1. Introduction
Alzheimer's Disease (AD), the predominant cause of dementia worldwide, poses a considerable challenge in biomedical research due to the aging global population. Characterized by cognitive decline, memory loss, and behavioural changes, AD is defined by the accumulation of amyloid-beta (Aβ) plaques and neurofibrillary tangles composed of hyperphosphorylated tau proteins. These pathological hallmarks are associated with synaptic dysfunction and neuronal loss, pivotal in the disease’s progression. The amyloid cascade hypothesis articulates that insufficient clearance of Aβ, rather than overproduction, initiates neuroinflammatory responses that lead to synaptic degradation and further neurodegeneration [1,2]. This inflammation is intensified by the activation of the NLRP3 inflammasome in microglia, which reacts to Aβ aggregates, releasing pro-inflammatory cytokines that exacerbate AD's symptoms [3]. Therapeutic strategies thus target the enhancement of Aβ clearance through key transporters like RAGE, ABCA1, LRP1, and P-glycoprotein at the blood-brain barrier, aiming to decelerate the disease’s progression [4,5].
The identification of Klotho (KL) by Kuro-o et al. in 1997 [6] marked a significant advancement in aging research, revealing its profound role in longevity and neurodegeneration. Named after the Greek goddess who spins the thread of life, Klotho functions as both a transmembrane protein and a secreted hormone, primarily expressed in the kidney and the choroid plexus. It regulates calcium and phosphate homeostasis through its interaction with fibroblast growth factor 23 (FGF23). The hormone, present in cerebrospinal fluid, affects several physiological processes crucial to aging, including ion channel modulation, insulin/IGF-1 signalling, and oxidative stress regulation. Notably, mice deficient in KL exhibit premature aging and reduced lifespans, while overexpression has been shown to extend life. Furthermore, reduced levels of Klotho in the aging brain and in AD patients suggest its therapeutic potential, with studies indicating that enhancing Klotho expression can alleviate cognitive deficits in AD models [7].
The interaction between Klotho and Alzheimer's Disease (AD) has garnered significant scientific attention, marking Klotho as a promising therapeutic target due to its neuroprotective and cognitive-enhancing properties. Klotho's ability to mitigate aging-related phenotypes and cognitive deficits in AD has been extensively studied, particularly through the enhancement of autophagy, reduction of oxidative stress, and improved amyloid-beta clearance. These mechanisms suggest Klotho's potential to counteract the pathophysiological processes in AD, notably by modulating amyloid-beta dynamics and neuroinflammatory pathways via the autophagy lysosomal pathway (ALP). Recent investigations, including the work of Zhao et al. (2020) [8], using murine models like APP/PS1, have further elucidated Klotho's impact on AD pathology. These studies highlight Klotho's capacity to influence central features of AD, such as amyloid-beta dynamics and neuroinflammation, reinforcing its role in modulating aging mechanisms and enhancing cognitive functions, with varying outcomes that shape its translational potential in neurodegenerative disease management.
However, current research into Klotho's role in AD, including pivotal studies like Zhao et al. (2020), encounters methodological challenges such as the lack of long-term observational data, significant variability in the age and genetic backgrounds of mouse models, and inconsistent Klotho administration. These issues cloud the efficacy of Klotho interventions and limit the generalizability of findings. This review aims to critically assess Klotho-related AD research in mouse models, spotlighting the investigation by Zhao et al. (2020) to identify experimental limitations and suggest improvements that enhance the reliability and applicability of future research, advancing our understanding and therapeutic application of Klotho in AD.
