1.Introduction
Even with all of the advancements in cancer treatment over the last few decades, the heterogeneity and drug resistance characteristics of cancer cells continue to present significant challenges to effective cancer therapy [1]. On February 2, 2024, IARC once again emphasized the growing global burden of cancer, a problem that requires global attention. Based on existing data, it can be projected that the number of new cancer cases in 2050 will exceed 30 million, a significant increase from the 2022 figure of 2 million (Figure 1). This represents an increase of 77% in new cases compared to the 2,000,000 cases recorded in 2022. Furthermore, it is likely that the mortality rate from cancer will have doubled by 2050 [2].

Figure 1. The annual number of cancer-related deaths and new cases.
To achieve effective cancer treatment, several different treatment methods have been the subject of recent research. In the long run, the research produced monoclonal antibodies that can bind directly to markers on the surface of tumor cells and trigger an immune response, further destroying tumor cells. The structure of a monoclonal antibody comprises two parts: the heavy chain (CH1 and CL) and the light chain (VH and VL). The heavy and light chains are formed by the variable region (VH and VL) and the constant region (CH1 and CL), respectively. The variable region is responsible for antigen recognition, while the constant region determines the specificity and function of the antibody (Figure 2). The function of a monoclonal antibody is largely determined by its structure. Each B cell clone produces a single antibody, resulting in high specificity and affinity. Additionally, monoclonal antibodies can be modified by altering the constant region (Fc region) to alter their functional properties. This includes enhancing ADCC, inhibiting complement activation, and other effects. Currently, an essential part of tumor therapy is the use of monoclonal antibodies, along with surgery, radiotherapy, and chemotherapy [3], [4], [5]. As the number of monoclonal antibodies in development continues to develop, the potential for using monoclonal antibodies to treat cancer is becoming increasingly evident.
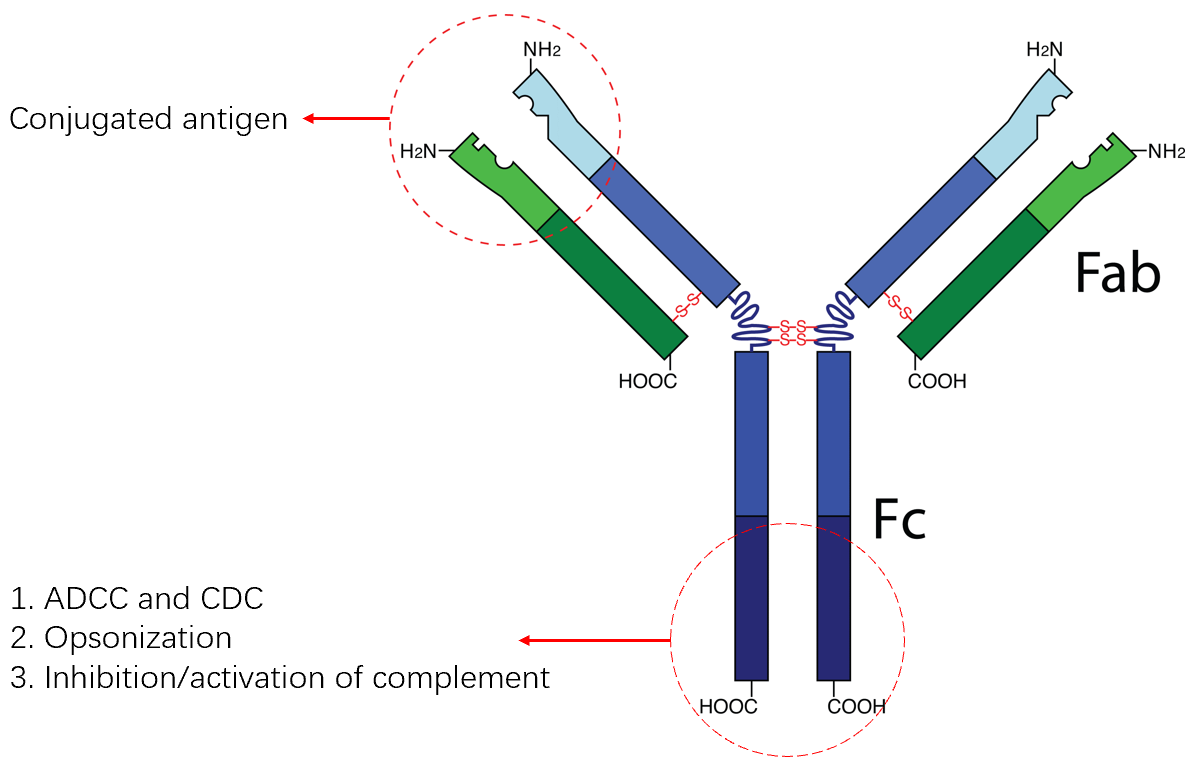
Figure 2. The structure and function of monoclonal antibody.
