1. Introduction
Worldwide, cancer ranks as the second most common cause of death. As stated by the International Agency for Research on Cancer (IARC) data from 2022, 20 million new cases of cancer are thought to have been diagnosed, with 9.7 million cancer-related deaths worldwide. Approximately 1 in 5 individuals will develop cancer during their lifetime, with a mortality rate of about 11% in males and 8.3% in females [1].
While many effective cancer therapies have been developed, significantly reducing global incidence and mortality rates, their limitations—including drug instability and tumor heterogeneity—remain significant challenges. Cytotoxic chemotherapy, a widely used approach, involves administering cytotoxic agents based on body surface area (BSA) dosing [2]. These agents are highly effective against cancer cells but also induce severe side effects by targeting DNA synthesis and protein expression in both malignant and healthy cells. The side effects, such as nausea, infertility, hair loss, and immunosuppression, originate from these medications’ non-specific effects on constantly dividing cells, such as bone marrow and hair follicles [2].
The advent of monoclonal antibodies (mAbs) has significantly mitigated the issue of off-target toxicity associated with chemotherapy. By specifically targeting antigens on the surface of cancer cells, mAbs can inhibit tumor growth, induce apoptosis, and block immune checkpoints [3].
The concept of Antibody-Drug Conjugates (ADCs), first introduced by Paul Ehrlich in the early 1900s, has ushered targeted cancer therapy into a new and improved era. ADCs combine the powerful killing potential of cytotoxic drugs with the specificity of mAbs, linked together to achieve a synergistic effect. The core of an ADC is the mAb, which targets specific epitopes on the cancer cell surface. Upon binding, the ADC is internalized, leading to the release of the cytotoxic payload via linker cleavage, ultimately resulting in the death of the cancer cell [4].
T-DM1 is an ADC that received FDA approval over a decade ago. It targets the human epidermal growth factor receptor 2 (HER2) on cancer cells, which is often overexpressed in HER2-positive breast cancer. T-DM1 effectively eliminates cancer cells by binding to HER2 with its mAb (trastuzumab), forming the HER2-T-DM1 complex. Once internalized, the linker is degraded, releasing emtansine (DM1), a potent cytotoxic drug that inhibits microtubule assembly and induces apoptosis [5].
In comparative studies of T-DM1 versus trastuzumab, ADCs have demonstrated notable advantages over mAbs. T-DM1 increased the disease-free survival rate of HER2+ breast cancer patients by 13.7%, with an overall survival improvement of 34% compared to trastuzumab, and a statistically significant 46% risk reduction [6].
While ADCs offer reduced side effects and increased specificity, numerous challenges remain in improving their efficacy, stability, and accessibility. This review will comprehensively explore the advances, limitations, and future developments in ADC technology.
2. Current clinical status of ADC
According to data from clinicaltrials.gov, there are currently 646 clinical trials investigating ADCs as a therapeutic approach for treating cancers. Among these, 5 trials are in early phase 1, 288 are in phase 1, 281 in phase 2, 62 in phase 3, 5 in phase 4, and 5 are categorized as non-applicable. Of these trials, 547 involve interventional studies, 4 are observational, and 3 are designed for expanded access. Results have been posted for 103 trials, while 451 trials do not yet have posted results. Funding sources include the National Institutes of Health (NIH) for 84 trials, industry funding for 432 trials, and other sources such as individuals, universities, or organizations for 198 trials. The following sections will focus on FDA-approved ADCs and their mechanisms of action.
2.1. Trastuzumab Emtansine
Trastuzumab Emtansine, commonly known as T-DM1, was the earliest ADC received approval from the FDA in 2013. It targets HER2, a receptor that is usually overexpressed on the surface of cancerous breast cells. T-DM1 is composed of three main parts: the mAb trastuzumab, which acts as a biological missile targeting HER2; a stable thioether linker; and the cytotoxic drug emtansine (DM1) [5].
T-DM1 is effective against both HER2+ solid tumors and metastatic tumors [7]. Its mechanism of action begins with the high-affinity binding of trastuzumab to the HER2 receptor [8]. This binding downregulates HER2 expression and inhibits its signaling pathways, thereby reducing cancer cell growth [9]. Additionally, trastuzumab triggers Antibody-Dependent Cellular Cytotoxicity (ADCC), which recruits immune cells, such as natural killer cells, to the cancer site.
After binding, receptor-mediated endocytosis allows the HER2-T-DM1 complex to be internalized into the cancer cell, leading to the formation of an early endosome (Figure 1). The thioether linker remains stable throughout this process, preventing premature drug release. Inside the early endosome, T-DM1 can either be return to the surface of the cell via recycling or degraded by lysosomal enzymes. When the linker is eventually degraded, it releases the cytotoxic drug DM1, which then kills the cancer cells by inducing apoptosis, inhibiting microtubule assembly, and causing mitotic arrest, ultimately reducing tumor size [9],[10].