2. Results
The general workflow of Zhao et al. 2020 included the following steps. First, GFP-tagged lentiviral plasmids carrying Klotho cDNA were prepared for gene delivery, generating four experimental groups: WT/LV-GFP, WT/LV-KL, APP|PS1/LV-GFP, and APP|PS1/LV-KL (WT, wide type; LV, lentivirus; GFP, green fluorescent protein; APP/PS1, a model of early AD carrying mutated Precursor Protein (APP) and Presenilin 1 (PS1) genes). Secondly, neurobehavioral assessments included Y-maze, passive avoidance, and Morris water maze tests. When mice reached 13 months, their tissues and blood were collected for biochemical analysis—immunohistochemistry was used to detect the distribution or localisation of targets, while accurate quantification was achieved using Quantitative real-time Polymerase Chain Reaction (qRT-PCR), Enzyme-linked Immunosorbent Assay (ELISA), and Western blot. Lastly, they generated an in vitro model, evaluating the effect of Klotho on Aβ clearance across blood-cerebrospinal fluid barrier (BCSFB).
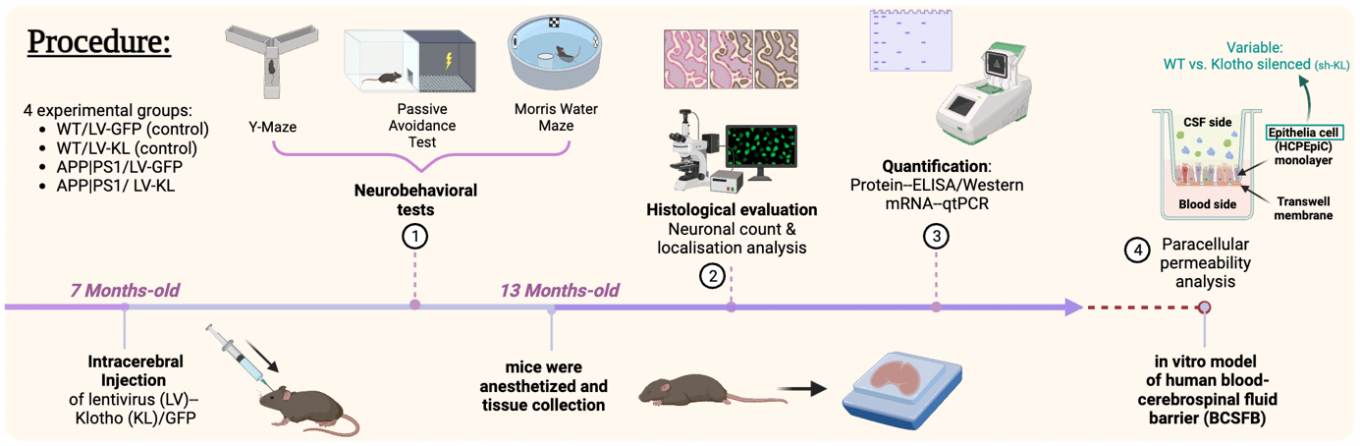
Figure 1. Major steps in Zhao et al. 2020 depicted over a timeline (made in Biorender by Evelyn Chen)
The results can be divided into two sections: confirming Klotho’s proposed neuroprotective effect and investigating hypothesised Klotho’s mechanism.
In the first section, researchers systematically confirmed the general belief that KL overexpression ameliorates AD pathology from three aspects: cognitive deficit improvement, Aβ burden alleviation, and neuroprotective effect.
Cognitive function was evaluated using neurobehavioral tests. APP|PS1/LV-GFP group performed the worst in all three tests, indicating their impaired cognitive function. KL overexpression improved AD mice cognitive deficit but cannot restore to the equal of WT mice. No significant differences were found between the WT/LV-GFP and WT/LV-KL groups, suggesting KL overexpression was more effective at mitigating pathological cognitive deficits rather than boosting unimpaired cognition, in this particular case.
Regarding Aβ pathology, a notable decrease in Aβ aggregates was observed in aged APP/PS1 mice with KL overexpression. Specifically, the treatment mitigated amyloid deposition in cerebral vasculature, which in AD can lead to cerebral amyloid angiopathy (CAA) and subsequent vascular dysfunction or haemorrhagic strokes [9]. This finding was obtained via the double-staining technique with antibodies against Aβ and laminin, which revealed a marked reduction in amyloid plaque deposition in cortical blood vessels following LV-KL treatment, compared to APP/PS1/LV-GFP mice, underscoring the potential of KL overexpression in alleviating Aβ burden.