2.The development of monoclonal antibodies
Monoclonal antibody technology has evolved from murine to human sources. Since its inception in the 1970s, monoclonal antibody technology has undergone a significant transformation, evolving from murine to human antibodies. This evolution has not only reflected advancements in scientific and technological fields but has also brought about revolutionary changes in clinical treatment. The following sections will delve into the details of this technological development.
2.1.Murine monoclonal antibodies
Monoclonal antibodies of murine origin were the first of their kind to be developed, the first successful production of the compound was achieved in 1975. They were initially hailed as a revolutionary treatment for cancer due to their high specificity and low toxicity. Moreover, it experienced a period of rapid development during the 1980s. However, their use in humans has been severely limited by several factors. Firstly, murine antibodies are recognized as foreign bodies within the human body, triggering an immune response that results in their rapid clearance and subsequent reduction in efficacy. Secondly, the high cost of production and the susceptibility of the antibodies to chemical degradation during antigen processing further compromise their therapeutic potential and stability.
2.2.Human-mouse chimeric monoclonal antibodies
To address the issue of immunogenicity associated with murine antibodies, scientists have developed a technology known as chimeric monoclonal antibodies in 1984s. The possibility of the antibody being identified as a foreign material in the human body is decreased by combining the constant portion of a human immunoglobulin with the variable area of the animal's antibody. This results in a longer half-life for the drug and a reduction in the incidence of immune reactions [6]. Compared to fully human monoclonal antibodies, chimeric antibodies are produced at a lower cost, which is beneficial in reducing the overall expense of cancer treatment. Cetuximab, the first chimeric antibody to be approved globally, has demonstrated good tolerability and the absence of enhanced toxicity in patients with metastatic colorectal cancer [7].
2.3.Humanized monoclonal antibody
In the 1990s, scientists in the United States successfully produced the first human monoclonal antibody using the recombinant DNA technology known as "phage display." They exhibit greater specificity than conventional monoclonal antibodies, enabling more precise identification and attaching to certain antigens on cancerous cells. This reduces the adverse effects on normal cells and improves treatment efficacy. Compared to traditional anti-tumor drugs, humanized monoclonal antibodies often have fewer adverse effects, which is beneficial for patients during treatment. Additionally, humanized monoclonal antibodies have reduced immunogenicity. The process involves the transfer of mouse antibodies' complementarity determining regions (CDRs), which oversee recognizing antigens, to the human antibody framework. This results in a higher proportion of humanized antibodies [8]. This method allows for the preservation of the antibody's specificity and affinity while significantly reducing the incidence of adverse effects.
2.4.Whole human monoclonal antibodies
The 1990s saw the development of monoclonal antibodies for the treatment of cancer. Many monoclonal antibodies have been created since then, such as trastuzumab and rituximab. Currently, monoclonal antibodies are a crucial tool in cancer therapy, with applications in the treatment of various types of cancer. Whole human monoclonal antibodies are antibodies that are entirely composed of human genes. Their variable and constant regions originate from humans. The development of techniques such as bacterial display, yeast display, and transgenic animals has enabled the complete resolution of immunogenicity, resulting in enhanced efficacy and safety of pharmaceuticals [9]. Consequently, these agents demonstrate enhanced specificity, directionality, and reduced adverse effects. However, there are still some potential drawbacks, such as the possibility that higher production costs may limit their applicability in certain scenarios.
Since the first monoclonal antibody drug was successfully developed in 1975 for the treatment of cancer, monoclonal antibody technology has consistently demonstrated advancements in overcoming the limitations of its predecessors, exhibiting enhanced efficacy and reduced toxicity. These developments have opened new avenues for the treatment of various diseases, including cancer and autoimmune disorders.