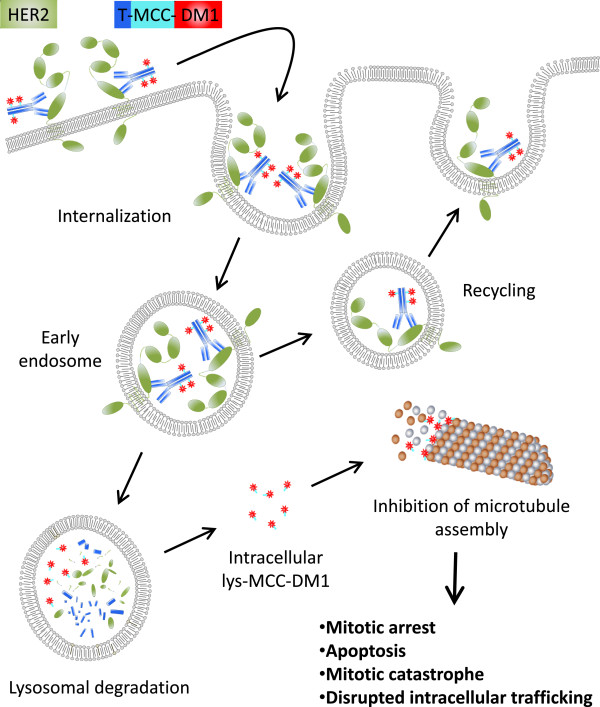
Figure 1. A schematic representation of HER2-T-DM1 complex internalization process into cancer cells [5].
T-DM1 has demonstrated outstanding efficacy in treating HER2+ breast cancer. For instance, a clinical trial (NCT03530696) conducted a randomized test of T-DM1 in conjugation with palbociclib for patients with metastatic HER2+ breast cancer. Over a 4-year period, the therapy achieved a 42.9% overall response rate (OR), an 85.7% disease control rate (DCR), and only 14.3% of patients experienced disease progression. These results further substantiate the substantial therapeutic efficacy of T-DM1 in treating HER2+ breast cancer.
However, as with all treatments, T-DM1 has its limitations. Resistance to the drug can develop in several ways: reduced expression of HER2 on the cellular surface, which impairs T-DM1 binding and internalization; alterations in intracellular trafficking, such as increased efflux of T-DM1 back to the cell surface, reducing its intracellular concentration; and increased activity of drug efflux transporters, which can expel DM1 from the cell, allowing cancer cells to avoid apoptosis and continue proliferating [11]. T-DM1 is still among the best treatments for HER2+ breast cancer tumour targeting, despite these difficulties.
2.2. Brentuximab Vedotin
Brentuximab Vedotin was approved by the FDA in 2018 and is a mouse-human chimeric ADC used for the treatment of Hodgkin’s lymphoma and anaplastic large-cell lymphoma (ALCL). It consists of a mAb, Brentuximab, which selectively targets cancer cells expressing CD30 antigens on their surface, linked to the anti-microtubule cytotoxic agent monomethyl auristatin E (MMAE), also known as vedotin) via a cleavable linker [12].
When Brentuximab binds to the CD30 epitope on the surface of cancerous cell, it triggers internalization, followed by intracellular trafficking. The cleavable linker is rapidly degraded by lysosomal enzymes, which then releases vedotin. Vedotin inhibits tubulin polymerization, a key process in the construction of microtubules, which results in M phase cell cycle arrest and eventually inducing apoptosis [12].
Brentuximab Vedotin has shown promising results in treating CD30+ lymphoma cancers. In a clinical trial (NCT01578499), a comparative study was conducted between Brentuximab Vedotin and methotrexate or bexarotene in patients with CD30+ T-cell lymphoma. The study revealed that Brentuximab Vedotin achieved a 54.7% OR, a 17.2% complete response rate (CR), and a 16.7% progression-free survival (PFS), significantly outperforming the methotrexate or bexarotene treatment group, which achieved only a 12.5% OR, 1.6% CR, and 3.5% PFS. This indicates the substantial benefit of Brentuximab Vedotin in treating CD30+ lymphoma [13].
Some common side effects associated with Brentuximab Vedotin include diarrhea, nausea, peripheral sensory neuropathy, neutropenia, constipation, alopecia, vomiting, and fatigue. However, ongoing research is focusing on combination therapies aimed at improving the therapeutic efficacy of Brentuximab Vedotin while minimizing treatment-emergent adverse events (TEAEs) [14].
3. Advances
3.1. Conjugation techniques
In addition to selecting the optimal mAb and the most effective cytotoxic agent based on the type of targeted cancer, the role of the linker is crucial. For an ADC to successfully deliver its drug to the tumor site, the linker must meet the following criteria to ensure safe and effective delivery: 1) The linker must maintain optimal stability to secure the cytotoxic payload and prevent its premature release into the bloodstream, which could cause unwanted damage to healthy cells. 2) The linker must also be able to degrade quickly and cleave the payload as soon as the ADC has been internalized [15].
Developing ADC linkers that balance safety and efficacy is challenging, and extensive research has focused on linker conjugation techniques. The primary conjugation techniques currently in use are chemical and enzymatic conjugations. The following sections provide a more detailed review of these conjugation methods.
3.1.1. Chemical conjugations. One of the most commonly used chemical conjugations is lysine amide coupling. This method involves binding the amino acid lysine residue to a carboxyl group via an amide bond (Figure 2). The reaction is often facilitated by reagents like N-hydroxy succinimidyl (NHS) or sulfo-NHS esters [16].
