1. Introduction
EC is among the most prevalent cancers all over the world, characterized by its aggressive characteristics and low survival rate [1]. but with higher rates in East Asia, Southern Africa, and parts of Iran, which are often referred to as the “esophageal cancer belt.” The esophagus is a tube-shaped organ linking the throat to the tummy, facilitating the movement of food for digestion. It is divided into three segments: cervical (neck), thoracic (chest) and abdominal. Every section contributes to moving food from the mouth to the stomach.
The cervical segment begins in the pharynx and ends in the suprasternal notch behind the trachea. The thoracic segment extends from the suprasternal notch to the diaphragm. The ventral segment extends from the diaphragm to the stomach. Contributing risk factors include smoking, excessive dramatic alcohol intake, gastroesophageal reflux disease (GERD), Barrett's esophagus (BE), obesity, and dietary factors. Esophageal cancer is a malignant tumor originating from the esophagus, which is the muscular tube responsible for transporting food from the throat to the stomach. This type of cancer is marked by the unchecked growth of cells within the esophageal lining. Esophageal cancer primarily manifests in two forms: squamous cell carcinoma, typically found in the upper and middle sections of the esophagus, and adenocarcinoma, which is more frequently observed in the lower portion, close to the stomach. Treatment depends on the stage of the cancer. It may include surgery, chemotherapy, radiation, or a combination. Chemotherapy is recommended for all stages of the disease. The ECF drug combination is very effective. For early-stage esophageal cancer, surgery is the best option. Both EMR and ESD are effective. ESD has a higher rate of complete resection [2,3].
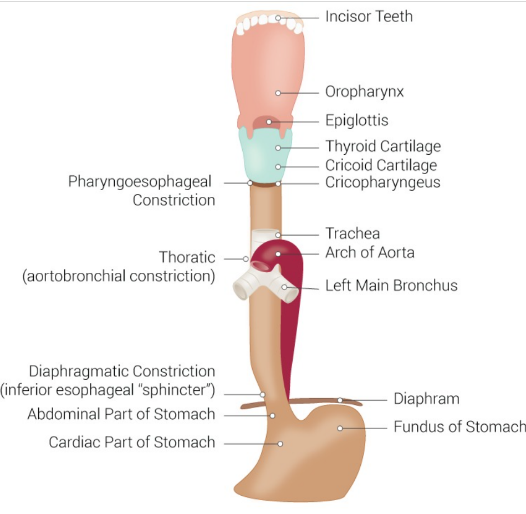
Figure 1. Esophageal and Gastric Junction Configuration [Chaudhry SR, Bordoni B. Anatomy, Thorax, Esophagus. StatPearls Publishing; 2024 Jan-.]
2. Content
2.1. Mechanism
The ECF stands for Epirubicin, Cisplatin and 5-Fluorouracil. Epirubicin is an anthracycline antibiotic that acts primarily by inserting itself into the DNA strand, thereby disrupting the basic processes of DNA replication and transcription. It inhibits topoisomerase II, an enzyme that is essential for DNA deconvolution during replication. This inhibition leads to the accumulation of double-strand breaks in DNA, which results in apoptosis in rapidly dividing cancer cells. In addition, Epirubicin generates free radicals that cause oxidative damage to cellular components, further exacerbating its cytotoxic effects. Cisplatin is a platinum-based chemotherapeutic agent that causes crosslinking of DNA strands, preventing proper DNA replication and transcription. The platinum molecule in cisplatin forms covalent bonds with purine bases (adenine and guanine) in DNA, leading to intra- and inter-strand crosslinks. These crosslinks interfere with the DNA repair mechanisms and induce apoptosis in cancer cells. Cisplatin's efficacy is particularly notable in rapidly proliferating cells, which are less able to repair the extensive DNA damage induced by the drug. The last drug is 5-FU. 5-FU is a pyrimidine simulation that disrupts nucleotide metabolism. It is metabolized to its active form within the cell and binds to RNA and DNA, thereby inhibiting nucleic acid synthesis and function. In addition, 5-FU inhibits thymidylate synthase which is a significant enzyme in the synthesis of thymidine, a nucleotide crucial for DNA replication. Prohibition of thymidylate synthase leads to thymidine depletion, which results in DNA strand breaks and apoptosis. 5-FU is particularly effective in cells that are actively synthesizing DNA. Combination therapy with Epirubicin, cisplatin and 5-fluorouracil (ECF) are used to treat diverse cancers, including gastric cancer. These three drugs target cancer cells at different phase of the cell cycle through different biochemical pathways with complementary mechanisms of action. This combination therapy enhances the chances of eradicating cancer cells while also reducing the risk of resistance developing. The combined action enhances cytotoxicity and thus improves therapeutic efficacy compared to the use of each drug alone.