3.Mechanism of action of monoclonal antibodies
The discovery of monoclonal antibodies has significantly improved the effectiveness of cancer treatment in the past few decades. Through several methods, these antibodies have the ability to attack cancer cells, thereby improving the prognosis and survival rate of patients. In certain instances, they have emerged as a primary therapeutic option. An overview of the main methods that monoclonal antibodies have recently been used in cancer therapy will be given in this article.
3.1.Specific targeting of cancer cells
The high specificity, high accuracy, and low immunogenicity of monoclonal antibodies make them ideal for identifying and binding to specific antigens on cancer cells, thereby initiating cell death and directly killing cancer cells. However, as research into tumor molecular biology and genomics has progressed, it has become evident that, except for CD20, HER2, and EGFR, many monoclonal antibodies lack antitumor activity [10]. Furthermore, the cost and complexity of designing unique epitopes may also impact the stability of therapeutic outcomes.
3.2.The capacity to regulate the immune response to cancer
A cancer treatment called monoclonal antibody therapy stimulates the patient's immune system in an attempt to eradicate or damage cancer cells. The immune system may be stimulated by certain monoclonal antibodies., particularly natural killer cells and macrophages, which can identify and destroy cancer cells [10]. Research has shown that the expression of FcγR on immune effector cells is necessary for the response of tumors to monoclonal antibody therapy [11]. Furthermore, monoclonal antibodies can not only recognize and bind to cancer cells but also interact with immune cells through their Fc receptors, enhancing the efficacy of cancer treatment.
3.3.Signal Transduction Interference
Signal transduction interference is a mechanism whereby receptors and ligands interact to block or activate specific signal pathways, thereby exerting anti-tumor effects. Stimulatory signals can activate immune cells, enhancing their ability to attack cancer cells, while inhibitory signals often interact with tumor cells, impeding their growth, differentiation, and other functions. The most prevalent method of signal transduction interference in cancer therapy is the disruption of growth signals required for tumor cell proliferation, which ultimately inhibits tumor growth. An illustrative example is the use of cetuximab in the treatment of Inhibition of EGFR signaling prevented the activation of the receptor, which subsequently inhibited the proliferation of tumor cells [10].
4.Monoclonal Antibodies' Application in Cancer Treatment
To further investigate the role of single-chain antibodies in cancer therapy, this review presents a summary of the efficacy of different single-chain antibody drugs in treating cancer (Table 1) and targeting different receptors (Table 2). It can be observed that the most effective single-chain antibody drugs are immune checkpoint inhibitors and immune stimulators. This is because many single-chain antibodies lack antitumor activity, and thus, can only stimulate immunological responses to increase the immune system's capacity to eliminate cancerous cells. However, a limited number of single-chain antibodies can also inhibit tumor cell proliferation or regulate angiogenesis, thereby further inhibiting tumor growth and metastasis.
Table 1. Monoclonal antibody medications are authorized for the management of cancer, and for their functions and targets.