Lysine is abundant on protein surfaces and remains stable at physiological pH, making it highly accessible for chemical modification. NHS or sulfo-NHS esters are commonly used reagents to facilitate lysine residue modification. They are highly reactive and commercially available, enabling the formation of amide bonds within a pH range of 7.0 to 9.0 in a short timeframe. The advantage of amide bonds in constructing ADC linkers lies in their ability to form resonating structures, which contributes to their high stability [17].
With more stable bonding, the average drug-to-antibody ratio (DAR) on each ADC can be increased, thereby improving the drug’s potency. This technique has proven successful in FDA-approved ADCs such as gemtuzumab ozogamicin and trastuzumab emtansine [15, 16].
Cysteine-based conjugation is another widely used chemical conjugation method for ADC construction. This approach involves the thiol group of cysteine binding with electrophilic reagents, quickly forming a stable thioether or disulfide bond [18].
A significant advantage of cysteine-based conjugation over lysine-based conjugation is its limited conjugation sites, which allows for site-specific antibody conjugation with a DAR distribution ranging from 0 to 8. This site-specificity improves the homogeneity of ADCs and enhances their pharmacokinetics and pharmacodynamics compared to lysine-based conjugation [15, 16].
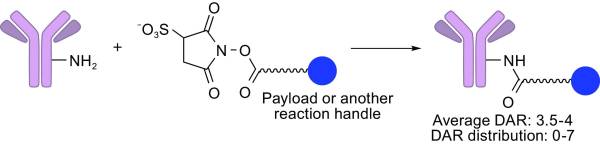
Figure 2. A schematic representation of lysine amide coupling [15].
3.1.2. Enzymatic conjugations. Enzymatic conjugation is a technique that uses enzymes to modify specific amino acid sequences in proteins. The binding reaction is catalyzed by the enzyme once the antibody has attached itself to the payload, forming site-specific conjugation with controlled DAR distribution [19].
Sortase A from Staphylococcus aureus is one commonly used enzyme in this process. It recognizes the LPXTG motif in proteins and attaches it to any molecules with oligoglycine [15].
Enzymatic conjugations offer several advantages over conventional chemical conjugations, including site-specific conjugation, controlled DAR distribution, and improved homogeneity, leading to better safety and efficacy.
3.2. Bispecific ADC (BsADC)
Since the advent of immunoglobulin antibodies and ADCs as targeted cancer therapies, significant advances have been made in both safety and efficacy. The rapid growth of the ADC field is largely due to its high specificity in delivering drugs to targeted cancer cells. However, targeting a single specific cancer cell is increasingly insufficient for achieving a complete cure, as complex diseases like cancer involve multiple cell types and signaling pathways for their proliferation and survival.
The introduction of Bispecific ADCs (BsADCs) marks the next generation of ADCs, capable of targeting two different cancer cell antigens simultaneously to deliver drugs and achieve synergistic therapeutic effects. The structural difference between a conventional ADC and a BsADC is minimal (Figure 3); the key distinction is that the BsADC’s linker-payload complex is connected to a bispecific antibody (bsAb) instead of a mAb.
The concept of bsAb was first proposed by Nisonoff and his collaborators in the 1960s, designed to target two distinct antigens from different cancer cells or co-expressed antigens from the same cancer cell. For example, bsAbs targeting Hepatocellular Carcinoma (HCC) can selectively bind to the GPC3-MUC13 pair, where both antigens are co-expressed in 30% of malignant hepatocytes but are scarcely present in other cells [20].
The advantage of dual-epitope binding is improved selectivity and reduced tumor escape. BsAbs are categorized into two main types: Fragment-based bsAbs and Fc-based bsAbs. Fragment-based bsAbs are smaller in size compared to Fc-based bsAbs, leading to improved clearance and tissue penetration in vivo. In contrast, Fc-based bsAbs, despite their larger size, achieve longer half-life by binding to FcRn, enabling FcRn recycling [21].
The effectiveness of BsADCs holds great promise in addressing cancer heterogeneity, with phase III studies for more than 400 variants are presently underway and ten already approved. Zanidatamab, a BsADC targeting two distinct epitopes on HER2, is a notable example [22].
According to data presented in 2022 by Zymeworks Inc., a biopharmaceutical company, a study involving 45 patients with HER2+ metastatic breast cancer who received Zanidatamab in combination with palbociclib and fulvestrant showed a 33% confirmed objective response rate (cORR), a 92% DCR, and a median PFS of 9.6 months [23].
In summary, BsADC represents not just a different type of ADC, but an enhanced version with improved efficacy. This promising research field is becoming mainstream in the exploration of targeted cancer treatments, with the potential to overcome many limitations of current therapies.
