1. Introduction
Polycystic ovary syndrome (PCOS) is a common endocrine disorder, occurring in about 5-10% of women in the reproductive age group. It is characterized by a group of various symptoms that include hyperandrogenism, ovulatory dysfunction, and polycystic ovarian morphology [2]. Traditional therapeutic approaches for PCOS are mainly directed toward symptom relief via lifestyle changes, hormonal contraceptives, and insulin-sensitizing agents [3]. However, these treatments very often give partial relief and do not revert the metabolic disturbances at the base of PCOS.
Glucagon-Like Peptide-1 (GLP-1) receptor agonists has recently emerged as a promising therapy to address PCOS pathophysiology by addressing insulin resistance and hyperinsulinemia as key pathogenic drivers worsening the condition [4]. Initially developed for type 2 diabetes, GLP-1 receptor agonists show potential metabolic benefits and weight loss properties, potentially correcting reproductive abnormalities in PCOS [5]. GLP-1, an incretin hormone, enhances glucose-dependent insulin secretion, suppresses glucagon secretion, and slows gastric emptying, providing better glycemic control and weight reduction [6]. GLP-1 receptor agonists benefit glycemic regulation, cardiovascular health, and weight management, all critical in managing PCOS [7]. Increasing evidence suggests that GLP-1 receptor agonists may also improve hyperandrogenism and ovulatory dysfunction, making them a promising therapy for women with PCOS.
While many emerging papers experiment with GLP-1 as interventions for PCOS patients, most are limited by small sample sizes and inconsistent results across different study durations and groups. This meta-analysis examines the impact of GLP-1 on PCOS patients across various parameters, including anthropometric, hormonal, glycemic, blood lipid, endocrine, and metabolic factors. Specifically, data on weight, BMI, SHBG, total testosterone, free testosterone, total cholesterol, HOMA-IR score, VAT area, LH, FSH, and DHEA-S from 669 participants across 13 published papers were analyzed. The analysis is divided into two subgroups: GLP-1 intervention compared to placebo and GLP-1 intervention compared to metformin.
2. Method
2.1. Literary Identification
Author identified 170 records from four electronic databases (PubMed, Frontier, Oxford academic, Cochrane library) of randomized controlled trials or randomized clinical trials (RCT) that studies the effect of GLP-1 on weight and key metabolic or inflammatory factors of adults diagnosed with polycystic ovarian syndrome. The search key terms used include randomized controlled trial, GLP-1 receptor agonist, and polycystic ovarian syndrome.
2.2. Selection Criteria
The search strategies for each database were as follows:
PubMed: ((GLP-1 receptor agonist) AND (polycystic ovary syndrome)) AND (randomized controlled trials)
Oxford Academic: CRT, RCT, polycystic ovary syndrome, GLP-1 receptor agonist
Cochrane library: "polycystic ovary syndrome", Glucagon-Like Peptide-1 Receptor Agonists in Title Abstract Keyword
Frontier: CRT, RCT, polycystic ovary syndrome, Glucagon-Like Peptide-1 Receptor Agonists
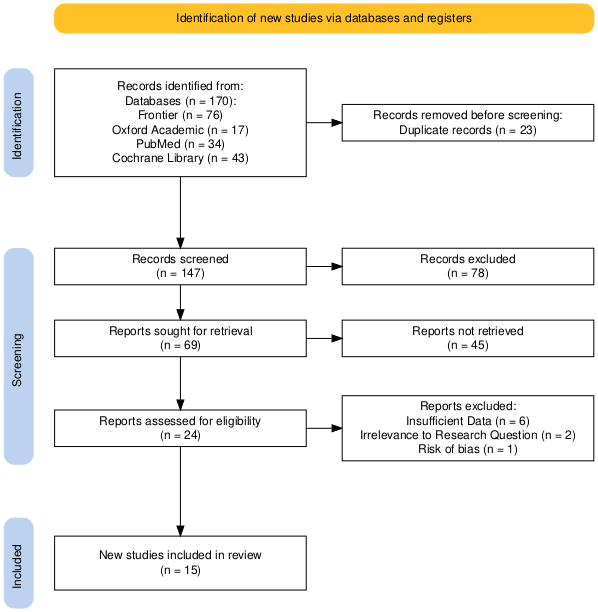
Figure 1. PRISMA flow diagram of study selection