|
Type of cancer |
Kind of cancer |
Brand name |
Interna-tional non-proprie-tary name |
Target |
The mechanism of action |
Ref-eren-ce |
|
Epithelial cancer |
Lung cancer |
OPDIVO |
Nivolumab |
PD-1 |
binds to PD-1 and inhibit PD-L1 and PD-L2 from interacting with it. inhibits the immunological checkpoint blockage. doesn't cause ADCC |
[12] |
|
Keytruda |
Pembrolizumab |
PD-1 |
binds to PD-1 and inhibit PD-L1 and PD-L2 from interacting with it. inhibits the immunological checkpoint blockage. doesn't cause ADCC |
[13] |
||
|
Breast cancer |
Herceptin |
Trastuzumab |
HER2 |
suppresses HER dimerization and stops HER2 from forming ligand-induced heterodimers with other members of the family. triggers phagocytosis and ADCC. |
[14] |
|
|
Perjeta |
Pertuzumab |
HER2 |
suppresses HER dimerization and stops HER2 from forming ligand-induced heterodimers with other members of the family. triggers phagocytosis and ADCC. |
[15] |
||
|
Colorectal cancer |
Avastin |
Bevacizumab |
VEGF |
bind to VEGFA to stop it from interacting with its binding sites and triggering them later. |
[16] |
|
|
MABp1c |
Xilonix |
IL-1α |
binds to IL-2α and prevents IL-1R from connecting with it. |
[17] |
||
|
Prostatic cancer |
Bavencio |
Avelumab |
PD-L1 |
prevents PD-L1 from interacting with the protein PD-1 as well as the compound CD80. inhibits the immunological checkpoint |
[18] |
|
|
Lymphatic system cancer |
Lymphoma |
Rituxan |
Rituximab |
CD20 |
bind to CD20, activate B-cell lysis-promoting immune effector cells. Switches on the ADCC, and the ADCP, and CDC |
[19] |
|
Hematopoi-etic system cancer |
Leukemia |
Mylotarg |
Gemtuzumab ozogamicin |
CD33 |
connects with CD33 cells. The toxin causes apoptosis and double-stranded DNA breaks after internalization. cannot be turned on ADCC |
[20] |
|
Skin cancer |
Melanoma |
Keytruda |
Pembrolizumab |
PD-1 |
binds to PD-1 and inhibits PD-L1 and PD-L2 from interacting with it. inhibits the immunological checkpoint blockage. doesn't cause ADCC |
[21] |
|
Yervoy |
Ipilimumab |
CTLA-4 |
enhances the stimulation of T cells and multiplication by binding to CTLA-4 and inhibiting its interaction with CD80 and CD86. |
[22] |
||
|
respiratory system cancer |
NSCLC |
Tecentriq |
Atezolizumab |
PD-L1 |
prevents PD-L1 from interacting with PD-1 and CD80. inhibits the immunological checkpoint blockage. has an altered Fc region to restrict CDC or ADCC |
[23] |
|
Digestive system cancer |
Gastric cancer |
Cryamza |
Ramucirumab |
VEGFR2 |
binds to VEGFR2 and prevents its ligands from binding, preventing receptor signaling. |
[24] |
Table 2. Therapeutic effects of different targets on cancer.
|
Antibody target |
The effect of cancer therapy |
|
PD-1 |
immunological checkpoint blockage |
|
PD-L1 |
immunological checkpoint blockage |
|
CTLA-4 |
immunological checkpoint blockage |
|
HER2 |
suppression of cell proliferation |
|
VEGF |
angiogenesis inhibition |
|
VEGFR2 |
angiogenesis inhibition |
|
IL-1α |
cell growth inhibition and anti-inflammatory |
|
CD20 |
activates CDC, ADCC, and ADCP |
|
CD33 |
ADC |
A list of the nine most important target points and 13 most potent drugs used in the management of solid tumors was provided, with six monoclonal antibodies (mAbs) that utilize the destruction of immune checkpoint signals, three mAbs that inhibit cancer cell proliferation, two mAbs that inhibit angiogenesis, and two mAbs that utilize cell-killing mechanisms to destroy cancer cells. The relationship between ligands and T cell-bound programmed cell death protein 1 (PD-1), such as Nivolumab, Pembrolizumab, and Pembrolizumab, these drugs work by attaching themselves to the CTLA-4 receptor on T cells (Xilonix) or the PD-L1 receptor on tumor cells (Avelumab and Tecentriq)., thereby disrupting immune checkpoint signals. They also inhibit HER dimerization (Trastuzumab and Pertuzumab) or impede IL-1R inhibits cell proliferation by binding to PD-L1 (Avelumab). Additionally, by binding to PDGF (Ramucirumab) or VEGF (Bevacizumab), it prevents tumor angiogenesis. Furthermore, it induces cell death in cancer cells through the induction of apoptosis (Rituximab and Gemtuzumab ozogamicin).
As can be observed, monoclonal antibodies have made significant advances in the treatment of cancer. However, to control or treat cancer, it is necessary to maintain a considerable number of monoclonal antibodies, which also results in a reduction in drug efficacy and an increase in cancer cell resistance. To enhance the efficacy of the treatment, these antibodies are often combined with alternative treatment approaches, such as cell therapies and vaccinations, or with chemotherapy, radiation, and other focused treatments. As our knowledge of cancer immunotherapy expands, new targeted combinations and treatment approaches are being investigated.