Aβ aggregates in Alzheimer’s disease tend to cluster in the CA1 area of the hippocampus, leading to neuronal dysfunction and death which correlates with the cognitive deficits observed [10]. Zhao et al. 2020 observed APP/PS1/LV-GFP mice experienced ~30% neuronal loss in the hippocampal CA1 area compared with WT/LV-GFP mice, while KL treatment (LV-KL) was effective in preventing such neuronal loss in AD mice.
Next, two hypothesised KL’s mechanisms were investigated: neuroinflammation amelioration and Aβ transporters regulation to promote Aβ clearance.
In aged APP/PS1 mouse models, KL overexpression was found to significantly reduce brain inflammation. This was evidenced by the reduced activity of the NLRP3/caspase-1 signalling pathway, with a notable decrease in interleukin-1 beta (IL-1β) levels, as identified through Western blot, qRT-PCR, and ELISA. IL-1β is a pro-inflammatory cytokine instrumental in activating inflammatory responses, and its diminished presence indicates suppressed inflammatory signalling [7]. Furthermore, Klotho facilitated a shift in microglial phenotypes from the pro-inflammatory M1 state towards the anti-inflammatory M2 state, indicated by decreased CD86 (a marker of M1 microglia) and increased CD206 (a marker of M2 microglia) expression ratios in the hippocampal CA1 area.
KL overexpression altered Aβ transporter levels, increasing the expression of efflux transporters (LRP1 and P-gp) and decreasing that of influx transporters (ABCA1 and RAGE), promoting Aβ clearance across the blood-brain barrier. This effect was further validated through an in vitro model. Human choroid plexus epithelial cells, either has KL knocked-down or expressing normally, were cultured on collagen-coated transwell membranes to mimic the BCSFB. The movement of fluorescent FITC-Aβ1-42 across this barrier was tracked, revealing a significant decrease in Aβ clearance when KL was silenced, accompanied by altered mRNA expression of crucial transporters. These changes were consistent with the findings on mice model, reinforcing the role of KL in Aβ transporters regulation and Aβ clearance from the brain.
These findings collectively underscore the therapeutic potential of KL overexpression in ameliorating the pathological aspects of AD without affecting the cognitive function of healthy neurons, presenting a potential target to treatment.
3. Discussion
Klotho, widely recognized as an anti-aging gene, exhibits decreased expression in Alzheimer's disease (AD) patients and animal models. Earlier studies [11,12] have indicated that Klotho overexpression can counteract AD pathology and cognitive impairment in murine models. The current research corroborates these observations in 7-month-old APP/PS1 mice.
Zhao et al. (2020) pioneered the exploration of Klotho's role in modulating AD pathology, illustrating its contribution to the transition of microglia from the pro-inflammatory M1 phenotype to the reparative M2 phenotype and its enhancement of Aβ clearance through BCSFB transporter regulation. While no subsequent studies have directly supported or contested these findings, the theoretical framework for Klotho's mechanisms aligns with existing literature. For instance, Zhao et al. (2020) provided evidence that Klotho is involved in neuroinflammatory regulation within the context of AD, a finding indirectly corroborated by Zeng et al. (2019), who demonstrated that Klotho activation leads to enhanced autophagy and reduced Aβ accumulation by inhibiting the Akt/mTOR signalling pathway. This pathway is known to suppress autophagy when overactivated, further supporting Klotho's potential role in mitigating AD pathology.
Furthermore, Zhao et al. (2020) noted that Klotho overexpression normalizes Aβ transporter expression at the blood-brain barrier (BBB), facilitating Aβ efflux and limiting influx—critical processes for reducing Aβ accumulation in the brain. These observations align with studies by Wahrle et al. (2008) [16] and Aykac & Sehirli (2021) [17] which reported diminished Aβ deposition in AD models through targeted, selective manipulation of Aβ transporters.