2.2. Pharmacology
2.2.1. Epirubicin
Topoisomerase II is a type of topoisomerase, a class of enzymes that modulate the topological states of DNA during cellular processes such as replication, transcription, and chromosome segregation. There are 3 general functions of Topoisomerase II. The first one is DNA unwinding, which helps alleviate the torsional strain that occurs in front of replication forks and transcription complexes by inducing transient double-strand breaks in the DNA molecule. This allows DNA strands to pass through each other, effectively reducing supercoiling and tangling. Topoisomerase II ensures proper chromosome segregation during cell division, especially during mitosis. The other function is relief of supercoiling, which manages DNA supercoiling, which is the overwinding or underwinding of DNA strands. The mechanism of action of Epirubicin is analogous to that of doxorubicin, an anthracycline chemotherapy drug that impedes the function of topoisomerase II. The chemical structure of the compound includes a planar anthracycline ring system that intercalates into DNA, whereby it inserts itself between the base pairs of the DNA double helix. This intercalation disrupts the normal function of topoisomerase II by stabilizing the DNA-topoisomerase II complex. Instead of re-ligating the cleaved DNA strands, the enzyme remains bound to the DNA, leading to the accumulation of DNA breaks, ultimately triggering cell death. This is how Epirubicin exerts its cytotoxic effects, particularly against rapidly dividing cancer cells. It also suppresses topoisomerase II, hindering the recombination of the DNA double helix and halting DNA replication. An additional suggested mechanism involves the production of free radicals, which can damage DNA and lead to cell death. Moreover, Epirubicin increases nucleosome turnover around promoters due to its ability to intercalate into DNA. These changes in nucleosome structure are thought to influence the mechanisms of cell death during chemotherapy [5].
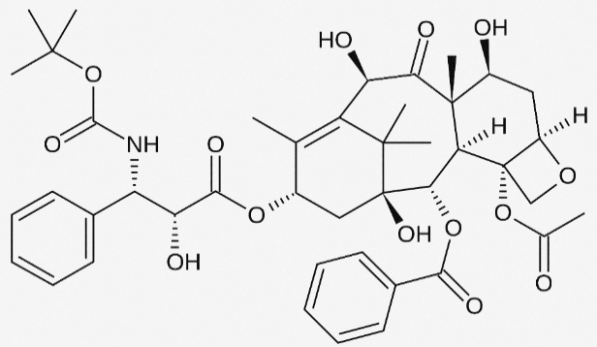
Figure 2. Structure of Epirubicin
2.2.2. Cisplatin
Upon entering the cell, cisplatin undergoes hydrolysis, resulting in the loss of one chloride ligand. This structural alteration allows cisplatin to form covalent bonds with DNA, specifically creating intra-strand cross-links between adjacent guanine bases, which disrupts DNA synthesis and subsequently inhibits cell growth [6]. The DNA damage caused by cisplatin triggers a DNA repair response through the nuclear excision repair (NER) system, which halts cell death by activating the ataxia telangiectasia mutated (ATM) pathway. Studies have shown that the p53 gene is also included in the DNA damage response and repair processes. When activated, ATM phosphorylates the tumor suppressor gene p53, leading to the transactivation of several genes. Additionally, p53 can bind directly to Bax-xL, negating its anti-apoptotic activity and reducing the effectiveness of FLICE-like prohibitor (FLIP), thereby promoting cisplatin-induced apoptosis. The DNA crosslinks caused by cisplatin also activate the mismatch repair (MMR) system, leading to the activation of tyrosine kinase c-Abl in response to DNA damage stress. Activated c-Abl then stimulates extracellular signals such as JNK and p38 MAPK, which keep tumor protein p73, culminating in programed cell death. Figure 3 illustrates the cytotoxic mechanisms of cisplatin chemotherapy within the cell membrane.