5.Conclusions and Upcoming Projects
Since cancer is the 2nd most common cause of death worldwide, research is now heavily focused on developing effective therapies. With the development of monoclonal antibodies as a mainstay of cancer treatment, the area of tumor molecular biology has made tremendous strides from forward to reverse pharmacology. It is indisputable that monoclonal antibodies have been essential in the therapy of cancer, despite our current lack of information regarding their targets, methods of action, and clinical significance. They provide a very focused, comparatively low-toxicity, and highly specialized treatment alternative. Furthermore, monoclonal antibody therapy has been effective in several cancer treatments, both on its own and in conjunction with other techniques. However, with the increasing use of monoclonal antibody therapy, the emergence of drug resistance and other challenges have become increasingly apparent. To improve clinical results and overcome medication resistance, future research must explore the mechanisms of action of monoclonal antibodies in greater detail, develop novel combination therapies, and find biological markers linked to antibody efficacy and resistance.
References
[1]. Zahavi, D.; Weiner, L. Monoclonal Antibodies in Cancer Therapy. Antibodies 2020, 9, 34. https://doi.org/10.3390/antib9030034
[2]. World Health Organization. Global Cancer Burden Growing, Amidst Mounting Need for Services. Available online: https://www.who.int/news/item/01-02-2024-global-cancer-burden-growing--amidst-mounting-need-for-services (accessed on 21 June 2024).
[3]. Singh, J. (2022). Antibodies. In An Interplay of Cellular and Molecular Components of Immunology (pp. 109–131). CRC Press. https://doi.org/10.1201/9781003286424-5
[4]. Galmarini D, Galmarini C M, Galmarini F C. Cancer chemotherapy: a critical analysis of its 60 years of history[J]. Critical reviews in oncology/hematology, 2012, 84(2): 181-199. https://doi.org/10.1016/j.critrevonc.2012.03.002
[5]. Tamargo, J., Caballero, R. & Delpón, E. Cancer Chemotherapy and Cardiac Arrhythmias: A Review. Drug Saf 38, 129–152 (2015). https://doi.org/10.1007/s40264-014-0258-4
[6]. Morrison SL, Johnson MJ, Herzenberg LA, Oi VT. Chimeric human antibody molecules: mouse antigen-binding domains with human constant region domains. Proc Natl Acad Sci U S A. 1984 Nov;81(21):6851-5. doi: 10.1073/pnas.81.21.6851. PMID: 6436822; PMCID: PMC392030.
[7]. Galizia, G., Lieto, E., De Vita, F., Orditura, M., Castellano, P., Troiani, T., … Ciardiello, F. (2007, May 28). Cetuximab, a chimeric human mouse anti-epidermal growth factor receptor monoclonal antibody, in the treatment of human colorectal cancer. Oncogene. https://doi.org/10.1038/sj.onc.1210381
[8]. Jones, P., Dear, P., Foote, J. et al. Replacing the complementarity-determining regions in a human antibody with those from a mouse. Nature 321, 522–525 (1986). https://doi.org/10.1038/321522a0
[9]. Nelson, A., Dhimolea, E. & Reichert, J. Development trends for human monoclonal antibody therapeutics. Nat Rev Drug Discov 9, 767–774 (2010). https://doi.org/10.1038/nrd3229
[10]. Zahavi, D.; Weiner, L. Monoclonal Antibodies in Cancer Therapy. Antibodies 2020, 9, 34. https://doi.org/10.3390/antib9030034
[11]. Veronique Minard-Colin, Yan Xiu, Jonathan C. Poe, Mayuka Horikawa, Cynthia M. Magro, Yasuhito Hamaguchi, Karen M. Haas, Thomas F. Tedder; Lymphoma depletion during CD20 immunotherapy in mice is mediated by macrophage FcγRI, FcγRIII, and FcγRIV. Blood 2008; 112 (4): 1205–1213. doi: https://doi.org/10.1182/blood-2008-01-135160
[12]. U.S. Food and Drug Administration. (2017). OPDIVO highlights of prescribing information. Warnings and Precautions, 7(25), 1–66. Retrieved from https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/125554s034lbl.pdf#page=64.