Klotho's potential as a therapeutic agent for AD is substantial, acting as a central regulator of multiple AD-related pathways. Notably, its anti-inflammatory properties are crucial in counteracting age-related neuroinflammation, a process that significantly contributes to cognitive decline in AD. Klotho's ability to inhibit pro-inflammatory cytokines like TNFα, as observed in studies of both neurodegenerative diseases and chronic inflammatory conditions, suggests that it could play a protective role in maintaining cognitive function and white matter integrity during aging.
Despite these promising findings, the precise molecular and cellular mechanisms underlying Klotho's neuroprotective actions warrant further investigation. Outstanding questions include the identification of its neuronal receptor, the specific signaling pathways Klotho activates for cognitive enhancement, and how peripherally administered Klotho confers neuroprotection [17]. Additionally, the development of Klotho enhancers that can cross the BBB presents a promising avenue for therapeutic intervention in AD and other neurodegenerative diseases. The discovery of small molecule Klotho enhancers capable of increasing Klotho expression in the brain could potentially offer new treatments for these conditions, addressing the challenges posed by Klotho's large molecular size and its limited bioavailability when administered directly.
4. Critical Analysis
This paper aims to establish the therapeutic relevance of the "anti-aging" gene Klotho in AD. The significant difference observed between the APP|PS1/LV-GFP and APP|PS1/LV-KL groups strongly support the main hypothesis. However, comparisons within and between groups, particularly between APP|PS1/LV-KL and WT mice, raise questions about the study's design, primarily due to the different lifespans of WT and APP|PS1 mice. The research involved four strategically chosen experimental groups: WT/LV-GFP, WT/LV-KL, APP|PS1/LV-GFP, and APP|PS1/LV-KL, intended for in-group and between-group matched pair comparisons, enhancing the reliability of the results.
Nevertheless, while WT C57BL/6 mice typically live around 2 to 3 years, APP|PS1 mice have a shorter lifespan of 12-15 months (Zeng et al., 2019). Consequently, at 13 months old, the APP|PS1 mice, when their brain tissues were examined, likely exhibited neuronal damage due to both AD pathology and natural aging. In contrast, the 13-month-old WT mice were relatively young, displaying no neuronal deterioration. This age disparity introduces a confounding variable that influences the certainty of data obtained.
Specifically, how did this experimental design limitation affect results? First, with both WT/LV-GFP and WT/LV-KL groups showing similar cognitive outcomes across all tests, the authors failed to demonstrate KL's long-term cognitive enhancement effects in wild-type mice, as proposed in many previous studies (Zeng et al., 2019). This discrepancy can be easily resolved by inspecting the age range of models selected—20-24 months mice were commonly experimented on in previous literature, not 13 months. This observation suggests that KL's neuroprotective effects might not be discernible in younger mice with normal KL expression. This was later confirmed by Castner et al. (2023), who found that there is a functional dosage threshold for KL's cognitive enhancement effects; over-expressing KL in young, WT mice with sufficient KL demonstrated little beneficial effect.
On the other hand, the inability of KL to restore performance or neuronal health in APP|PS1 mice to WT levels prompts further questions about whether KL's effects are primarily anti-aging or AD-specific and whether its anti-aging and AD therapeutic roles are complementary or conflicting. Addressing these questions necessitates a longitudinal study comparing the lifespans and molecular pathways in aged APP|PS1 and WT mice.
Moreover, while the development of Klotho enhancers presents a promising therapeutic strategy, it is important to critically assess the challenges associated with their application. Klotho's large molecular size and its limited ability to cross the BBB pose significant obstacles for direct therapeutic use. The identification and optimization of small molecule Klotho enhancers that can effectively increase Klotho levels in the brain while avoiding potential off-target effects remain crucial areas of ongoing research. As such, the potential for Klotho-based therapies in neurodegenerative diseases like AD is vast, but the path from bench to bedside will require rigorous testing and validation in preclinical and clinical settings.