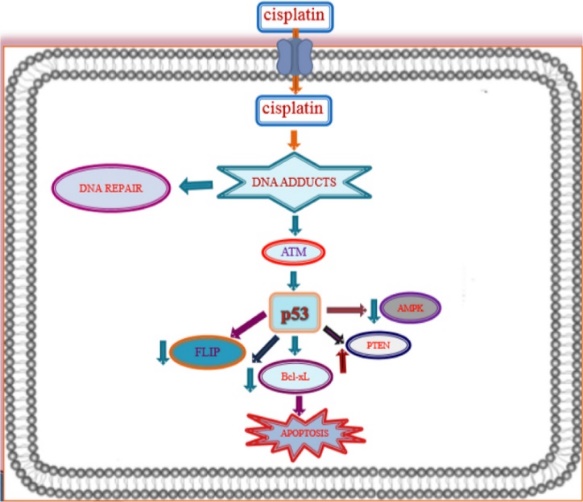
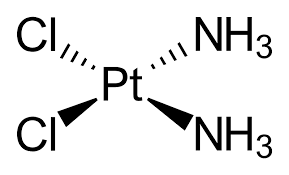
Figure 3. Induction of Apoptosis by Cisplatin [8]
2.2.3. 5-fluorouracil
Thymidine is a nucleotide that forms part of RNA, while deoxythymidine is a nucleotide found in DNA. Thymidylate synthase (TS) is an enzyme that catalyzes the conversion of deoxyuridine monophosphate (dUMP) to deoxythymidine monophosphate (dTMP). This process is essential for the resynthesis of deoxythymidine monophosphate (dTMP) from deoxyuridine monophosphate (dUMP), which serves as a methyl donor for 5,10-methylenetetrahydrofolate (CH2-THF). Thymidylate synthase, a key target of chemotherapy, can be inhibited by folate and nucleotide analogs to form a stable ternary complex, which is further stabilized by leucovorin (LV) [9].
2.2.4. Docetaxel
Docetaxel is a kind of chemotherapy drug used to treat a variety of cancers, including esophageal and stomach cancers. Docetaxel belongs to a class of drugs known as "paclitaxel", which works by disrupting the good functioning of microtubules during cell division, thereby inhibiting the growth and spread of cancer cells. Shown in Figure 4, docetaxel works by stabilizing microtubules and preventing their breakdown, hence interfering with t-cell division, resulting in cell cycle arrest programmed apoptosis. This stabilizing effect prevents cancer cells from dividing and proliferating, thus reducing tumor growth.
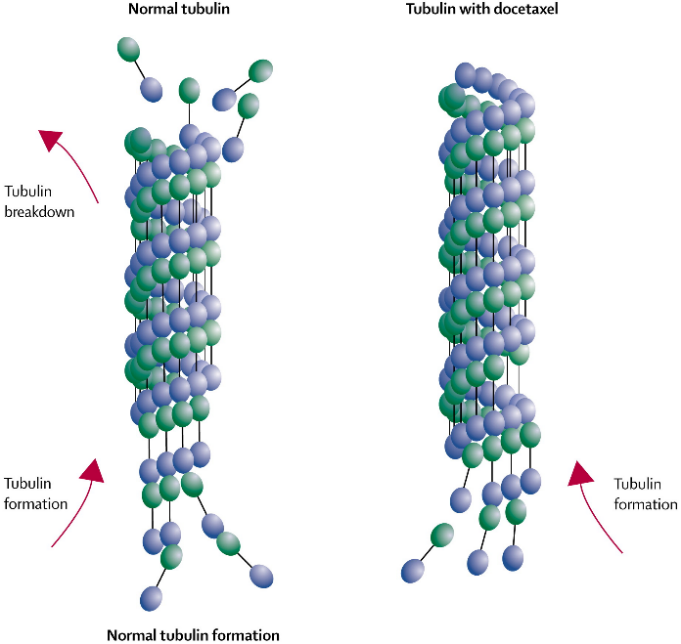
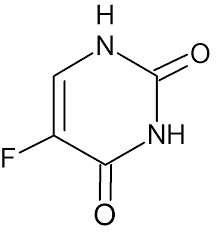
Figure 4. Docetaxel Stabilizes Microtubules and Blocks Cell Division [10]
2.3. Pathophysiology
Esophageal cancer can be separated into2 main groups: squamous cell carcinoma and adenocarcinoma. Squamous cell carcinomas arise from squamous cells in the lining of the esophagus and are more common in the upper and middle esophagus. In contrast, adenocarcinomas arise from glandular cells and are found in the deep esophagus and near the esophagogastric connection.