Inclusion criteria: (1) Participant: All patients with either PCOS diagnosed with Rotterdam criteria [8], National Institute of Child Health and Human Development (NICHD) criteria [9]. There is no limit on region, age, duration, and ethnicity. (2) Intervention: Liraglutide, Exenatide, Sitagliptin, Glimepiride, Dulaglutide, and Semaglutide are considered. (3) Control: Metformin and placebo. (4) Main outcomes: weight, body mass index (BMI), sex hormone-binding globulin (SHBG), total testosterone, homeostatic model assessment for insulin resistance (HOMA IR) score, visceral adipose tissue (VAT) area, and dehydroepiandrosterone (DHEA). (5) Study design: CRT or RCT
3. Result
3.1. Demographic Characteristic
This meta-analysis included a total of 13 studies [10-23], comprising 669 participants. The studies were published between 2008 to 2023 and were conducted across four different countries, including United States, China, Slovenia, and Denmark. The majority were conducted in Slovenia (6 studies), followed by the United States (3 studies), and China (3 studies). A few studies were conducted in Denmark (1 study). The sample sizes of the included studies varied, ranging from 24 to 67, with a median sample size of 28.
The studies primarily focused on populations diagnosed with Polycystic Ovary Syndrome (PCOS). For eligible patient assessment, 7 studies used the Rotterdam Criteria; 2 used the National Institutes of Health (NIH) 1990 Criteria; and 4 used the ASRM-ESHRE (American Society for Reproductive Medicine - European Society of Human Reproduction and Embryology) Criteria.
Age range of participants across the studies was 18 to 40 years, with a focus on adult females in their late 20s to early 30s. The mean age of participants reported across the studies was 31.7 ± 6.0 years. 7 studies had the subject inclusion criteria of Body Mass Index (BMI) greater than 25 kg/m² and/or insulin resistance, and 7 other studies used BMI greater than 30 kg/m². The BMI across the 13 studies ranged from 27 kg/m² to 39.5 kg/m², with a combined mean BMI of 37.94 ± 5.27 kg/m².
3.2. Meta-Analysis Primary Outcomes: Body Mass Index (BMI)
The analysis in Fig.2. a. using a random effects model indicates that GLP-1 receptor agonists do not lead to a statistically significant reduction in BMI compared to placebo in PCOS patients, with a standardized mean difference (SMD) of -3.13 (95% CI: -6.44 to 0.17), but high heterogeneity (I^2 = 96%) indicate a larger sample size is needed to confirm the conclusion. Notably, metformin treatments and GLP-1 receptor agonists exhibit comparable effects on BMI, with SMD of -0.10 (95% CI: -0.48 to 0.28).
3.3. Meta-Analysis Secondary Outcome
3.3.1. Weight (kg)
The analysis in Fig.2.b indicates no statistically significant weight reduction in the outcome measure among PCOS patients treated with GLP-1 receptor agonists compared to placebo, with an SMD of -2.22 (95% CI: -2.68 to -1.76). However, the high heterogeneity (I² = 96%) suggests variability in the results across different studies. There is no statistically significant difference in weight reduction (kg) among PCOS patients treated with GLP-1 receptor agonists compared to those treated with metformin, with SMD of -0.17 kg (95% CI: -0.55 to 0.21).
3.3.2. Testosterone
The random effects model analysis in Fig.2.c on reduction of total testosterone levels (nmol/L) between GLP-1 receptor agonists and metformin shows SMD of 0.17 (95% CI: -0.60 to 0.93), with a high heterogeneity (I^2 = 74%).
3.3.3. HOMA-IR Score
The analysis in Fig.2. d. on the effect of intervention versus placebo on HOMA-IR scores shows SMD of -1.47 (95% CI: -3.24 to 0.30), with a high heterogeneity (I^2 = 94%). The comparison between GLP-1 receptor agonists and metformin on HOMA-IR scores in PCOS patients yields SMD of -0.56 (95% CI: -1.60 to 0.49), with a high heterogeneity (I^2 = 80%).
