1. Introduction
For many years, scientists believed that neurogenesis only happened during embryonic development. However, recent studies have provided evidence of adult neurogenesis, especially in the hippocampus [1, 2]. Additionally, adult neurogenesis has been linked to the formation of memory and the development of neurological diseases [3, 4].
Adult neurogenesis is highly regulated. The Reelin pathway and S1P pathway have been reported in separate studies to function in adult neurogenesis regulation. The Reelin gene was discovered in 1995, encoding a large extracellular protein that activates the downstream signaling via binding to the receptors apolipoprotein E receptor 2 (ApoER2) and very-low-density lipoprotein receptor (VLDLR) [5-7]. The adaptor Disabled -1 (Dab1) is another major component in the downstream of Reelin [7]. Activation of Reelin pathway promotes radial neuronal migration and dendritic growth in the adult neurons and is required for the maturation of newborn dentate gyrus cells (DGCs) [8-10]. S1P is a metabolite that can bind to S1P receptors 1-5, known as S1PR1-S1PR5 [11]. S1PR1, a G-protein coupled receptor, has been shown to be upregulated in the newborn DGCs, as well as SPHK1, the kinase that phosphorylates sphingosine into S1P [12]. In addition, knockdown of S1PR1 reduced the number of new DGCs that undergo horizontal-to-radial transitioning during the initial phase of neuronal circuit integration, suggesting that S1PR1 regulates the maturation of newborn DGCs [12].
Since both the Reelin and S1P pathways contribute to the maturation of newborn DGCs, it is possible that they work cooperatively in the process of horizontal-to-radial transitioning. Previous research on this topic has shown that knocking down either Reelin or S1PR1 reduced neurite orientation, though not a complete loss [10, 12]. Here, I will determine whether knocking down both Reelin and S1PR1 results in a more significant decline in neurite orientation and whether one gene regulates the expression of the other.
2. Materials and Methods
2.1. Animals
In brief, the C57BL/6 mice (6–8-week-old) will be ordered from Charles River Laboratories (Wilmington, MA) as previously described [12]. They will be injected with a cocktail of ketamine/xylazine at the dose of 200 mg/kg body weight for anesthesia. Buprenorphine HCl will be administered for postoperative analgesia at the dose of 0.05 mg/kg. Following surgery, the animals will be placed on a heating pad of 37°C for recovery.
2.2. Virus and infection
Retroviral production and infection have been described previously [12]. High titer viruses will be infused into two sites of the dentate gyrus, with 0.5 mL per injection site. Expression of shRNAs and GFP will be induced 3 days post infection (dpi) by addition of 5 mM doxycycline to the mice’s drinking water.
2.3. Immunostaining and fluorescence microscopy
Mice handling will follow the previously established methods [12]. Briefly, the brains will be extracted and fixed with 4% formaldehyde overnight. After fixation, the brain will be sliced into sections, covering the entire DG axis. Immunostaining will begin with blocking at room temperature in PBS containing 1% donkey serum and 0.025% Triton. Following the blocking step, the sections will be incubated overnight at 4°C with primary antibodies, then incubated with secondary antibodies at room temperature. The following antibodies will be used: anti-Reelin (goat polyclonal antibody, Thermo Fisher Scientific), anti-S1PR1 (rabbit polyclonal antibody, Abcam), anti-GFP (rabbit polyclonal antibody, Sigma-Aldrich), Alexa Fluor 555 donkey anti-goat (Thermo Fisher Scientific), Alexa Fluor 647 donkey anti-rabbit antibody (Thermo Fisher Scientific), and Alexa 488-conjugated donkey anti-rabbit antibody (Thermo Fisher Scientific). Images will be captured on a confocal microscope, and ImageJ will be used to analyze the morphology of the new DGCs.