2.3.1. Squamous Cell Carcinoma
Squamous cell carcinoma (SCC) is a form of skin cancer resulting from the abnormal proliferation of squamous cells in the epidermis, the outermost layer of the skin. Prolonged exposure to the sun's ultraviolet rays, among other factors, significantly increases the risk of developing this type of skin cancer. There is a strong association between SCC and esophageal cancer. Squamous cell carcinoma of the esophagus begins with chronic irritation, which causes squamous cells to mutate. This irritation can develop into squamous cell carcinoma in situations, which is a localized, non-invasive cancer. If left side fail to heal, carcinoma in situ can offend the deeper layers of the esophagus and spread to surrounding tissues and distant organs, leading to invasive squamous cell carcinoma.
2.3.2. Adenocarcinoma
Adenocarcinoma is a type of cancer that starts in glands inside organs and can affect different parts of the body. Adenocarcinoma forms in the epithelial cells of glands that secrete mucus, digestive juices such as gastric acid or other fluids. GERD is an important risk factor because chronic reflux can result in BE, a situation where the normal squamous cells in the esophagus are substituted by glandular cells, significantly elevating the risk of developing adenocarcinoma.
3. Method
In this trial, the quality-of-life index (QOL) of patients with further esophageal cancer was compared with that of patients with advanced malignant tumors after using two combinations of ECF and DCF, respectively. This is attributable to the fact that patients with metastatic disease typically have a less favorable prognosis, with a median survival of 3 to 5 months when receiving optimal supportive care and 7 to 9 months with systemic chemotherapy. Although there is currently no standardized definition of QOL. However, it is generally recognized that social, psychological and physiological factors influence the QOL.
3.1. Patients in Experiment
Patients were all adults over 18 years of age and all had advanced inoperable adenocarcinoma of the stomach or esophagus. Secondly, they had normal liver and kidney function. They excluded individuals with bloodshot heart fault and those with a secondary malignancy. Eventually, a total of 65 sicks were enrolled in this trial, of which 34 received the ECF treatment group and the remaining 31 received the DCF treatment group.
3.2. ECF Dosing Procedures
The first experiment involved patients receiving ECF chemotherapy. The chemotherapy was administered through a subclavian vein with a 5-FU measurement of 250 mg per day and Epirubicin and cisplatin measurements of 50 mg/square meter and 60 mg/square meter, respectively. Grading was based on the National Cancer Institute (NCI) Common Toxicity Criteria. For patients experiencing grade III or IV non-hematologic toxicity, ECF administration was suspended until symptoms resolved.
3.3. DCF Dosing Procedures
The DCF regimen was administered as follows: docetaxel 75 mg/m² was administered via a one-hour intravenous infusion on day 1, in conjunction with cisplatin and 5-fluorouracil (5-FU), which were administered at the same dosages as in the ECF regimen. All patients received appropriate hydration, pre-treatment conditioning, and primary prophylaxis with granulocyte colony-stimulating factor (G-CSF) at a dose of 5 μg/kg/day, delivered subcutaneously for five consecutive days commencing on day 6. The treating physician was permitted to make dose adjustments and implement treatment delays at their discretion. In the event of a grade 4 or life-threatening toxic reaction, a 25% reduction in chemotherapy dosage may be considered. The treatment was continued until one of the following occurred: disease progression, the occurrence of unacceptable toxicity, death, or patient withdrawal.
4. Result
A total of 184 courses were conducted, with a median of 6 courses per sick (range: 1-9 courses). Overall, 92% of the planned dose of Epirubicin, 93% of the planned dose of 5-FU, and 94% of the planned dose of cisplatin were used per course. However, a majority of patients required dose reductions. Toxicity data are shown in Table 1. Grade 3 or 4 vomiting occurred in 3% of cycles, mucositis in 2%, anemia in 10%, and leukopenia in 3%. A small percentage of cycles required delayed treatment due to myelosuppression. Central venous catheter-related complications requiring catheter removal occurred in a significant proportion of patients. Other catheter-related complications included shoulder pain, infection, thrombosis, catheter slippage, and pneumothorax. None of these complications were life-threatening or resulted in long-term morbidity.