3.3.4. Total cholesterol
The analysis in Fig.2.e indicates no statistically significant difference in total cholesterol reduction (mmol/L) among PCOS patients between GLP-1 receptor agonists and metformin, with a SMD of -0.28 (95% CI: -0.93 to 0.38).
3.3.5. DHEA
The analysis in Fig.2.f shows no statistically significant difference in DHEA reduction (μmol/L) among PCOS patients between GLP-1 receptor agonists and metformin, with SMD of -0.24 (95% CI: -0.63 to 0.14).
3.3.6. SHBG
The analysis in Fig.2.g indicates no statistically significant difference in SHBG reduction (nmol/L) among PCOS patients between GLP-1 receptor agonists and metformin, with SMD of -0.04 (95% CI: -0.50 to 0.43).
4. Discussion
Metformin is a common type II diabetes treatment known for its glucose regulation and safety [24]. Due to metformin’s ability to regular insulin levels, it has become commonly recognized as beneficial to PCOS patients in ways that it promotes spontaneous menstruation, better hormonal, and lipid profile [25]. Though, there is still a limited number of papers that focuses on large sample of PCOS patients or human models. Increasingly, scientists began to test or combine alternative interventions, such as GLP-1 RA due to its metabolic and hormonal regulation abilities [26]. In this meta-analysis, primary and secondary outcomes show no statistically significant difference between intervention’s effect on PCOS patients in comparison to that of placebo and metformin. In primary outcome, standardized mean difference (SMD) between GLP-1 RA and placebo yields -3.13 (95% CI: -6.44 to 0.17), in addition, metformin treatments and GLP-1 receptor agonists exhibit comparable effects on BMI, with SMD of -0.10 (95% CI: -0.48 to 0.28). This pattern of high similarity between placebo and GLP-1 effect, as well as between GLP-1 RA and metformin can be also observed in secondary outcomes.
One reason that could result in this statistical outcome is that while GLP-1 RAs are effective in weight management and improving insulin sensitivity, their impact on directly regulating ovarian hormones and menstrual irregularities in PCOS is less clear. Research by G. Pugliese et.al suggests that GLP-1 RAs may not significantly influence estrogen synthesis in granulosa cells, which are essential for normal ovarian function [1]. A 2023 paper by L. Zhou et. Al also suggests that GLP-1 RA’s influence on reproductive hormones like estrogen and progesterone is a less pronounced. Although they may help in reducing androgens due to improved insulin sensitivity, they do not significantly alter estrogen levels or directly affect ovarian steroidogenesis – essential to regulate hormonal modulation. One reason that could result in this statistical outcome is that while GLP-1 RAs are effective in weight management and improving insulin sensitivity, their impact on directly regulating ovarian hormones and menstrual irregularities in PCOS is less clear. Research by G. Pugliese et.al suggests that GLP-1 RAs may not significantly influence estrogen synthesis in granulosa cells, which are essential for normal ovarian function. A 2023 paper by L. Zhou et. Al also suggests that GLP-1 RA’s influence on reproductive hormones like estrogen and progesterone is a less pronounced. Although they may help in reducing androgens due to improved insulin sensitivity, they do not significantly alter estrogen levels or directly affect ovarian steroidogenesis – essential to regulate hormonal modulation [27].
Additionally, PCOS patients may not respond to GLP-1 RA in the same way normal people do. Research by S J Henderson et.al suggest that obese patients exhibit lower postprandial GLP-1 levels compared to healthy controls, which could reduce the effectiveness of GLP-1 RAs in managing glucose metabolism and weight loss [28]. Side effects like nausea, vomiting, and diarrhea are more severe in patients with metabolic dysregulation such as PCOS [29].
Combined with the high heterogeneity across primary and secondary outcomes, it is also likely that the limited sample size and experimental data lead to this statistically insignificant outcome. The duration of the RCTs examined in this meta-analysis range from 32 to 12 weeks, with an average of 16.4 weeks. Current data lack comprehensive long-term measures evaluating the impact of GLP-1 RAs on reproductive outcomes such as fertility and pregnancy rates. Most research has focused on short-term metabolic improvements, leaving gaps in understanding their long-term effects on hormonal health and reproductive functions.