2.4. Western blotting
The dentate gyrus will be homogenized in a lysis buffer with protease inhibitors as described in a previous study [7]. The proteins will be extracted from DGCs and separated on an SDS-PAGE gel followed by membrane transfer. The membrane will then be blocked in blocking buffer (1X TBS buffer containing 5% nonfat dry milk and 0.1% Tween-20), incubated with primary antibodies, and then by horseradish peroxidase (HRP)-conjugated secondary antibodies. The following primary antibodies will be used: anti-Reelin antibody (Thermo Fisher Scientific), anti-S1PR1 antibody (Abcam), and anti-GAPDH antibody (Thermo Fisher Scientific). The anti-GAPDH blotting will be used as a loading control.
2.5. Electrophysiological recordings
A bipolar electrode will be used (100 µs duration) as previously described [12] to stimulate evoked glutamatergic transmission and GABAergic synaptic transmission. The stimulus intensity will be set at 30 µA and maintained consistent throughout all experiments. A total of twenty stimuli will be delivered at a frequency of 0.1 Hz to assess evoked synaptic transmission.
2.6. Data analysis
Data will be analyzed with independent-samples Student’s t tests. All data will be represented as the mean ± standard deviations. Two-tailed P values of < 0.05 will be considered statistically significant.
3. Results
3.1. Reelin and S1PR1 contribute to horizontal-to-radial transitioning of new DGCs cooperatively
To understand if there is any correlation between Reelin pathway and S1PR1 pathway, I first want to find out if Reelin and S1PR1 contribute to horizontal-to-radial transitioning of newly-generated DGCs cooperatively or independently. In other words, the goal is to determine if Reelin and S1PR1 are in the same pathway. In this experiment, I plan to compare the phenotypes resulting from the knockdown of either Reelin or S1PR1 individually, and then both together. If the phenotypes are similar in both single and combined knockdowns, this would suggest that Reelin and S1PR1 function within the same pathway.
I plan to infect the mouse DGCs with inducible shRNA vectors to knock down Reelin (shReelin), S1PR1 (shS1PR1), and both Reelin and S1PR1 (shReelin + shS1PR1). A shCTR will be used as the control. At 3 dpi, shRNA expression will be induced by adding 5mM doxycycline to the drinking water (Figure 1A). In Figure 1B, immunostaining is used to assess the expressions of Reelin and S1PR1 in DGCs infected with shReelin, shS1PR1, and shCTR. The knockdown efficiency for Reelin is shown in Figure 1C, while the knockdown efficiency for S1PR1 is shown in Figure 1D.