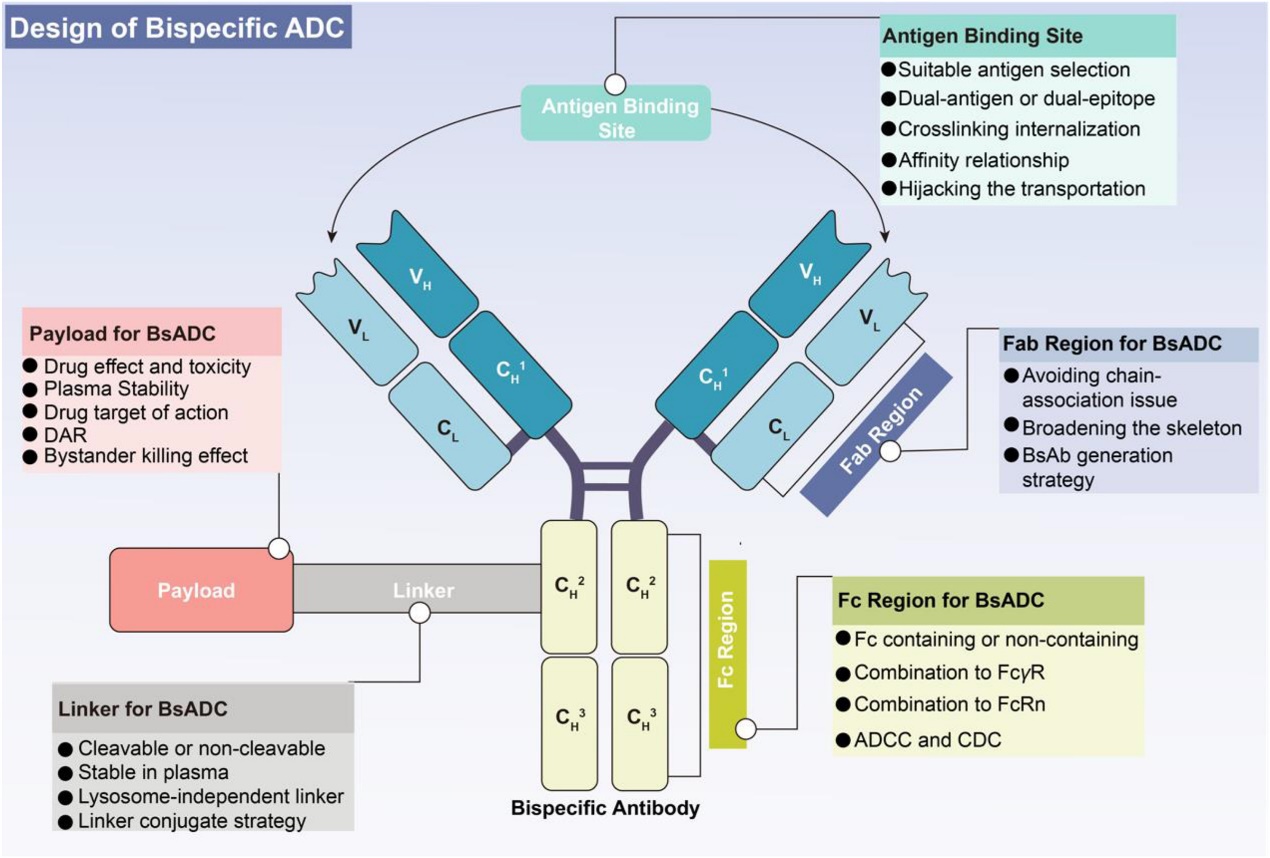
Figure 3. A schematic representation of the structural design of BsADCs [10].
4. Limitations
The most significant advantage of ADCs compared to conventional chemotherapy agents is their specificity. ADCs are designed to precisely target a selective range of cancer cells without causing unwanted toxicity to the human body. However, one of the biggest challenges encountered in many clinical trials is off-target toxicity. This could happen when the drug payload is released prematurely into the bloodstream before reaching the targeted tumor site, damaging healthy tissue cells. This issue often results from instability in linkers.
Linkers in ADCs could be classified into two types: cleavable and non-cleavable linkers. Cleavable linkers are designed to remain stable and be quickly released by lysosomal enzymes after the ADC enters early endosomes. However, in practical trial, these linkers are often hydrolyzed at a significant rate, leading to premature payload release. Lipophilic payloads, which have higher permeability to the plasma membrane, can be particularly problematic if released prematurely, as they may be transported to nearby healthy cells through membrane diffusion, causing off-target toxicity [24].
For example, the hydrazone linker used in some first-generation ADCs, such as gemtuzumab ozogamicin, was designed to be cleaved in an acidic environment after entering the plasma through receptor-mediated endocytosis. However, it was found that this linker hydrolyzed long before the ADC interacted with the tumor cell. This premature release led to a substantial loss of available payload delivered to targeted cells and an increase in off-target toxicity [24].
In general, non-cleavable linkers are more commonly used in modern ADCs, as they offer a wider therapeutic window than cleavable linkers. Non-cleavable linkers have better plasma stability, providing enhanced safety and efficacy compared to their cleavable counterparts [25].
Another limitation frequently encountered in ADC therapy is the bystander effect. The bystander effect occurs when the payload, after being delivered to the cancer cell, diffuses out of the plasma membrane and causes off-target toxicity by affecting neighboring non-target cells. This efflux of payloads is most common among lipophilic molecules with high membrane permeability, facilitated by transporter-mediated diffusion or passive diffusion. For example, hepatic toxicity was observed in patients who received cantuzumab mertansine (Can-M), with the bystander effect on adjacent normal cells suggested as a possible cause [26].