[13]. Ren, L. (2023). The Role of Pembrolizumab (Keytruda) in Treating Non-small Cell Lung Cancer. Highlights in Science, Engineering and Technology, 36, 608–613. https://doi.org/10.54097/hset.v36i.5743
[14]. Liu, Y., Zuo, W.-J., Wang, R.-X., Wang, Z.-H., & Shao, Z.-M. (2023). Abstract P1-11-20: Trastuzumab (HLX02) plus Pertuzumab as Dual-target Neoadjuvant Therapy for HER2-positive Breast Cancer: A Real-World Study. Cancer Research, 83(5_Supplement), P1-11-20-P1-11–20. https://doi.org/10.1158/1538-7445.sabcs22-p1-11-20
[15]. Robert, M., Frenel, J. S., Bourbouloux, E., Berton Rigaud, D., Patsouris, A., Augereau, P., … Campone, M. (2020). Pertuzumab for the treatment of breast cancer. Expert Review of Anticancer Therapy, 20(2), 85–95. https://doi.org/10.1080/14737140.2019.1596805
[16]. Garcia, J., Hurwitz, H. I., Sandler, A. B., Miles, D., Coleman, R. L., Deurloo, R., & Chinot, O. L. (2020, June 1). Bevacizumab (Avastin®) in cancer treatment: A review of 15 years of clinical experience and future outlook. Cancer Treatment Reviews. W.B. Saunders Ltd. https://doi.org/10.1016/j.ctrv.2020.102017
[17]. Hickish T, Andre T, Wyrwicz L, Saunders M, Sarosiek T, Kocsis J, et al. MABp1 as a novel antibody treatment for advanced colorectal cancer: a randomised, double-blind, placebo-controlled, phase 3 study. Lancet Oncol (2017) 18(2):192–201. https://doi.org/10.1016/S1470-2045(17)30006-2
[18]. Madan, R. A., Redman, J. M., Karzai, F., Dahut, W. L., Cordes, L., Fakhrejahani, F., … Gulley, J. L. (2023). Avelumab in Men with Metastatic Castration-Resistant Prostate Cancer, Enriched for Patients Treated Previously with a Therapeutic Cancer Vaccine. Journal of Immunotherapy, 46(4), 145–151. https://doi.org/10.1097/CJI.0000000000000459
[19]. Si, T., Ma, X., Zhu, W., & Zhou, Y. (2023). Clinical efficacy and safety of subcutaneous rituximab in non-Hodgkin lymphoma: a systematic literature review and meta-analysis. Hematology (United Kingdom). Taylor and Francis Ltd. https://doi.org/10.1080/16078454.2023.2284047
[20]. Gilardi, M., Kaushik, G., Walling, B., Sambandam, V., Schiavini, P., Cairo, S., … Ritchie, M. (2023). Correlation Revealed between Gemtuzumab-Ozogamicin Efficacy and CD33+ Expression in AML Primary Samples through a Novel AML in Vitro Model. Blood, 142(Supplement 1), 7151–7151. https://doi.org/10.1182/blood-2023-186200
[21]. Kwok, G., Yau, T. C. C., Chiu, J. W., Tse, E., & Kwong, Y. L. (2016, November 1). Pembrolizumab (Keytruda). Human Vaccines and Immunotherapeutics. Taylor and Francis Inc. https://doi.org/10.1080/21645515.2016.1199310
[22]. Yan, Y. (2023). The Role Ipilimumab Plays in the Treatment of Melanoma. Theoretical and Natural Science, 3(1), 701–708. https://doi.org/10.54254/2753-8818/3/20220435
[23]. Zhou, J. G., Wong, A. H. H., Wang, H., Jin, S. H., Tan, F., Chen, Y. Z., … Gaipl, U. S. (2022). Definition of a new blood cell count score for early survival prediction for non-small cell lung cancer patients treated with atezolizumab: Integrated analysis of four multicenter clinical trials. Frontiers in Immunology, 13. https://doi.org/10.3389/fimmu.2022.961926
[24]. Chan, M. M. K., Sjoquist, K. M., & Zalcberg, J. R. (2015, September 22). Clinical utility of ramucirumab in advanced gastric cancer. Biologics: Targets and Therapy. Dove Medical Press Ltd. https://doi.org/10.2147/BTT.S62777
Cite this article
Fang,Z. (2025). The mechanisms of monoclonal antibodies and their utilization in cancer therapy. Theoretical and Natural Science,77,34-42.