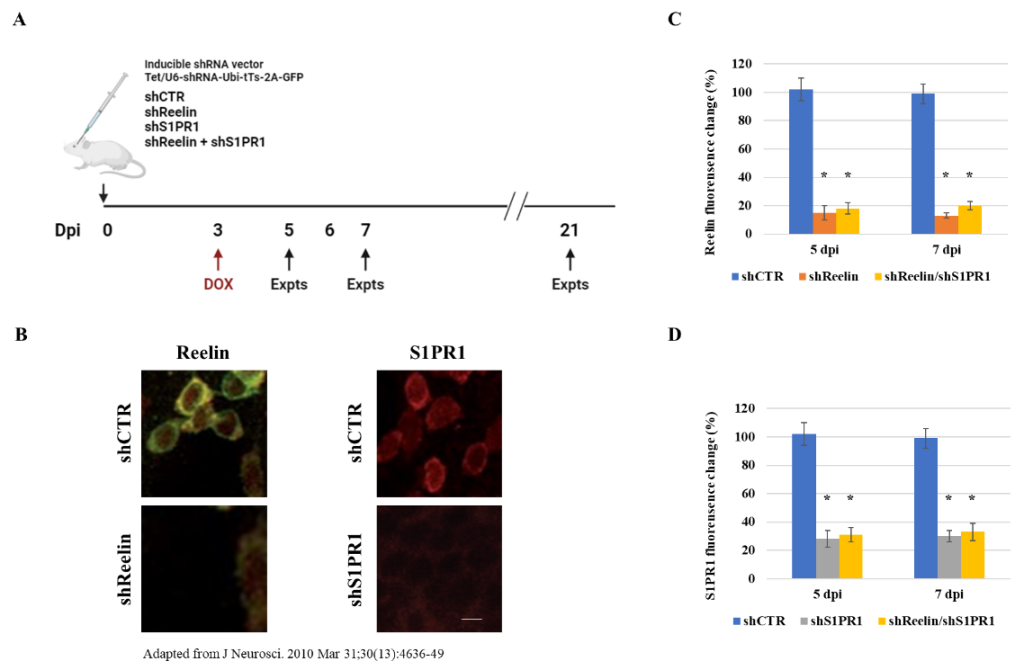
Figure 1. The shRNAs knock down Reelin and S1PR1 expression. (A) Description of the flow scheme and vectors of shRNA viral infection and doxycycline treatment. (B) Representative images of Reelin expression in shCTR and shReelin infected DGCs (left) and S1PR1 expression in shCTR and shS1PR1 infected DGCs (right) at 5 dpi. The images were adapted from a previous study [13]. Scale bar: 10 µM. (C) Plot showing Reelin fluorescence signal intensities at 5 dpi and 7 dpi, respectively. The * indicates P < 0.05. (D) Plot showing S1PR1 fluorescence signal intensities at 5 dpi and 7 dpi, respectively. The * indicates P < 0.05.
Once I identify shRNAs for Reelin and S1PR1 that successfully knock them down, I will analyze the phenotypes of newly generated DGCs infected with shCTR, DGCs infected with shReelin, DGCs infected with shS1PR1, and DGCs infected with both shReelin and shS1PR1 at 5 dpi, 7 dpi, and 21 dpi, respectively. I will first determine the percentage of horizontal cells (<20 degrees as defined previously) [12] at 5 dpi and 7 dpi. In DGCs infected with control shRNA (shCTR), most cells are expected to undergo a horizontal-to-radial transition after 5 dpi, as shown by representative images in Figure 2. In DGCs infected with either shReelin or shS1PR1, I expect a decrease in the number of cells undergoing this transition. However, I do not expect a further reduction in the number of cells undergoing the horizontal-to-radial transition after knocking down both Reelin and S1PR1, suggesting that Reelin and S1PR1 may function cooperatively. The expected percentages of DGCs remaining in the horizontal position at 5 and 7 dpi are displayed in Figure 3A. Additionally, I will count the number of neurites per DGC at 5 dpi and 7 dpi. DGCs with Reelin knockdown, S1PR1 knockdown, and both knockdowns are expected to show similar neurite counts (Figure 3B), further supporting the hypothesis that Reelin and S1P signaling pathways work cooperatively.
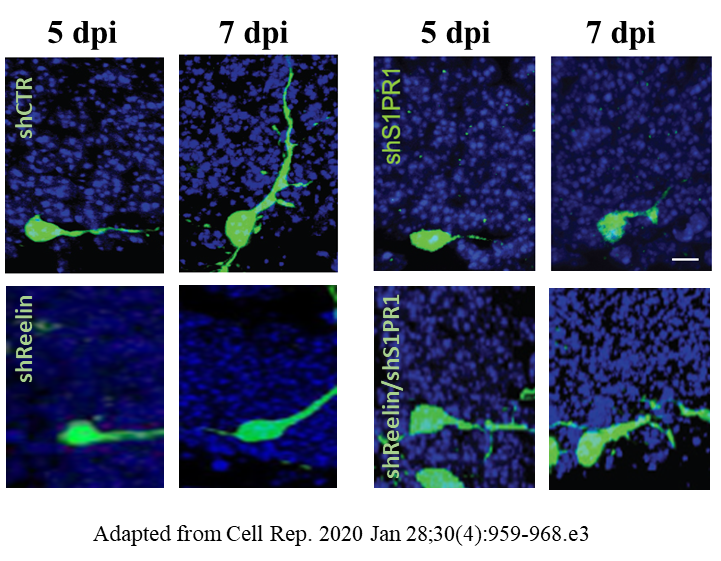
Figure 2. Representative images of DGCs infected with designated shRNAs at 5 dpi and 7 dpi. Scale bar: 10 µM.