To fully understand the mechanisms behind this phenomenon, whether it is due to GLP-1 RAs being less effective in PCOS patients, functioning similarly to metformin, or the need for more long-term studies, additional comprehensive data is required to support these hypotheses.
4.1. Limitation
This meta-analysis included 13 studies with a total of 669 participants. To draw more robust conclusions, additional studies with larger sample sizes are necessary. A critical limitation is the significant variation in the quality and setup of the included studies. The GLP-1 RA interventions used across studies included Semaglutide (1.0 mg daily), Liraglutide (1.8 mg/day), Exenatide (10 µg BID), Sitagliptin (100 mg daily), and Beinaglutide (0.2 mg TID). These disparities in GLP-1 RA interventions may contribute to the high heterogeneity observed in the analysis. As an initial, exploratory investigation, this meta-analysis suggests that the current randomized controlled trials (RCTs) on GLP-1 RA interventions are not sufficient to provide definitive and persuasive conclusions. Therefore, more high-quality, long-term RCTs are needed in this field.
5. Conclusion
In conclusion, this meta-analysis indicates that there is no significant difference in outcomes between GLP-1 receptor agonists and placebo in PCOS patients, and it also shows no statistically significant difference compared to metformin. The current randomized controlled trials (CRTs) assessing the effects of GLP-1 receptor agonists in PCOS patients do not provide compelling evidence for their effectiveness in regulating or improving weight reduction, HOMA-IR scores, or hormonal levels, including testosterone, cholesterol, and DHEA. To draw more definitive conclusions, future studies should include larger sample sizes, adopt more rigorous designs, and extend the duration of trials to generate more robust clinical data.
References
[1]. Pugliese, G., de Alteriis, G., and Muscogiuri, G. “Liraglutide and Polycystic Ovary Syndrome: Is It Only a Matter of Body Weight?” Journal of Endocrinological Investigation, vol. 46, 2023, pp. 1761-1774. doi:10.1007/s40618-023-02084-6.
[2]. Goodarzi, M. O., et al. “The Polycystic Ovary Syndrome: A Position Statement from the Androgen Excess and Polycystic Ovary Syndrome Society.” Journal of Clinical Endocrinology & Metabolism, vol. 96, no. 11, 2011, pp. 3541-3550.
[3]. Teede, H. J., et al. “Recommendations from the International Evidence-Based Guideline for Assessing and Managing Polycystic Ovary Syndrome.” Human Reproduction, vol. 33, no. 9, 2018, pp. 1602-1618.
[4]. Diamanti-Kandarakis, E., et al. “Insulin Resistance in PCOS.” Fertility and Sterility, vol. 88, no. 1, 2007, pp. 83-88.
[5]. Drucker, D. J., et al. “The Biology of Incretin Hormones.” Cell Metabolism, vol. 3, no. 3, 2006, pp. 153-165.
[6]. Vilsbøll, T., et al. “Effects of Glucagon-Like Peptide-1 on the Endocrine Pancreas.” Diabetes, Obesity, and Metabolism, vol. 14, no. 2, 2012, pp. 175-186.
[7]. Holst, J. J. “The Physiology of Glucagon-Like Peptides 1.” Physiological Reviews, vol. 87, no. 4, 2007, pp. 1409-1439.
[8]. Smet, Maria-Elisabeth, and Andrew McLennan. “Rotterdam criteria, the end.” Australasian journal of ultrasound in medicine vol. 21,2 59-60. 17 May. 2018, doi:10.1002/ajum.12096
[9]. Christ, Jacob P, and Marcelle I Cedars. “Current Guidelines for Diagnosing PCOS.” Diagnostics (Basel, Switzerland) vol. 13,6 1113. 15 Mar. 2023, doi:10.3390/diagnostics13061113
[10]. Elkind-Hirsch, Karen et al. “Comparison of Single and Combined Treatment with Exenatide and Metformin on Menstrual Cyclicity in Overweight Women with Polycystic Ovary Syndrome.” The Journal of Clinical Endocrinology and Metabolism, vol. 93, no. 7, 2008, pp. 2670-8. doi:10.1210/jc.2008-0115.
