-Naraine, A. M., Guaqueta, D., & Ergle, A. (2024). Molecular Targeting of the Human Epidermal Growth Factor Receptor-2 (HER2) Genes across Various Cancers. International journal of molecular sciences, 25(2), 1064. https://doi.org/10.3390/ijms25021064
[5]. Swain, S. M., Shastry, M., & Hamilton, E. (2023). Targeting HER2-positive breast cancer: advances and future directions. Nature reviews. Drug discovery, 22(2), 101–126. https://doi.org/10.1038/s41573-022-00579-0
[6]. Najminejad, Z., Dehghani, F., Mirzaei, Y., Mer, A. H., Saghi, S. A., Abdolvahab, M. H., Bagheri, N., Meyfour, A., Jafari, A., Jahandideh, S., Gharibi, T., Amirkhani, Z., Delam, H., Mashatan, N., Shahsavarani, H., & Abdollahpour-Alitappeh, M. (2023). Clinical perspective: Antibody-drug conjugates for the treatment of HER2-positive breast cancer. Molecular therapy, 31(7), 1874–1903. https://doi.org/10.1016/j.ymthe.2023.03.019
[7]. Wang, J., & Xu, B. (2019). Targeted therapeutic options and future perspectives for HER2-positive breast cancer. Signal transduction and targeted therapy, 4, 34. https://doi.org/10.1038/s41392-019-0069-2
[8]. Lai, H. Z., Han, J. R., Fu, X., Ren, Y. F., Li, Z. H., & You, F. M. (2022). Targeted Approaches to HER2-Low Breast Cancer: Current Practice and Future Directions. Cancers, 14(15), 3774. https://doi.org/10.3390/cancers14153774
[9]. Curigliano, G., Dent, R., Earle, H., Modi, S., Tarantino, P., Viale, G., & Tolaney, S. M. (2024). Open questions, current challenges, and future perspectives in targeting human epidermal growth factor receptor 2-low breast cancer. ESMO open, 9(4), 102989. https://doi.org/10.1016/j.esmoop.2024.102989
[10]. Hou, Y., Nitta, H., & Li, Z. (2023). HER2 Intratumoral Heterogeneity in Breast Cancer, an Evolving Concept. Cancers, 15(10), 2664. https://doi.org/10.3390/cancers15102664
Cite this article
Qi,Q. (2025). Breast cancer targeting HER2: mechanisms, resistance, and emerging therapies. Theoretical and Natural Science,77,70-79.
Data availability
The datasets used and/or analyzed during the current study will be available from the authors upon reasonable request.
Disclaimer/Publisher's Note
The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of EWA Publishing and/or the editor(s). EWA Publishing and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
About volume
Volume title: Proceedings of ICBioMed 2024 Workshop: Computational Proteomics in Drug Discovery and Development from Medicinal Plants
© 2024 by the author(s). Licensee EWA Publishing, Oxford, UK. This article is an open access article distributed under the terms and
conditions of the Creative Commons Attribution (CC BY) license. Authors who
publish this series agree to the following terms:
1. Authors retain copyright and grant the series right of first publication with the work simultaneously licensed under a Creative Commons
Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this
series.
2. Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the series's published
version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial
publication in this series.
3. Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and
during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See
Open access policy for details).
References
[1]. Gámez-Chiachio, M., Sarrió, D., & Moreno-Bueno, G. (2022). Novel Therapies and Strategies to Overcome Resistance to Anti-HER2-Targeted Drugs. Cancers, 14(18), 4543. https://doi.org/10.3390/cancers14184543
[2]. Yu, J., Fang, T., Yun, C., Liu, X., & Cai, X. (2022). Antibody-Drug Conjugates Targeting the Human Epidermal Growth Factor Receptor Family in Cancers. Frontiers in molecular biosciences, 9, 847835. https://doi.org/10.3389/fmolb.2022.847835
[3]. Goutsouliak, K., Veeraraghavan, J., Sethunath, V., De Angelis, C., Osborne, C. K., Rimawi, M. F., & Schiff, R. (2020). Towards personalized treatment for early stage HER2-positive breast cancer. Nature reviews. Clinical oncology, 17(4), 233–250. https://doi.org/10.1038/s41571-019-0299-9
[4]. Rubin, E., Shan, K. S., Dalal, S., Vu, D. U. D., Milillo-Naraine, A. M., Guaqueta, D., & Ergle, A. (2024). Molecular Targeting of the Human Epidermal Growth Factor Receptor-2 (HER2) Genes across Various Cancers. International journal of molecular sciences, 25(2), 1064. https://doi.org/10.3390/ijms25021064
[5]. Swain, S. M., Shastry, M., & Hamilton, E. (2023). Targeting HER2-positive breast cancer: advances and future directions. Nature reviews. Drug discovery, 22(2), 101–126. https://doi.org/10.1038/s41573-022-00579-0
[6]. Najminejad, Z., Dehghani, F., Mirzaei, Y., Mer, A. H., Saghi, S. A., Abdolvahab, M. H., Bagheri, N., Meyfour, A., Jafari, A., Jahandideh, S., Gharibi, T., Amirkhani, Z., Delam, H., Mashatan, N., Shahsavarani, H., & Abdollahpour-Alitappeh, M. (2023). Clinical perspective: Antibody-drug conjugates for the treatment of HER2-positive breast cancer. Molecular therapy, 31(7), 1874–1903. https://doi.org/10.1016/j.ymthe.2023.03.019
[7]. Wang, J., & Xu, B. (2019). Targeted therapeutic options and future perspectives for HER2-positive breast cancer. Signal transduction and targeted therapy, 4, 34. https://doi.org/10.1038/s41392-019-0069-2
[8]. Lai, H. Z., Han, J. R., Fu, X., Ren, Y. F., Li, Z. H., & You, F. M. (2022). Targeted Approaches to HER2-Low Breast Cancer: Current Practice and Future Directions. Cancers, 14(15), 3774. https://doi.org/10.3390/cancers14153774
[9]. Curigliano, G., Dent, R., Earle, H., Modi, S., Tarantino, P., Viale, G., & Tolaney, S. M. (2024). Open questions, current challenges, and future perspectives in targeting human epidermal growth factor receptor 2-low breast cancer. ESMO open, 9(4), 102989. https://doi.org/10.1016/j.esmoop.2024.102989
[10]. Hou, Y., Nitta, H., & Li, Z. (2023). HER2 Intratumoral Heterogeneity in Breast Cancer, an Evolving Concept. Cancers, 15(10), 2664. https://doi.org/10.3390/cancers15102664