I will then determine the synaptic transmission in the knockdown cells by measuring the frequency and amplitude of spontaneous excitatory post-synaptic currents (sEPSCs) at 21 dpi using electrophysiological recordings at 32°C-34°C. The Reelin knockdown cells, S1PR1 knockdown cells, and Reelin/S1PR1 knockdown cells are expected to exhibit fewer sEPSCs compared to the control at 21 dpi, but at similar levels to each other (Figures 3C and 3D). Overall, the results of these experiments would suggest that the phenotypes of shReelin, shS1PR1, and shReelin/shS1PR1 cells are very similar, indicating that Reelin and S1P likely operate within the same pathway.
3.2. Reelin functions upstream of S1PR1 in regulating the horizontal-to-radial transitioning of new DGCs
Next, I aim to investigate whether Reelin functions upstream or downstream of S1PR1 in regulating the horizontal-to-radial transition of new DGCs. To achieve this, I will examine whether Reelin affects S1PR1 expression, or vice versa. Specifically, I will analyze S1PR1 expression following Reelin knockdown, as well as Reelin expression following S1PR1 knockdown. Newly generated DGCs will be infected with either shReelin or shS1PR1, as illustrated in Figure 2A. I expect to observe reduced S1PR1 level in the western blotting after Reelin knockdown, but not the reverse (Figure 4A), suggesting that Reelin regulates S1PR1 expression and functions upstream of S1PR1. Additionally, I will determine if Reelin overexpression can rescue the horizontal-to-radial transitioning in shS1PR1-infected DGCs, and whether S1PR1 overexpression can rescue this transition in shReelin-infected DGCs. I anticipate that S1PR1 overexpression will restore DGC orientation following Reelin knockdown, but not vice versa (Figures 4B and 4C), further suggesting that S1PR1 acts downstream of Reelin.
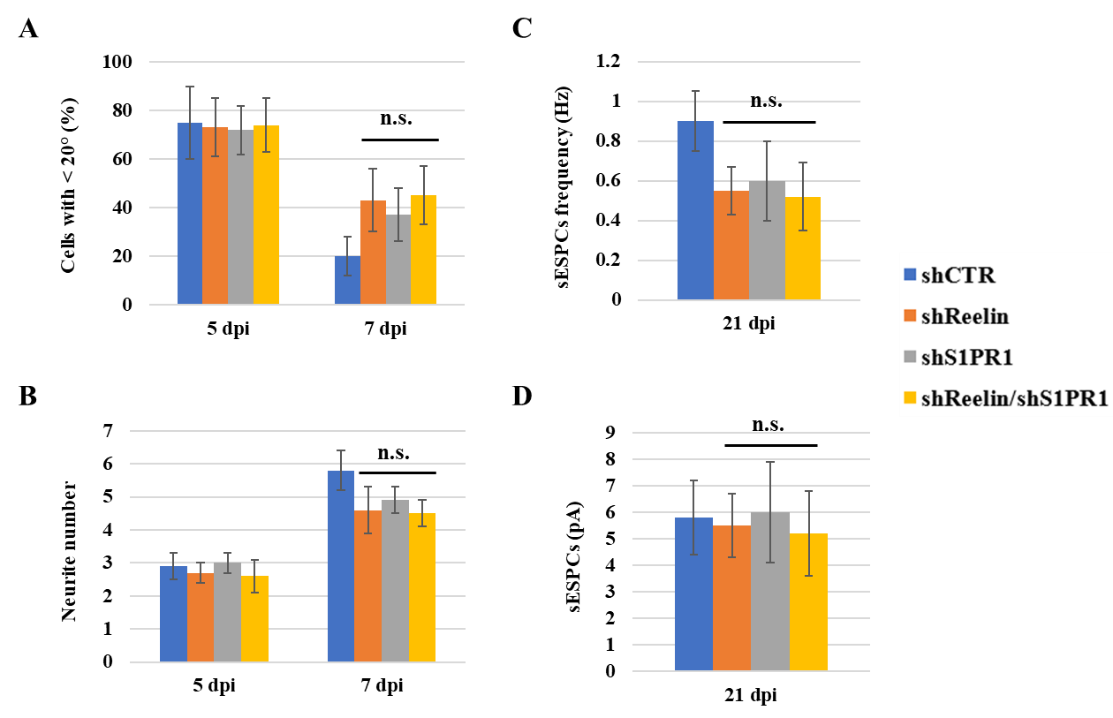
Figure 3. The shReelin, shS1PR1, and shReelin/shS1PR1 result in similar phenotypes. (A) The percentages of cells infected with indicated shRNA with an angle <20° at 5 dpi and 7 dpi. (B) The neurite number per cell in DGCs infected with indicated shRNA at 5 dpi and 7 dpi. (C and D) Glutamatergic synaptic transmission obtained from DGCs at 21 dpi. The n.s. indicates P > 0.05.