Data availability
The datasets used and/or analyzed during the current study will be available from the authors upon reasonable request.
Disclaimer/Publisher's Note
The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of EWA Publishing and/or the editor(s). EWA Publishing and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
About volume
Volume title: Proceedings of ICBioMed 2024 Workshop: Computational Proteomics in Drug Discovery and Development from Medicinal Plants
© 2024 by the author(s). Licensee EWA Publishing, Oxford, UK. This article is an open access article distributed under the terms and
conditions of the Creative Commons Attribution (CC BY) license. Authors who
publish this series agree to the following terms:
1. Authors retain copyright and grant the series right of first publication with the work simultaneously licensed under a Creative Commons
Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this
series.
2. Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the series's published
version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial
publication in this series.
3. Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and
during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See
Open access policy for details).
References
[1]. Zahavi, D.; Weiner, L. Monoclonal Antibodies in Cancer Therapy. Antibodies 2020, 9, 34. https://doi.org/10.3390/antib9030034
[2]. World Health Organization. Global Cancer Burden Growing, Amidst Mounting Need for Services. Available online: https://www.who.int/news/item/01-02-2024-global-cancer-burden-growing--amidst-mounting-need-for-services (accessed on 21 June 2024).
[3]. Singh, J. (2022). Antibodies. In An Interplay of Cellular and Molecular Components of Immunology (pp. 109–131). CRC Press. https://doi.org/10.1201/9781003286424-5
[4]. Galmarini D, Galmarini C M, Galmarini F C. Cancer chemotherapy: a critical analysis of its 60 years of history[J]. Critical reviews in oncology/hematology, 2012, 84(2): 181-199. https://doi.org/10.1016/j.critrevonc.2012.03.002
[5]. Tamargo, J., Caballero, R. & Delpón, E. Cancer Chemotherapy and Cardiac Arrhythmias: A Review. Drug Saf 38, 129–152 (2015). https://doi.org/10.1007/s40264-014-0258-4
[6]. Morrison SL, Johnson MJ, Herzenberg LA, Oi VT. Chimeric human antibody molecules: mouse antigen-binding domains with human constant region domains. Proc Natl Acad Sci U S A. 1984 Nov;81(21):6851-5. doi: 10.1073/pnas.81.21.6851. PMID: 6436822; PMCID: PMC392030.
[7]. Galizia, G., Lieto, E., De Vita, F., Orditura, M., Castellano, P., Troiani, T., … Ciardiello, F. (2007, May 28). Cetuximab, a chimeric human mouse anti-epidermal growth factor receptor monoclonal antibody, in the treatment of human colorectal cancer. Oncogene. https://doi.org/10.1038/sj.onc.1210381
[8]. Jones, P., Dear, P., Foote, J. et al. Replacing the complementarity-determining regions in a human antibody with those from a mouse. Nature 321, 522–525 (1986). https://doi.org/10.1038/321522a0
[9]. Nelson, A., Dhimolea, E. & Reichert, J. Development trends for human monoclonal antibody therapeutics. Nat Rev Drug Discov 9, 767–774 (2010). https://doi.org/10.1038/nrd3229
[10]. Zahavi, D.; Weiner, L. Monoclonal Antibodies in Cancer Therapy. Antibodies 2020, 9, 34. https://doi.org/10.3390/antib9030034
[11]. Veronique Minard-Colin, Yan Xiu, Jonathan C. Poe, Mayuka Horikawa, Cynthia M. Magro, Yasuhito Hamaguchi, Karen M. Haas, Thomas F. Tedder; Lymphoma depletion during CD20 immunotherapy in mice is mediated by macrophage FcγRI, FcγRIII, and FcγRIV. Blood 2008; 112 (4): 1205–1213. doi: https://doi.org/10.1182/blood-2008-01-135160
[12]. U.S. Food and Drug Administration. (2017). OPDIVO highlights of prescribing information. Warnings and Precautions, 7(25), 1–66. Retrieved from https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/125554s034lbl.pdf#page=64.