Table 1. Toxic Reactions
Grade (%) | 1 | 2 | 3 | 4 |
Hematologic | ||||
Leukopenia | 58(32) | 36(20) | 4(2) | 2(1) |
Anemia | 64(34) | 44(24) | 16(9) | 2(1) |
Thrombocytopenia | 12(7) | 4(2) | 1(0.5) | N/A |
Nonhematologic | ||||
Nausea | 76(41) | 78(42) | 6(3) | N/A |
Vomiting | 29(16) | 24(13) | 4(2) | 1(0.5) |
Mucositis | 17(9) | 13(7) | 4(2) | N/A |
Diarrhea | 8(4) | 2(1) | 1(0.5) | N/A |
Neuropathy* | 8(4) | 2(1) | 1(0.5) | N/A |
Hair loss* | 24(69) | 9(26) | N/A | N/A |
Hand-foot skin reaction | 6(17) | 3(9) | N/A | N/A |
Total | 184 cycles | |||
*Maximum toxicity experienced by each patient (%) | ||||
According to Tables 2, the baseline quality of life (QOL) scores for the DCF group showed a decrease in overall quality of life and most functional scores, with the exception of cognitive function. Notably, the DCF group reported significantly lower levels of pain (P = 0.04) and fewer sleep disturbances compared to the other groups. Tables 3 and 4 present the mean scores for various quality of life (QOL) after three and six cycles of DCF chemotherapy. Following three cycles, most QOL in the DCF group showed improvement from baseline, though issues like constipation and diarrhea worsened. Statistically and clinically significant enhancements were observed in overall QOL (P = 0.013), pain (P = 0.007), and sleeping disabled. After six cycles, most QOL indicators continued to improve in the DCF group, with the exceptions of constipation and diarrhea, which further deteriorated. The improvements in pain (P = 0.04) and sleep difficulties (P = 0.08) remained significant, while overall QOL scores were better than baseline but did not reach statistical significance (P = 0.27). Sicks in the DCF-treated group had a significantly longer median survival before significant deterioration in overall quality of life (7.1 months, P = 0.000). Overall QOL scores tended to improve in patients with confirmed tumor regression after chemotherapy, with mean overall QOL scores significantly better in responding patients (63.2 points) than in non-responders (51.7 points) after three cycles. The overall response rate (ORR) in the DCF group was 48.3%. Most adverse events, both hematologic and non-hematologic, were of grade 1 or 2 severity. However, grade 3-4 toxic reactions were more frequently observed with the DCF regimen, including neutropenia (41.9%, P = 0.12), febrile neutropenia (19.3%, P = 0.75), mucositis (19.3%, P = 0.26), and diarrhea (16.1%, P = 0.43). Despite these occurrences, the differences were not statistically important.
Table 2. Baseline Quality of Life Scores
Baseline Quality of Life Scores of QOL I | |||
QOL parameters | Mean (SD) | P | |
Functional scores | 46.5 (13.9) | 0.31 | |
Physical functioning | 61.7 (13.2) | 0.8 | |
Role functioning | 67.2 (11.4) | 0.89 | |
Emotional functioning | 55.2 (13.3) | 0.43 | |
Cognitive functioning | 82.7 (14.1) | 0.78 | |
Social functioning | 65.5 (11.7) | 0.57 | |
Baseline Quality of Life Scores of QOL II | |||
QOL parameters | Mean (SD) | P | |
Fatigue | 45.2 (11.2) | 0.51 | |
Nausea and vomiting | 23.8 (19.1) | 0.64 | |
Pain | 53.8 (8.5) | 0.04 | |
Dyspnea | 8.3 (20.7) | 0.53 | |
Sleep difficulties | 44.7 (12.1) | 0.03 | |
Appetite loss | 44.8 (9.4) | 0.38 | |
Constipation | 11.5 (21.3) | 0.71 | |
Diarrhea | 7.8 (17.4) | 0.44 | |
Table 3. Changes in Mean volume of QOL in 3 and 6 Cycles of ECF Chemotherapy
QOL parameters | At baseline (n=34) | At 3 months | P (3 months) | At 6 months (n=26) | P (6 months) |
Global QOL | 51.2 (11.8) | 56.8 (10.2) | 0.11 | 47.8 (9.7) | 0.29 |