[11]. Frøssing, Signe, et al. “Effect of Liraglutide on Atrial Natriuretic Peptide, Adrenomedullin, and Copeptin in PCOS.” Endocrine Connections, vol. 7, no. 1, 2018, pp. 115-123. doi:10.1530/EC-17-0327.
[12]. Jensterle, M., et al. “Short-Term Intervention with Liraglutide Improved Insulin Sensitivity More Effectively than Metformin in Obese Women with Polycystic Ovary Syndrome: A Randomized, Open-Label, Cross-Over Study.” BMC Endocrine Disorders, vol. 15, 2015, p. 20.
[13]. Jensterle, Mojca, et al. “A 12-Week Treatment with the Long-Acting Glucagon-Like Peptide 1 Receptor Agonist Liraglutide Leads to Significant Weight Loss in a Subset of Obese Women with Newly Diagnosed Polycystic Ovary Syndrome.” Hormones (Athens, Greece), vol. 14, no. 1, 2015, pp. 81-90. doi:10.1007/BF03401383.
[14]. Jensterle, Mojca, et al. “Short Term Monotherapy with GLP-1 Receptor Agonist Liraglutide or PDE 4 Inhibitor Roflumilast is Superior to Metformin in Weight Loss in Obese PCOS Women: A Pilot Randomized Study.” Journal of Ovarian Research, vol. 8, 2015, p. 32. doi:10.1186/s13048-015-0161-3.
[15]. Elkind-Hirsch, Karen E., et al. “Liraglutide 3 mg on Weight, Body Composition, and Hormonal and Metabolic Parameters in Women with Obesity and Polycystic Ovary Syndrome: A Randomized Placebo-Controlled-Phase 3 Study.” Fertility and Sterility, vol. 118, no. 2, 2022, pp. 371-381. doi:10.1016/j.fertnstert.2022.04.027.
[16]. Jensterle Sever, Mojca, et al. “Short-Term Combined Treatment with Liraglutide and Metformin Leads to Significant Weight Loss in Obese Women with Polycystic Ovary Syndrome and Previous Poor Response to Metformin.” European Journal of Endocrinology, vol. 170, no. 3, 2014, pp. 451-459. doi:10.1530/EJE-13-0797.
[17]. Jensterle, Mojca, et al. “Semaglutide Reduces Fat Accumulation in the Tongue: A Randomized Single-Blind, Pilot Study.” Diabetes Research and Clinical Practice, vol. 178, 2021, p. 108935. doi:10.1016/j.diabres.2021.108935.
[18]. Jensterle, Mojca et al. “Metformin as an initial adjunct to low-dose liraglutide enhances the weight-decreasing potential of liraglutide in obese polycystic ovary syndrome: Randomized control study.” Experimental and therapeutic medicine vol. 11,4 (2016): 1194-1200. doi:10.3892/etm.2016.3081
[19]. Ma, Rui-Lin et al. “Short-term combined treatment with exenatide and metformin for overweight/obese women with polycystic ovary syndrome.” Chinese medical journal vol. 134,23 2882-2889. 3 Nov. 2021, doi:10.1097/CM9.0000000000001712
[20]. Jensterle, Mojca et al. “Short-term effectiveness of low dose liraglutide in combination with metformin versus high dose liraglutide alone in treatment of obese PCOS: randomized trial.” BMC endocrine disorders vol. 17,1 5. 31 Jan. 2017, doi:10.1186/s12902-017-0155-9
[21]. Ferjan, Simona et al. “Dipeptidyl Peptidase-4 Inhibitor Sitagliptin Prevented Weight Regain in Obese Women with Polycystic Ovary Syndrome Previously Treated with Liraglutide: A Pilot Randomized Study.” Metabolic syndrome and related disorders vol. 15,10 (2017): 515-520. doi:10.1089/met.2017.0095
[22]. Wen, Qing et al. “Short-term effect of beinaglutide combined with metformin versus metformin alone on weight loss and metabolic profiles in obese patients with polycystic ovary syndrome: a pilot randomized trial.” Frontiers in endocrinology vol. 14 1156521. 6 Jun. 2023, doi:10.3389/fendo.2023.1156521
[23]. Xing, Chuan et al. “Effect of metformin versus metformin plus liraglutide on gonadal and metabolic profiles in overweight patients with polycystic ovary syndrome.” Frontiers in endocrinology vol. 13 945609. 17 Aug. 2022, doi:10.3389/fendo.2022.945609
[24]. LaMoia, T. E., and Shulman, G. I. “Cellular and Molecular Mechanisms of Metformin Action.” Endocrine Reviews, vol. 42, no. 1, 2021, pp. 77-96. doi:10.1210/endrev/bnaa023.