But there are two sides to the bystander effect. While it can cause off-target toxicity, it may also provide therapeutic benefits by killing heterogeneous cancer cells, especially in tumors that express variable antigens [26].
Overall, the bystander effect must be carefully managed. When implemented correctly, it can enhance tumor-killing efficacy, but if not controlled, it can lead to off-target toxicities.
5. Conclusion
ADCs are increasingly important in targeted cancer therapies, integrating mAb’s specificity with the formidable antitumor effects of chemotherapy agents. Their success in clinical applications, such as with T-DM1 targeting HER2+ solid tumors and metastatic breast cancer, has significantly improved patient outcomes and raised the likelihood of disease remission. Despite these successes, challenges such as off-target toxicity and cancer heterogeneity persist.
Advancements in conjugation techniques and the development of BsADCs offer promising solutions to these limitations. These innovations have the potential to optimize therapeutic outcomes and minimize adverse events, thereby enhancing the overall efficacy of ADC-based treatments.
The field of ADCs is rapidly evolving, particularly with the emergence of combination therapies and personalized ADCs. Combination therapy, where ADCs are used alongside other immunotherapies, such as immune checkpoint inhibitors, offers a multifaceted approach to improving therapeutic efficacy. By targeting multiple pathways, combination therapies address the heterogeneous nature of cancer and overcome drug resistance. For example, a recent clinical trial combining a HER2-targeted ADC with a PD-1 inhibitor in patients with recurrent HER2+ breast cancer exceeded expectations in treating low-expressing tumors, surpassing previous treatments like T-DM1. The potent antitumor effect was attributed to the unique dual mechanisms of action targeting low-expressing HER2. However, the lack of phase III clinical trials highlights the urgent need for further research to refine these therapies [27].
Personalized ADCs represent another frontier in cancer treatment, tailored to the unique tumor profile of individual patients. This approach focuses on the safe delivery of payloads and the elimination of off-target toxicities by customizing the antibody, linker, payload, and DAR to best match the patient's health system.
The full potential of ADCs has yet to be realized. Ongoing research into conjugation techniques, bispecific antibody-drug conjugates, combination therapies, and personalized ADCs promises to address current limitations. As these advancements continue, the future of cancer treatment with more effective and safer therapies looks increasingly promising.
References
[1]. World Health Organization. (2024). https://www.who.int/news-room/fact-sheets/detail/breast-cancer.
[2]. Amjad, M. (2023). Cancer chemotherapy. StatPearls. https://www.ncbi.nlm.nih.gov/books/NBK564367/
[3]. Bayer V. (2019). An Overview of Monoclonal Antibodies. Seminars in oncology nursing, 35(5), 150927. https://doi.org/10.1016/j.soncn.2019.08.006
[4]. Shastry, M., Gupta, A., Chandarlapaty, S., Young, M., Powles, T., & Hamilton, E. (2023). Rise of antibody-drug conjugates: The present and future. American Society of Clinical Oncology Educational Book. https://ascopubs.org/doi/10.1200/EDBK_390094
[5]. Barok, M., Joensuu, H., & Isola, J. (2014). Trastuzumab emtansine: mechanisms of action and drug resistance. Breast cancer research : BCR, 16(2), 209. https://doi.org/10.1186/bcr3621
[6]. Goodman, A. (2024). Long-term follow-up supports postneoadjuvant T-DM1 over trastuzumab in early, high-risk, HER2-positive breast cancer. The ASCO Post. https://ascopost.com/issues/january-25-2024/long-term-follow-up-supports-postneoadjuvant-t-dm1-over-trastuzumab-in-early-high-risk-her2-positive-breast-cancer/#:~:text=The%20absolute%20invasive%20disease%E2%80%93free, 0001
[7]. Peddi, P. F., & Hurvitz, S. A. (2014). Ado-trastuzumab emtansine (T-DM1) in human epidermal growth factor receptor 2 (HER2)-positive metastatic breast cancer: latest evidence and clinical potential. Therapeutic advances in medical oncology, 6(5), 202–209. https://doi.org/10.1177/1758834014539183
[8]. Malik, B. (2023). Understanding how monoclonal antibodies work. StatPearls. https://www.ncbi.nlm.nih.gov/books/NBK572118/
[9]. Gajria, D., & Chandarlapaty, S. (2011). HER2-amplified breast cancer: mechanisms of trastuzumab resistance and novel targeted therapies. Expert review of anticancer therapy, 11(2), 263–275. https://doi.org/10.1586/era.10.226
[10]. Gu, Y., Wang, Z., & Wang, Y. (2024). Bispecific antibody drug conjugates: Making 1+1>2. Acta Pharmaceutica Sinica B, 14(5), 1965–1986. https://doi.org/10.1016/j.apsb.2024.01.009