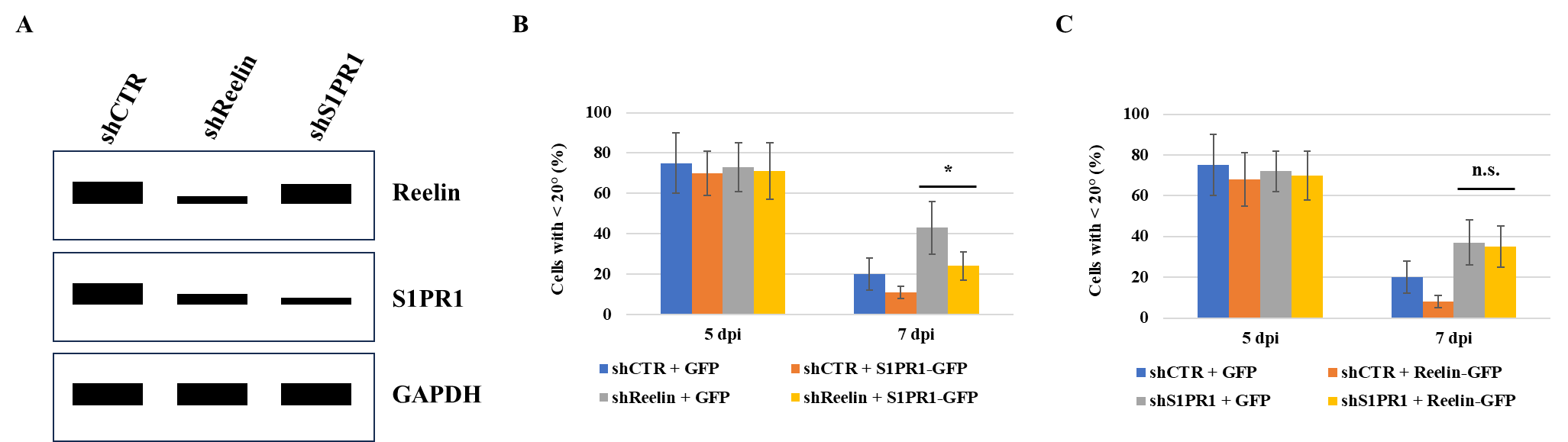
Figure 4. S1PR1 overexpression restores morphological maturation in shReelin-infected DGCs. (A) Western blotting showing the expressions of Reelin and S1PR1 in newly generated DGCs infected with indicated shRNAs at 5 dpi. (B) Cells will be infected with virus containing pTet/U6-shCTR-Ubi-tTs-2A-GFP (shCTR + GFP), pTet/U6-shCTR-Ubi-tTs-2A-S1PR1-GFP (shCTR + S1PR1-GFP), pTet/U6-shReelin-Ubi-tTs-2A-GFP (shReelin + GFP), pTet/U6-shReelin-Ubi-tTs-2A-S1PR1-GFP (shReelin + S1PR1-GFP), respectively. The percentages of DGCs with an angle less than 20° at 5 dpi and 7 dpi are shown. The * indicates P < 0.05. (C) Cells will be infected with virus containing pTet/U6-shCTR-Ubi-tTs-2A-GFP (shCTR + GFP), pTet/U6-shCTR-Ubi-tTs-2A-Reelin-GFP (shCTR + Reelin-GFP), pTet/U6-shS1PR1-Ubi-tTs-2A-GFP (shS1PR1 + GFP), pTet/U6-shS1PR1-Ubi-tTs-2A-Reelin-GFP (shS1PR1 + Reelin-GFP), respectively. The percentages of DGCs with an angle less than 20° at 5 dpi and 7 dpi are presented. The n.s. indicates P > 0.05.