Physical functioning | 62.5 (11.4) | 65.4 (9.5) | 0.28 | 57.3 (12.3) | 0.37 |
Role functioning | 66.8 (12.6) | 67.3 (11.6) | 0.71 | 61.5 (13.7) | 0.29 |
Emotional functioning | 58.3 (9.7) | 59.2 (10.3) | 0.96 | 54.9 (10.8) | 0.18 |
Cognitive functioning | 84.1 (10.5) | 85.7 (11.1) | 0.83 | 85.2 (12.4) | 0.87 |
Social functioning | 67.7 (12.9) | 65.9 (10.7) | 0.66 | 61.8 (8.8) | 0.8 |
Fatigue | 41.7 (13.8) | 46.5 (14.0) | 0.12 | 44.5 (13.1) | 1.3 |
Nausea and vomiting | 18.2 (18.4) | 15.8 (17.6) | 0.35 | 20.2 (20.2) | 0.77 |
Pain | 44.9 (13.0) | 34.3 (12.4) | 0.02 | 46.7 (15.3) | 0.13 |
Dyspnea | 6.5 (17.6) | 5.3 (15.7) | 0.88 | 8.1 (15.2) | 0.69 |
Sleep difficulties | 33.8 (14.6) | 20.3 (17.5) | 0.01 | 34.4 (16.9) | 0.77 |
Appetite loss | 41.7 (9.3) | 34.7 (11.5) | 0.84 | 48.3 (9.2) | 0.21 |
Constipation | 12.8 (18.7) | 10.3 (15.6) | 0.64 | 11.5 (14.8) | 0.82 |
Diarrhea | 5.4 (15.8) | 13.7 (12.3) | 0.11 | 11.2 (13.7) | 0.25 |
Table 4. Δx of QOL after 3 and 6 cycles of DCF
QOL parameters | At baseline (n=31) | At 3 months (n=31) | P (3 months) | At 6 months (n=31) | P (6 months) |
Global QOL | 46.5 (13.9) | 55.8(11.6) | 0.013 | 50.8 (10.3) | 0.27 |
Physical functioning | 61.7 (13.2) | 64.3 (11.7) | 0.44 | 62.3 (8.5) | 0.78 |
Role functioning | 67.2 (11.4) | 70.8 (8.2) | 0.62 | 71.5 (17.9) | 0.47 |
Emotional functioning | 55.2 (13.3) | 61.2 (10.3) | 0.17 | 58.9 (10.8) | 0.38 |
Cognitive functioning | 82.7 (14.1) | 84.3 (13.5) | 0.65 | 85.3 (11.5) | 0.51 |
Social functioning | 65.5 (11.7) | 68.3 (13.7) | 0.43 | 67.8 (11.4) | 0.58 |
Fatigue | 45.2 (11.2) | 41.3 (11.7) | 0.28 | 42.8 (13.1) | 0.42 |
Nausea and vomiting | 23.8 (19.1) | 21.8 (15.2) | 0.45 | 22.2 (17.5) | 0.67 |
Pain | 53.8 (8.5) | 44.8 (13.4) | 0.007 | 44.8 (13.4) | 0.04 |
Dyspnea | 8.3 (20.7) | 6.2 (15.3) | 0.47 | 7.9 (10.2) | 0.8 |
Sleep difficulties | 44.7 (12.1) | 32.3 (15.4) | 0.004 | 37.4 (16.9) | 0.08 |
Appetite loss | 44.8 (9.4) | 39.4 (12.7) | 0.18 | 42.7 (11.9) | 0.61 |
Constipation | 11.5 (21.3) | 13.5 (14.6) | 0.44 | 13.8 (11.4) | 0.42 |
Diarrhea | 7.8 (17.4) | 15.2 (12.3) | 0.08 | 15.8 (17.3) | 0.07 |
The five domains of functioning include physical, role, emotional, cognitive, and social aspects. These are followed by symptom scales that assess issues such as fatigue, nausea, pain, dyspnea, and so on, with an overall quality of life (QOL) scale. On the Functional Scale and Overall QOL, higher scores indicate improvement, whereas dramatic standards on the Symptom Scale suggest worsening conditions. The current prospective study yielded similar findings, with the DCF regimen being preferred. The primary goal of this study was to assess whether DCF offers better efficacy and improves QOL, which is related to health, compared to the standard ECF therapy. The study results indicated no significant improvement in overall response rates but showed a marked progression in non-progressional survival and overall survival, with no significant increase in grade 3 and 4 toxicities associated with the DCF regimen.
While around 26.5% of patients responded primarily to ECF chemotherapy and 48.3% to DCF chemotherapy, the QOL assessment revealed that a huge rate of sicks experienced enhanced HRQOL, largely due to the palliative benefits of the chemotherapy. Toxicity did not have a significant impact on the overall QOL of responding patients, and this data is clinically significant and may help clinicians make diverse treatment decisions. Based on the current data, it is clinically significant that the DCF regimen continued to improve health QOL and overall functioning in patients even after several cycles of treatment.