[25]. Santana, L. F., de Sá, M. F., Ferriani, R. A., de Moura, M. D., Foss, M. C., and dos Reis, R. M. “Effect of Metformin on the Clinical and Metabolic Assessment of Women with Polycystic Ovary Syndrome.” Gynecological Endocrinology, vol. 19, no. 2, 2004, pp. 88-96. doi:10.1080/09513590400002342.
[26]. Cena, H., Chiovato, L., and Nappi, R. E. “Obesity, Polycystic Ovary Syndrome, and Infertility: A New Avenue for GLP-1 Receptor Agonists.” Journal of Clinical Endocrinology & Metabolism, vol. 105, no. 8, 2020, pp. e2695–709. doi:10.1210/clinem/dgaa285.
[27]. Zhou, L., Qu, H., and Yang, L., et al. “Effects of GLP1RAs on Pregnancy Rate and Menstrual Cyclicity in Women with Polycystic Ovary Syndrome: A Meta-Analysis and Systematic Review.” BMC Endocrine Disorders, vol. 23, 2023, p. 245. doi:10.1186/s12902-023-01500-5.
[28]. Henderson, S. J., et al. “Robust Anti-Obesity and Metabolic Effects of a Dual GLP-1/Glucagon Receptor Peptide Agonist in Rodents and Non-Human Primates.” Diabetes, Obesity and Metabolism, vol. 18, no. 12, 2016, pp. 1176-1190. doi:10.1111/dom.12735.
[29]. Elvert, Ralf et al. “Team Players or Opponents: Coadministration of Selective Glucagon and GLP-1 Receptor Agonists in Obese Diabetic Monkeys.” Endocrinology vol. 159,8 (2018): 3105-3119. doi:10.1210/en.2018-00399
Cite this article
Li,X. (2025). Glucagon-Like Peptide-1 Receptor Agonists' Effect on Polycystic Ovary Syndrome: A Meta Analysis. Theoretical and Natural Science,76,62-68.
Data availability
The datasets used and/or analyzed during the current study will be available from the authors upon reasonable request.
Disclaimer/Publisher's Note
The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of EWA Publishing and/or the editor(s). EWA Publishing and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
About volume
Volume title: Proceedings of the 4th International Conference on Biological Engineering and Medical Science
© 2024 by the author(s). Licensee EWA Publishing, Oxford, UK. This article is an open access article distributed under the terms and
conditions of the Creative Commons Attribution (CC BY) license. Authors who
publish this series agree to the following terms:
1. Authors retain copyright and grant the series right of first publication with the work simultaneously licensed under a Creative Commons
Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this
series.
2. Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the series's published
version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial
publication in this series.
3. Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and
during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See
Open access policy for details).
References
[1]. Pugliese, G., de Alteriis, G., and Muscogiuri, G. “Liraglutide and Polycystic Ovary Syndrome: Is It Only a Matter of Body Weight?” Journal of Endocrinological Investigation, vol. 46, 2023, pp. 1761-1774. doi:10.1007/s40618-023-02084-6.
[2]. Goodarzi, M. O., et al. “The Polycystic Ovary Syndrome: A Position Statement from the Androgen Excess and Polycystic Ovary Syndrome Society.” Journal of Clinical Endocrinology & Metabolism, vol. 96, no. 11, 2011, pp. 3541-3550.