[11]. Hunter, F. W., Barker, H. R., Lipert, B., Rothé, F., Gebhart, G., Piccart-Gebhart, M. J., Sotiriou, C., & Jamieson, S. M. F. (2020). Mechanisms of resistance to trastuzumab emtansine (T-DM1) in HER2-positive breast cancer. British journal of cancer, 122(5), 603–612. https://doi.org/10.1038/s41416-019-0635-y
[12]. van de Donk, N. W., & Dhimolea, E. (2012). Brentuximab vedotin. mAbs, 4(4), 458–465. https://doi.org/10.4161/mabs.20230
[13]. Horwitz, S., O'Connor, O. A., Pro, B., Illidge, T., Fanale, M., Advani, R., Bartlett, N. L., Christensen, J. H., Morschhauser, F., Domingo-Domenech, E., Rossi, G., Kim, W. S., Feldman, T., Lennard, A., Belada, D., Illés, Á., Tobinai, K., Tsukasaki, K., Yeh, S. P., Shustov, A., … ECHELON-2 Study Group (2019). Brentuximab vedotin with chemotherapy for CD30-positive peripheral T-cell lymphoma (ECHELON-2): a global, double-blind, randomised, phase 3 trial. Lancet (London, England), 393(10168), 229–240. https://doi.org/10.1016/S0140-6736(18)32984-2
[14]. Horwitz, S., O'Connor, O. A., Pro, B., Trümper, L., Iyer, S., Advani, R., Bartlett, N. L., Christensen, J. H., Morschhauser, F., Domingo-Domenech, E., Rossi, G., Kim, W. S., Feldman, T., Menne, T., Belada, D., Illés, Á., Tobinai, K., Tsukasaki, K., Yeh, S. P., Shustov, A., … Illidge, T. (2022). The ECHELON-2 Trial: 5-year results of a randomized, phase III study of brentuximab vedotin with chemotherapy for CD30-positive peripheral T-cell lymphoma. Annals of oncology : official journal of the European Society for Medical Oncology, 33(3), 288–298. https://doi.org/10.1016/j.annonc.2021.12.002
[15]. Tsuchikama, K., & An, Z. (2018). Antibody-drug conjugates: recent advances in conjugation and linker chemistries. Protein & cell, 9(1), 33–46. https://doi.org/10.1007/s13238-016-0323-0
[16]. Lysine based conjugation. Creative Biolabs. https://www.creative-biolabs.com/adc/lysine-based-conjugation.htm
[17]. Mahesh, S., Tang, K. C., & Raj, M. (2018). Amide Bond Activation of Biological Molecules. Molecules (Basel, Switzerland), 23(10), 2615. https://doi.org/10.3390/molecules23102615
[18]. You, J., Zhang, J., Wang, J., & Jin, M. (2021). Cysteine-based coupling: Challenges and solutions. ACS Publications. https://pubs.acs.org/doi/10.1021/acs.bioconjchem.1c00213
[19]. Enzymatic conjugation. BOC Sciences. https://www.bocsci.com/enzymatic-conjugation.html#:~:text=Enzymatic%20conjugation%20involves%20modification%20of, found%20in%20the%20tumor%20microenvironment
[20]. Chekalin, E., Paithankar, S., Shankar, R., Xing, J., Xu, W., & Chen, B. (2024). Computational discovery of co-expressed antigens as dual targeting candidates for cancer therapy through bulk, single-cell, and spatial transcriptomics. OUP Academic. https://academic.oup.com/bioinformaticsadvances/article/4/1/vbae096/7696853#469502195
[21]. Shim H. (2020). Bispecific Antibodies and Antibody-Drug Conjugates for Cancer Therapy: Technological Considerations. Biomolecules, 10(3), 360. https://doi.org/10.3390/biom10030360
[22]. Harding, J. J., Fan, J., Oh, D. Y., Choi, H. J., Kim, J. W., Chang, H. M., Bao, L., Sun, H. C., Macarulla, T., Xie, F., Metges, J. P., Ying, J., Bridgewater, J., Lee, M. A., Tejani, M. A., Chen, E. Y., Kim, D. U., Wasan, H., Ducreux, M., Bao, Y., … HERIZON-BTC-01 study group (2023). Zanidatamab for HER2-amplified, unresectable, locally advanced or metastatic biliary tract cancer (HERIZON-BTC-01): a multicentre, single-arm, phase 2b study. The Lancet. Oncology, 24(7), 772–782. https://doi.org/10.1016/S1470-2045(23)00242-5
[23]. Zymeworks Inc. (2022). New clinical data for Zanidatamab in her2+ /hr+ metastatic breast cancer presented today at 2022 SABCS. https://ir.zymeworks.com/news-releases/news-release-details/new-clinical-data-zanidatamab-her2-hr-metastatic-breast-cancer-0
[24]. Nguyen, T. D., Bordeau, B. M., & Balthasar, J. P. (2023). Mechanisms of ADC Toxicity and Strategies to Increase ADC Tolerability. Cancers, 15(3), 713. https://doi.org/10.3390/cancers15030713
[25]. Sheyi, R., de la Torre, B. G., & Albericio, F. (2022). Linkers: An Assurance for Controlled Delivery of Antibody-Drug Conjugate. Pharmaceutics, 14(2), 396. https://doi.org/10.3390/pharmaceutics14020396
[26]. Mahalingaiah, P. K., Ciurlionis, R., Durbin, K. R., Yeager, R. L., Philip, B. K., Bawa, B., Mantena, S. R., Enright, B. P., Liguori, M. J., & Vleet, T. R. V. (2019). Potential mechanisms of target-independent uptake and toxicity of antibody-drug conjugates. Pharmacology & Therapeutics, 200, 110–125. https://doi.org/10.1016/j.pharmthera.2019.04.008
[27]. Fan, S., He, L., & Sang, D. (2023). Combination therapy with antibody drug conjugate RC48 (disitamab vedotin) and zimberelimab (PD 1 inhibitor) successfully controlled recurrent HER2 positive breast cancer resistant to trastuzumab emtansine: A case report. Oncology letters, 26(2), 359. https://doi.org/10.3892/ol.2023.13945
Cite this article
Wang,Z. (2025). On-target off-tumor toxicity from HER2-targeting chimeric antigen receptor (CAR) engineered T cell therapy: current solutions. Theoretical and Natural Science,77,107-115.