However, there are some limitations of this study. First, it was not possible to accurately control for variables in both experimental groups, as patients are subject to a variety of unanticipated special circumstances in selection and treatment. Second, Post-treatment QOL was evaluated only after therapy of several circulars, leaving greater uncertainty regarding the long-term effects of the treatment. Finally, the sample size was small and did not allow reliable conclusions to be drawn. By banning the anthracycline-induced topoisomerase II catalytic cycle when using Epirubicin [11], dexrazoxane is effective in preventing or minimizing skin injury caused by localized extravasation of anthracyclines from peripheral or central veins [12-14]. So that specific manifestations of skin and abdominal inflammatory reactions and blood disorders may disappear completely within a month. Dexrazoxane may play a key role in limiting hepatic and gastrointestinal damage and blood disorders, eliminating skin damage and protecting heart function [15].
5. Conclusion
In conclusion, this study demonstrates that while both ECF and DCF chemotherapy regimens provide palliative benefits for patients with advanced esophageal cancer, the DCF regimen appears to provide greater improvements in health-related quality of life (HRQOL) and overall functioning, especially after multiple cycles of treatment. Despite the high incidence of certain grade 3-4 toxicities with DCF, the regimen's efficacy in improving non-progressional survival, whole survival, and result of the disease outcomes makes it the preferred regimen for first-line treatment in this patient population but some restrictions to this research, including the small sample size, variable patient condition, and short post-treatment evaluation period, suggesting the need for further studies. To corroborate these findings and refine therapeutic strategies for esophageal cancer, it is imperative to enroll a larger cohort and examine a broader spectrum of responses to the administered drugs.
References
[1]. Sio-Hou Wong, Endoscopic submucosal dissection for early esophageal squamous cell carcinoma after ligation and sclerotherapy of esophageal and gastric varices, Elsevier Masson SAS, 2024. DOI: 10.1016/j.dld.2024.01.001
[2]. Oyama T. Esophageal ESD: Technique and Prevention of Complications. Gastrointest Endosc Clin N Am. 2014;24(2):201–12.
[3]. Ahmed Y, Othman M. EMR/ESD: Techniques, Complications, and Evidence. Curr Gastroenterol Rep. 2020;22(8):39.
[4]. Mouridsen HT, Alfthan C, Bastholt L, Bergh J, Dalmark M, Eksborg S, et al. (January 1990). Current status of epirubicin (Farmorubicin) in the treatment of solid tumors. Acta Oncologica. 29(3):257–285.
[5]. Taymaz-Nikerel H, Karabekmez ME, Eraslan S, et al. Doxorubicin induces an extensive transcriptional and metabolic rewiring in yeast cells. Sci Rep. 2018;8:13672.
[6]. The American Society of Health-System Pharmacists. Archived from the original on 21 December 2016. Retrieved 8 December 2016.
[7]. Airley R. Cancer Chemotherapy: Basic Science to the Clinic. John Wiley & Sons; 2009. p. 76. ISBN: 9780470092569. Archived from the original on 5 November 2017.
[8]. Brown A. Cisplatin-Based Chemotherapy of Human Cancers. J Cancer Sci Ther. 2019;11(4):97. Epub 2019 Apr 8.
[9]. Peters GJ, Backus HH, Freemantle S, van Triest B, Codacci-Pisanelli G, van der Wilt CL, et al. Induction of thymidylate synthase as a 5-fluorouracil resistance mechanism. Biochim Biophys Acta. 2002;1587(2-3):194-205. DOI: 10.1016/s0925-4439(02)00082-0. PMID: 12084461.
[10]. Montero A. Docetaxel for Treatment of Solid Tumors: A Systematic Review of Clinical Data. Oncol Lancet. 2005;6(4):229-239.
[11]. Tyson AM, Gay WE. Successful experience utilizing dexrazoxane treatment for an anthracycline extravasation. Ann Pharmacother. 2010;44:922-5.
[12]. Langer SW, Sehested M, Jensen PB. Treatment of anthracycline extravasation with dexrazoxane. Clin Cancer Res. 2000;6:3680-6.
[13]. Muthuramalingam S, Gale J, Bradbury J. Dexrazoxane efficacy for anthracycline extravasation: Use in UK clinical practice. Int J Clin Pract. 2013;67:244-9.
[14]. Arroyo PA, Perez RU, Feijoo MA, Hernandez MA. Good clinical and cost outcomes using dexrazoxane to treat accidental Epirubicin extravasation. J Cancer Res Ther. 2010;6:573-4.