[13]. Ren, L. (2023). The Role of Pembrolizumab (Keytruda) in Treating Non-small Cell Lung Cancer. Highlights in Science, Engineering and Technology, 36, 608–613. https://doi.org/10.54097/hset.v36i.5743
[14]. Liu, Y., Zuo, W.-J., Wang, R.-X., Wang, Z.-H., & Shao, Z.-M. (2023). Abstract P1-11-20: Trastuzumab (HLX02) plus Pertuzumab as Dual-target Neoadjuvant Therapy for HER2-positive Breast Cancer: A Real-World Study. Cancer Research, 83(5_Supplement), P1-11-20-P1-11–20. https://doi.org/10.1158/1538-7445.sabcs22-p1-11-20
[15]. Robert, M., Frenel, J. S., Bourbouloux, E., Berton Rigaud, D., Patsouris, A., Augereau, P., … Campone, M. (2020). Pertuzumab for the treatment of breast cancer. Expert Review of Anticancer Therapy, 20(2), 85–95. https://doi.org/10.1080/14737140.2019.1596805
[16]. Garcia, J., Hurwitz, H. I., Sandler, A. B., Miles, D., Coleman, R. L., Deurloo, R., & Chinot, O. L. (2020, June 1). Bevacizumab (Avastin®) in cancer treatment: A review of 15 years of clinical experience and future outlook. Cancer Treatment Reviews. W.B. Saunders Ltd. https://doi.org/10.1016/j.ctrv.2020.102017
[17]. Hickish T, Andre T, Wyrwicz L, Saunders M, Sarosiek T, Kocsis J, et al. MABp1 as a novel antibody treatment for advanced colorectal cancer: a randomised, double-blind, placebo-controlled, phase 3 study. Lancet Oncol (2017) 18(2):192–201. https://doi.org/10.1016/S1470-2045(17)30006-2
[18]. Madan, R. A., Redman, J. M., Karzai, F., Dahut, W. L., Cordes, L., Fakhrejahani, F., … Gulley, J. L. (2023). Avelumab in Men with Metastatic Castration-Resistant Prostate Cancer, Enriched for Patients Treated Previously with a Therapeutic Cancer Vaccine. Journal of Immunotherapy, 46(4), 145–151. https://doi.org/10.1097/CJI.0000000000000459
[19]. Si, T., Ma, X., Zhu, W., & Zhou, Y. (2023). Clinical efficacy and safety of subcutaneous rituximab in non-Hodgkin lymphoma: a systematic literature review and meta-analysis. Hematology (United Kingdom). Taylor and Francis Ltd. https://doi.org/10.1080/16078454.2023.2284047
[20]. Gilardi, M., Kaushik, G., Walling, B., Sambandam, V., Schiavini, P., Cairo, S., … Ritchie, M. (2023). Correlation Revealed between Gemtuzumab-Ozogamicin Efficacy and CD33+ Expression in AML Primary Samples through a Novel AML in Vitro Model. Blood, 142(Supplement 1), 7151–7151. https://doi.org/10.1182/blood-2023-186200
[21]. Kwok, G., Yau, T. C. C., Chiu, J. W., Tse, E., & Kwong, Y. L. (2016, November 1). Pembrolizumab (Keytruda). Human Vaccines and Immunotherapeutics. Taylor and Francis Inc. https://doi.org/10.1080/21645515.2016.1199310
[22]. Yan, Y. (2023). The Role Ipilimumab Plays in the Treatment of Melanoma. Theoretical and Natural Science, 3(1), 701–708. https://doi.org/10.54254/2753-8818/3/20220435
[23]. Zhou, J. G., Wong, A. H. H., Wang, H., Jin, S. H., Tan, F., Chen, Y. Z., … Gaipl, U. S. (2022). Definition of a new blood cell count score for early survival prediction for non-small cell lung cancer patients treated with atezolizumab: Integrated analysis of four multicenter clinical trials. Frontiers in Immunology, 13. https://doi.org/10.3389/fimmu.2022.961926
[24]. Chan, M. M. K., Sjoquist, K. M., & Zalcberg, J. R. (2015, September 22). Clinical utility of ramucirumab in advanced gastric cancer. Biologics: Targets and Therapy. Dove Medical Press Ltd. https://doi.org/10.2147/BTT.S62777