Data availability
The datasets used and/or analyzed during the current study will be available from the authors upon reasonable request.
Disclaimer/Publisher's Note
The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of EWA Publishing and/or the editor(s). EWA Publishing and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
About volume
Volume title: Proceedings of ICBioMed 2024 Workshop: Computational Proteomics in Drug Discovery and Development from Medicinal Plants
© 2024 by the author(s). Licensee EWA Publishing, Oxford, UK. This article is an open access article distributed under the terms and
conditions of the Creative Commons Attribution (CC BY) license. Authors who
publish this series agree to the following terms:
1. Authors retain copyright and grant the series right of first publication with the work simultaneously licensed under a Creative Commons
Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this
series.
2. Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the series's published
version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial
publication in this series.
3. Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and
during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See
Open access policy for details).
References
[1]. World Health Organization. (2024). https://www.who.int/news-room/fact-sheets/detail/breast-cancer.
[2]. Amjad, M. (2023). Cancer chemotherapy. StatPearls. https://www.ncbi.nlm.nih.gov/books/NBK564367/
[3]. Bayer V. (2019). An Overview of Monoclonal Antibodies. Seminars in oncology nursing, 35(5), 150927. https://doi.org/10.1016/j.soncn.2019.08.006
[4]. Shastry, M., Gupta, A., Chandarlapaty, S., Young, M., Powles, T., & Hamilton, E. (2023). Rise of antibody-drug conjugates: The present and future. American Society of Clinical Oncology Educational Book. https://ascopubs.org/doi/10.1200/EDBK_390094
[5]. Barok, M., Joensuu, H., & Isola, J. (2014). Trastuzumab emtansine: mechanisms of action and drug resistance. Breast cancer research : BCR, 16(2), 209. https://doi.org/10.1186/bcr3621
[6]. Goodman, A. (2024). Long-term follow-up supports postneoadjuvant T-DM1 over trastuzumab in early, high-risk, HER2-positive breast cancer. The ASCO Post. https://ascopost.com/issues/january-25-2024/long-term-follow-up-supports-postneoadjuvant-t-dm1-over-trastuzumab-in-early-high-risk-her2-positive-breast-cancer/#:~:text=The%20absolute%20invasive%20disease%E2%80%93free, 0001
[7]. Peddi, P. F., & Hurvitz, S. A. (2014). Ado-trastuzumab emtansine (T-DM1) in human epidermal growth factor receptor 2 (HER2)-positive metastatic breast cancer: latest evidence and clinical potential. Therapeutic advances in medical oncology, 6(5), 202–209. https://doi.org/10.1177/1758834014539183
[8]. Malik, B. (2023). Understanding how monoclonal antibodies work. StatPearls. https://www.ncbi.nlm.nih.gov/books/NBK572118/
[9]. Gajria, D., & Chandarlapaty, S. (2011). HER2-amplified breast cancer: mechanisms of trastuzumab resistance and novel targeted therapies. Expert review of anticancer therapy, 11(2), 263–275. https://doi.org/10.1586/era.10.226
[10]. Gu, Y., Wang, Z., & Wang, Y. (2024). Bispecific antibody drug conjugates: Making 1+1>2. Acta Pharmaceutica Sinica B, 14(5), 1965–1986. https://doi.org/10.1016/j.apsb.2024.01.009
[11]. Hunter, F. W., Barker, H. R., Lipert, B., Rothé, F., Gebhart, G., Piccart-Gebhart, M. J., Sotiriou, C., & Jamieson, S. M. F. (2020). Mechanisms of resistance to trastuzumab emtansine (T-DM1) in HER2-positive breast cancer. British journal of cancer, 122(5), 603–612. https://doi.org/10.1038/s41416-019-0635-y
[12]. van de Donk, N. W., & Dhimolea, E. (2012). Brentuximab vedotin. mAbs, 4(4), 458–465. https://doi.org/10.4161/mabs.20230
[13]. Horwitz, S., O'Connor, O. A., Pro, B., Illidge, T., Fanale, M., Advani, R., Bartlett, N. L., Christensen, J. H., Morschhauser, F., Domingo-Domenech, E., Rossi, G., Kim, W. S., Feldman, T., Lennard, A., Belada, D., Illés, Á., Tobinai, K., Tsukasaki, K., Yeh, S. P., Shustov, A., … ECHELON-2 Study Group (2019). Brentuximab vedotin with chemotherapy for CD30-positive peripheral T-cell lymphoma (ECHELON-2): a global, double-blind, randomised, phase 3 trial. Lancet (London, England), 393(10168), 229–240. https://doi.org/10.1016/S0140-6736(18)32984-2