[15]. Andrea Giampreti, Maria Lucà, Mariapina Gallo, et al. Dexrazoxane for rapid extended livedo reticularis-like skin reaction due to systemic epirubicin diffusion during transcatheter arterial chemoembolization procedure for hepatocellular carcinoma. J Cancer Res Ther. 2021;22-Nov-2021.
Cite this article
Zhang,C. (2025). Epirubicin, cisplatin plus 5-fluorouracil (ECF) versus docetaxel, cisplatin with 5-fluorouracil (DCF) therapy as a regimen for locally further inoperable or metastatic esophageal and gastric cancer. Theoretical and Natural Science,78,38-46.
Data availability
The datasets used and/or analyzed during the current study will be available from the authors upon reasonable request.
Disclaimer/Publisher's Note
The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of EWA Publishing and/or the editor(s). EWA Publishing and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
About volume
Volume title: Proceedings of the 4th International Conference on Biological Engineering and Medical Science
© 2024 by the author(s). Licensee EWA Publishing, Oxford, UK. This article is an open access article distributed under the terms and
conditions of the Creative Commons Attribution (CC BY) license. Authors who
publish this series agree to the following terms:
1. Authors retain copyright and grant the series right of first publication with the work simultaneously licensed under a Creative Commons
Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this
series.
2. Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the series's published
version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial
publication in this series.
3. Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and
during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See
Open access policy for details).
References
[1]. Sio-Hou Wong, Endoscopic submucosal dissection for early esophageal squamous cell carcinoma after ligation and sclerotherapy of esophageal and gastric varices, Elsevier Masson SAS, 2024. DOI: 10.1016/j.dld.2024.01.001
[2]. Oyama T. Esophageal ESD: Technique and Prevention of Complications. Gastrointest Endosc Clin N Am. 2014;24(2):201–12.
[3]. Ahmed Y, Othman M. EMR/ESD: Techniques, Complications, and Evidence. Curr Gastroenterol Rep. 2020;22(8):39.
[4]. Mouridsen HT, Alfthan C, Bastholt L, Bergh J, Dalmark M, Eksborg S, et al. (January 1990). Current status of epirubicin (Farmorubicin) in the treatment of solid tumors. Acta Oncologica. 29(3):257–285.
[5]. Taymaz-Nikerel H, Karabekmez ME, Eraslan S, et al. Doxorubicin induces an extensive transcriptional and metabolic rewiring in yeast cells. Sci Rep. 2018;8:13672.
[6]. The American Society of Health-System Pharmacists. Archived from the original on 21 December 2016. Retrieved 8 December 2016.
[7]. Airley R. Cancer Chemotherapy: Basic Science to the Clinic. John Wiley & Sons; 2009. p. 76. ISBN: 9780470092569. Archived from the original on 5 November 2017.
[8]. Brown A. Cisplatin-Based Chemotherapy of Human Cancers. J Cancer Sci Ther. 2019;11(4):97. Epub 2019 Apr 8.
[9]. Peters GJ, Backus HH, Freemantle S, van Triest B, Codacci-Pisanelli G, van der Wilt CL, et al. Induction of thymidylate synthase as a 5-fluorouracil resistance mechanism. Biochim Biophys Acta. 2002;1587(2-3):194-205. DOI: 10.1016/s0925-4439(02)00082-0. PMID: 12084461.
[10]. Montero A. Docetaxel for Treatment of Solid Tumors: A Systematic Review of Clinical Data. Oncol Lancet. 2005;6(4):229-239.
[11]. Tyson AM, Gay WE. Successful experience utilizing dexrazoxane treatment for an anthracycline extravasation. Ann Pharmacother. 2010;44:922-5.
[12]. Langer SW, Sehested M, Jensen PB. Treatment of anthracycline extravasation with dexrazoxane. Clin Cancer Res. 2000;6:3680-6.
[13]. Muthuramalingam S, Gale J, Bradbury J. Dexrazoxane efficacy for anthracycline extravasation: Use in UK clinical practice. Int J Clin Pract. 2013;67:244-9.
[14]. Arroyo PA, Perez RU, Feijoo MA, Hernandez MA. Good clinical and cost outcomes using dexrazoxane to treat accidental Epirubicin extravasation. J Cancer Res Ther. 2010;6:573-4.
[15]. Andrea Giampreti, Maria Lucà, Mariapina Gallo, et al. Dexrazoxane for rapid extended livedo reticularis-like skin reaction due to systemic epirubicin diffusion during transcatheter arterial chemoembolization procedure for hepatocellular carcinoma. J Cancer Res Ther. 2021;22-Nov-2021.