4. Discussion
Previous studies have shown that newly generated DGCs undergo neurite remodeling before functional circuit integration, and both the Reelin and S1P pathways have been found to regulate neurite orientation and the maturation of new DGCs [10, 12]. The objective in this study is to investigate the correlation between Reelin and S1PR1. I expect similar percentages of new DGCs undergoing horizontal-to-radial transitioning following the knockdown of both genes compared to individual knockdowns (Figures 2 and 3). Additionally, I anticipate that Reelin regulates the expression of S1PR1, and that S1PR1 overexpression can restore neurite remodeling in Reelin knockdown DGCs (Figure 4). These observations would suggest that the Reelin and S1P pathways operate in the same pathway to facilitate the integration of newly generated DGCs into the neuronal circuit, with Reelin acting upstream of S1PR1. However, further investigation is needed to determine how Reelin functions upstream of S1PR1. It is important to note that these results are hypothetical, and future experiments must be conducted in a lab to determine whether the expected outcomes are correct.
5. Conclusion
This study will be the first to investigate the relationship between two signaling pathways, the Reelin pathway and the S1P pathway. These pathways are expected to work cooperatively to contribute to the maturation of new DGCs. More specifically, Reelin is hypothesized to work upstream of S1PR1 and affect its expression. These findings will not only reveal the new functions of the Reelin and S1P pathways but also provide insights into the mechanisms of adult neurogenesis. The knowledge gained from this study could potentially benefit the development of future treatments for neurological diseases.
References
[1]. Altman, J. and G.D. Das, Autoradiographic and histological evidence of postnatal hippocampal neurogenesis in rats. J Comp Neurol, 1965. 124(3): p. 319-35.
[2]. Kuhn, H.G., H. Dickinson-Anson, and F.H. Gage, Neurogenesis in the dentate gyrus of the adult rat: age-related decrease of neuronal progenitor proliferation. J Neurosci, 1996. 16(6): p. 2027-33.
[3]. Moreno-Jimenez, E.P., et al., Adult hippocampal neurogenesis is abundant in neurologically healthy subjects and drops sharply in patients with Alzheimer's disease. Nat Med, 2019. 25(4): p. 554-560.
[4]. Gage, F.H., Adult neurogenesis in mammals. Science, 2019. 364(6443): p. 827-828.
[5]. D'Arcangelo, G., et al., A protein related to extracellular matrix proteins deleted in the mouse mutant reeler. Nature, 1995. 374(6524): p. 719-23.
[6]. D'Arcangelo, G., et al., Reelin is a secreted glycoprotein recognized by the CR-50 monoclonal antibody. J Neurosci, 1997. 17(1): p. 23-31.
[7]. Niu, S., et al., Reelin promotes hippocampal dendrite development through the VLDLR/ApoER2-Dab1 pathway. Neuron, 2004. 41(1): p. 71-84.
[8]. Alexander, A., J. Herz, and L. Calvier, Reelin through the years: From brain development to inflammation. Cell Rep, 2023. 42(6): p. 112669.
[9]. Haas, C.A., et al., Role for reelin in the development of granule cell dispersion in temporal lobe epilepsy. J Neurosci, 2002. 22(14): p. 5797-802.
[10]. Teixeira, C.M., et al., Cell-autonomous inactivation of the reelin pathway impairs adult neurogenesis in the hippocampus. J Neurosci, 2012. 32(35): p. 12051-65.