[3]. Teede, H. J., et al. “Recommendations from the International Evidence-Based Guideline for Assessing and Managing Polycystic Ovary Syndrome.” Human Reproduction, vol. 33, no. 9, 2018, pp. 1602-1618.
[4]. Diamanti-Kandarakis, E., et al. “Insulin Resistance in PCOS.” Fertility and Sterility, vol. 88, no. 1, 2007, pp. 83-88.
[5]. Drucker, D. J., et al. “The Biology of Incretin Hormones.” Cell Metabolism, vol. 3, no. 3, 2006, pp. 153-165.
[6]. Vilsbøll, T., et al. “Effects of Glucagon-Like Peptide-1 on the Endocrine Pancreas.” Diabetes, Obesity, and Metabolism, vol. 14, no. 2, 2012, pp. 175-186.
[7]. Holst, J. J. “The Physiology of Glucagon-Like Peptides 1.” Physiological Reviews, vol. 87, no. 4, 2007, pp. 1409-1439.
[8]. Smet, Maria-Elisabeth, and Andrew McLennan. “Rotterdam criteria, the end.” Australasian journal of ultrasound in medicine vol. 21,2 59-60. 17 May. 2018, doi:10.1002/ajum.12096
[9]. Christ, Jacob P, and Marcelle I Cedars. “Current Guidelines for Diagnosing PCOS.” Diagnostics (Basel, Switzerland) vol. 13,6 1113. 15 Mar. 2023, doi:10.3390/diagnostics13061113
[10]. Elkind-Hirsch, Karen et al. “Comparison of Single and Combined Treatment with Exenatide and Metformin on Menstrual Cyclicity in Overweight Women with Polycystic Ovary Syndrome.” The Journal of Clinical Endocrinology and Metabolism, vol. 93, no. 7, 2008, pp. 2670-8. doi:10.1210/jc.2008-0115.
[11]. Frøssing, Signe, et al. “Effect of Liraglutide on Atrial Natriuretic Peptide, Adrenomedullin, and Copeptin in PCOS.” Endocrine Connections, vol. 7, no. 1, 2018, pp. 115-123. doi:10.1530/EC-17-0327.
[12]. Jensterle, M., et al. “Short-Term Intervention with Liraglutide Improved Insulin Sensitivity More Effectively than Metformin in Obese Women with Polycystic Ovary Syndrome: A Randomized, Open-Label, Cross-Over Study.” BMC Endocrine Disorders, vol. 15, 2015, p. 20.
[13]. Jensterle, Mojca, et al. “A 12-Week Treatment with the Long-Acting Glucagon-Like Peptide 1 Receptor Agonist Liraglutide Leads to Significant Weight Loss in a Subset of Obese Women with Newly Diagnosed Polycystic Ovary Syndrome.” Hormones (Athens, Greece), vol. 14, no. 1, 2015, pp. 81-90. doi:10.1007/BF03401383.
[14]. Jensterle, Mojca, et al. “Short Term Monotherapy with GLP-1 Receptor Agonist Liraglutide or PDE 4 Inhibitor Roflumilast is Superior to Metformin in Weight Loss in Obese PCOS Women: A Pilot Randomized Study.” Journal of Ovarian Research, vol. 8, 2015, p. 32. doi:10.1186/s13048-015-0161-3.
[15]. Elkind-Hirsch, Karen E., et al. “Liraglutide 3 mg on Weight, Body Composition, and Hormonal and Metabolic Parameters in Women with Obesity and Polycystic Ovary Syndrome: A Randomized Placebo-Controlled-Phase 3 Study.” Fertility and Sterility, vol. 118, no. 2, 2022, pp. 371-381. doi:10.1016/j.fertnstert.2022.04.027.
[16]. Jensterle Sever, Mojca, et al. “Short-Term Combined Treatment with Liraglutide and Metformin Leads to Significant Weight Loss in Obese Women with Polycystic Ovary Syndrome and Previous Poor Response to Metformin.” European Journal of Endocrinology, vol. 170, no. 3, 2014, pp. 451-459. doi:10.1530/EJE-13-0797.