[14]. Horwitz, S., O'Connor, O. A., Pro, B., Trümper, L., Iyer, S., Advani, R., Bartlett, N. L., Christensen, J. H., Morschhauser, F., Domingo-Domenech, E., Rossi, G., Kim, W. S., Feldman, T., Menne, T., Belada, D., Illés, Á., Tobinai, K., Tsukasaki, K., Yeh, S. P., Shustov, A., … Illidge, T. (2022). The ECHELON-2 Trial: 5-year results of a randomized, phase III study of brentuximab vedotin with chemotherapy for CD30-positive peripheral T-cell lymphoma. Annals of oncology : official journal of the European Society for Medical Oncology, 33(3), 288–298. https://doi.org/10.1016/j.annonc.2021.12.002
[15]. Tsuchikama, K., & An, Z. (2018). Antibody-drug conjugates: recent advances in conjugation and linker chemistries. Protein & cell, 9(1), 33–46. https://doi.org/10.1007/s13238-016-0323-0
[16]. Lysine based conjugation. Creative Biolabs. https://www.creative-biolabs.com/adc/lysine-based-conjugation.htm
[17]. Mahesh, S., Tang, K. C., & Raj, M. (2018). Amide Bond Activation of Biological Molecules. Molecules (Basel, Switzerland), 23(10), 2615. https://doi.org/10.3390/molecules23102615
[18]. You, J., Zhang, J., Wang, J., & Jin, M. (2021). Cysteine-based coupling: Challenges and solutions. ACS Publications. https://pubs.acs.org/doi/10.1021/acs.bioconjchem.1c00213
[19]. Enzymatic conjugation. BOC Sciences. https://www.bocsci.com/enzymatic-conjugation.html#:~:text=Enzymatic%20conjugation%20involves%20modification%20of, found%20in%20the%20tumor%20microenvironment
[20]. Chekalin, E., Paithankar, S., Shankar, R., Xing, J., Xu, W., & Chen, B. (2024). Computational discovery of co-expressed antigens as dual targeting candidates for cancer therapy through bulk, single-cell, and spatial transcriptomics. OUP Academic. https://academic.oup.com/bioinformaticsadvances/article/4/1/vbae096/7696853#469502195
[21]. Shim H. (2020). Bispecific Antibodies and Antibody-Drug Conjugates for Cancer Therapy: Technological Considerations. Biomolecules, 10(3), 360. https://doi.org/10.3390/biom10030360
[22]. Harding, J. J., Fan, J., Oh, D. Y., Choi, H. J., Kim, J. W., Chang, H. M., Bao, L., Sun, H. C., Macarulla, T., Xie, F., Metges, J. P., Ying, J., Bridgewater, J., Lee, M. A., Tejani, M. A., Chen, E. Y., Kim, D. U., Wasan, H., Ducreux, M., Bao, Y., … HERIZON-BTC-01 study group (2023). Zanidatamab for HER2-amplified, unresectable, locally advanced or metastatic biliary tract cancer (HERIZON-BTC-01): a multicentre, single-arm, phase 2b study. The Lancet. Oncology, 24(7), 772–782. https://doi.org/10.1016/S1470-2045(23)00242-5
[23]. Zymeworks Inc. (2022). New clinical data for Zanidatamab in her2+ /hr+ metastatic breast cancer presented today at 2022 SABCS. https://ir.zymeworks.com/news-releases/news-release-details/new-clinical-data-zanidatamab-her2-hr-metastatic-breast-cancer-0
[24]. Nguyen, T. D., Bordeau, B. M., & Balthasar, J. P. (2023). Mechanisms of ADC Toxicity and Strategies to Increase ADC Tolerability. Cancers, 15(3), 713. https://doi.org/10.3390/cancers15030713
[25]. Sheyi, R., de la Torre, B. G., & Albericio, F. (2022). Linkers: An Assurance for Controlled Delivery of Antibody-Drug Conjugate. Pharmaceutics, 14(2), 396. https://doi.org/10.3390/pharmaceutics14020396
[26]. Mahalingaiah, P. K., Ciurlionis, R., Durbin, K. R., Yeager, R. L., Philip, B. K., Bawa, B., Mantena, S. R., Enright, B. P., Liguori, M. J., & Vleet, T. R. V. (2019). Potential mechanisms of target-independent uptake and toxicity of antibody-drug conjugates. Pharmacology & Therapeutics, 200, 110–125. https://doi.org/10.1016/j.pharmthera.2019.04.008
[27]. Fan, S., He, L., & Sang, D. (2023). Combination therapy with antibody drug conjugate RC48 (disitamab vedotin) and zimberelimab (PD 1 inhibitor) successfully controlled recurrent HER2 positive breast cancer resistant to trastuzumab emtansine: A case report. Oncology letters, 26(2), 359. https://doi.org/10.3892/ol.2023.13945