[11]. Mendelson, K., T. Evans, and T. Hla, Sphingosine 1-phosphate signalling. Development, 2014. 141(1): p. 5-9.
[12]. Yang, C.H., et al., Circuit Integration Initiation of New Hippocampal Neurons in the Adult Brain. Cell Rep, 2020. 30(4): p. 959-968 e3.
[13]. Pujadas, L., et al., Reelin regulates postnatal neurogenesis and enhances spine hypertrophy and long-term potentiation. J Neurosci, 2010. 30(13): p. 4636-49.
Cite this article
Zhou,V. (2025). Reelin and S1P signaling contribute to horizontal-to-radial transitioning of new DGCs cooperatively. Theoretical and Natural Science,78,47-52.
Data availability
The datasets used and/or analyzed during the current study will be available from the authors upon reasonable request.
Disclaimer/Publisher's Note
The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of EWA Publishing and/or the editor(s). EWA Publishing and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
About volume
Volume title: Proceedings of the 4th International Conference on Biological Engineering and Medical Science
© 2024 by the author(s). Licensee EWA Publishing, Oxford, UK. This article is an open access article distributed under the terms and
conditions of the Creative Commons Attribution (CC BY) license. Authors who
publish this series agree to the following terms:
1. Authors retain copyright and grant the series right of first publication with the work simultaneously licensed under a Creative Commons
Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this
series.
2. Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the series's published
version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial
publication in this series.
3. Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and
during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See
Open access policy for details).
References
[1]. Altman, J. and G.D. Das, Autoradiographic and histological evidence of postnatal hippocampal neurogenesis in rats. J Comp Neurol, 1965. 124(3): p. 319-35.
[2]. Kuhn, H.G., H. Dickinson-Anson, and F.H. Gage, Neurogenesis in the dentate gyrus of the adult rat: age-related decrease of neuronal progenitor proliferation. J Neurosci, 1996. 16(6): p. 2027-33.
[3]. Moreno-Jimenez, E.P., et al., Adult hippocampal neurogenesis is abundant in neurologically healthy subjects and drops sharply in patients with Alzheimer's disease. Nat Med, 2019. 25(4): p. 554-560.
[4]. Gage, F.H., Adult neurogenesis in mammals. Science, 2019. 364(6443): p. 827-828.
[5]. D'Arcangelo, G., et al., A protein related to extracellular matrix proteins deleted in the mouse mutant reeler. Nature, 1995. 374(6524): p. 719-23.
[6]. D'Arcangelo, G., et al., Reelin is a secreted glycoprotein recognized by the CR-50 monoclonal antibody. J Neurosci, 1997. 17(1): p. 23-31.
[7]. Niu, S., et al., Reelin promotes hippocampal dendrite development through the VLDLR/ApoER2-Dab1 pathway. Neuron, 2004. 41(1): p. 71-84.
[8]. Alexander, A., J. Herz, and L. Calvier, Reelin through the years: From brain development to inflammation. Cell Rep, 2023. 42(6): p. 112669.
[9]. Haas, C.A., et al., Role for reelin in the development of granule cell dispersion in temporal lobe epilepsy. J Neurosci, 2002. 22(14): p. 5797-802.
[10]. Teixeira, C.M., et al., Cell-autonomous inactivation of the reelin pathway impairs adult neurogenesis in the hippocampus. J Neurosci, 2012. 32(35): p. 12051-65.
[11]. Mendelson, K., T. Evans, and T. Hla, Sphingosine 1-phosphate signalling. Development, 2014. 141(1): p. 5-9.
[12]. Yang, C.H., et al., Circuit Integration Initiation of New Hippocampal Neurons in the Adult Brain. Cell Rep, 2020. 30(4): p. 959-968 e3.
[13]. Pujadas, L., et al., Reelin regulates postnatal neurogenesis and enhances spine hypertrophy and long-term potentiation. J Neurosci, 2010. 30(13): p. 4636-49.