[17]. Jensterle, Mojca, et al. “Semaglutide Reduces Fat Accumulation in the Tongue: A Randomized Single-Blind, Pilot Study.” Diabetes Research and Clinical Practice, vol. 178, 2021, p. 108935. doi:10.1016/j.diabres.2021.108935.
[18]. Jensterle, Mojca et al. “Metformin as an initial adjunct to low-dose liraglutide enhances the weight-decreasing potential of liraglutide in obese polycystic ovary syndrome: Randomized control study.” Experimental and therapeutic medicine vol. 11,4 (2016): 1194-1200. doi:10.3892/etm.2016.3081
[19]. Ma, Rui-Lin et al. “Short-term combined treatment with exenatide and metformin for overweight/obese women with polycystic ovary syndrome.” Chinese medical journal vol. 134,23 2882-2889. 3 Nov. 2021, doi:10.1097/CM9.0000000000001712
[20]. Jensterle, Mojca et al. “Short-term effectiveness of low dose liraglutide in combination with metformin versus high dose liraglutide alone in treatment of obese PCOS: randomized trial.” BMC endocrine disorders vol. 17,1 5. 31 Jan. 2017, doi:10.1186/s12902-017-0155-9
[21]. Ferjan, Simona et al. “Dipeptidyl Peptidase-4 Inhibitor Sitagliptin Prevented Weight Regain in Obese Women with Polycystic Ovary Syndrome Previously Treated with Liraglutide: A Pilot Randomized Study.” Metabolic syndrome and related disorders vol. 15,10 (2017): 515-520. doi:10.1089/met.2017.0095
[22]. Wen, Qing et al. “Short-term effect of beinaglutide combined with metformin versus metformin alone on weight loss and metabolic profiles in obese patients with polycystic ovary syndrome: a pilot randomized trial.” Frontiers in endocrinology vol. 14 1156521. 6 Jun. 2023, doi:10.3389/fendo.2023.1156521
[23]. Xing, Chuan et al. “Effect of metformin versus metformin plus liraglutide on gonadal and metabolic profiles in overweight patients with polycystic ovary syndrome.” Frontiers in endocrinology vol. 13 945609. 17 Aug. 2022, doi:10.3389/fendo.2022.945609
[24]. LaMoia, T. E., and Shulman, G. I. “Cellular and Molecular Mechanisms of Metformin Action.” Endocrine Reviews, vol. 42, no. 1, 2021, pp. 77-96. doi:10.1210/endrev/bnaa023.
[25]. Santana, L. F., de Sá, M. F., Ferriani, R. A., de Moura, M. D., Foss, M. C., and dos Reis, R. M. “Effect of Metformin on the Clinical and Metabolic Assessment of Women with Polycystic Ovary Syndrome.” Gynecological Endocrinology, vol. 19, no. 2, 2004, pp. 88-96. doi:10.1080/09513590400002342.
[26]. Cena, H., Chiovato, L., and Nappi, R. E. “Obesity, Polycystic Ovary Syndrome, and Infertility: A New Avenue for GLP-1 Receptor Agonists.” Journal of Clinical Endocrinology & Metabolism, vol. 105, no. 8, 2020, pp. e2695–709. doi:10.1210/clinem/dgaa285.
[27]. Zhou, L., Qu, H., and Yang, L., et al. “Effects of GLP1RAs on Pregnancy Rate and Menstrual Cyclicity in Women with Polycystic Ovary Syndrome: A Meta-Analysis and Systematic Review.” BMC Endocrine Disorders, vol. 23, 2023, p. 245. doi:10.1186/s12902-023-01500-5.
[28]. Henderson, S. J., et al. “Robust Anti-Obesity and Metabolic Effects of a Dual GLP-1/Glucagon Receptor Peptide Agonist in Rodents and Non-Human Primates.” Diabetes, Obesity and Metabolism, vol. 18, no. 12, 2016, pp. 1176-1190. doi:10.1111/dom.12735.
[29]. Elvert, Ralf et al. “Team Players or Opponents: Coadministration of Selective Glucagon and GLP-1 Receptor Agonists in Obese Diabetic Monkeys.” Endocrinology vol. 159,8 (2018): 3105-3119. doi:10.1210/en.2018-00399