5. Suggested Improvements
Due to experimental constraints, the extent of Klotho's neuroprotection and therapeutic impact on Alzheimer's disease remains unclear. A longitudinal study, building upon the critical analysis, would be an apt approach to explore this. The objective is to determine if KL overexpression can equate the neuronal health of AD mouse models to that of age-matched WT controls to assess its efficacy and to discern if KL's effects are primarily anti-aging or AD-specific or both. Such a study could extend over 2-3 years, covering the mice's full lifespan, and replicate the four experimental groups used by Zhao et al. 2019. Consideration for additional groups with varied timings and methods of KL induction (hormonal injection or viral delivery) is also advised. Neurobehavioral tests will be conducted regularly, and post-mortem analyses of brain tissues at the end.
By comparing the trajectory of AD progression with and without KL intervention, and by examining its effects on age-matched healthy controls, the study will clarify the scope and limitations of KL's neuroprotective effects. Researchers can observe if APP|PS1/KL (LV deleted because the mode of delivery may not be lentivirus) obtained an elongated lifespan in addition to the improve cognitive function compared to APP|PS1/GFP, which will elucidate if KL’s general anti-ageing effect is maintained in AD individuals: a positive result will mean that KL's anti-aging and AD therapeutic effects are not in conflict but may be complementary. Additionally, when conducting between-group analysis, if cognitive and brain health in APP|PS1 mice with KL treatment are inferior to aged WT controls, it implies KL's limited capacity to fully reverse AD pathology. Equivalence would suggest that KL is capable of ameliorating AD-induced damage to a level commensurate with normal aging. Superior performance in KL-treated APP|PS1 mice over aged WT controls would imply that KL not only mitigates AD-related degeneration but also confers protection against the neurobiological insults associated with aging. Through these scenarios, the longitudinal study will systematically dissect the nuanced role of Klotho overexpression across different life stages and conditions of disease progression.
As mentioned in the Discussion section, future research should delve deeper into Klotho's mechanism of action, particularly its role in transforming microglia from a pro-inflammatory to a reparative state and regulating Aβ transporter function. High-throughput screening methods could be instrumental in identifying molecules interacting with Klotho in these pathways.
Given the limitations in extrapolating animal model results to humans, especially in AD research, transitioning to non-mice models is essential for broader applicability. These models can provide more relevant data on Klotho's safety and efficacy in diverse human AD types for the subsequent progression to human clinical trials.
The future research on KL presents significant implications for understanding its role in neuroprotection. The longitudinal and mechanistic studies could elucidate whether KL's benefits are exclusive to AD or can extend to general cognitive enhancement in aged individuals and to other neurodegenerative diseases. Furthermore, transitioning to non-mouse models will bridge the gap between animal and human studies, essential for translating these findings into clinical applications. All in all, research on KL has the potential to reshape our approach to neurodegenerative diseases, opening new avenues for treatment and prevention strategies beyond the realm of AD.
6. Conclusion
In conclusion, the exploration of KL as a therapeutic target in AD has revealed promising neuroprotective effects, particularly in mitigating amyloid-beta pathology and neuroinflammation, as demonstrated by studies such as Zhao et al. (2020). However, the current understanding is hindered by methodological challenges and the need for more comprehensive, long-term research. The proposed longitudinal studies, with careful consideration of age-matched controls and diverse experimental models, are crucial for elucidating KL's dual role in aging and AD. Further investigation into the molecular mechanisms by which Klotho modulates microglial phenotypes and Aβ clearance, alongside the development of Klotho enhancers capable of crossing the blood-brain barrier, will be vital. These efforts could not only solidify KL's role in AD therapy but also extend its potential application to other neurodegenerative conditions, ultimately contributing to a broader understanding of aging and cognitive decline. The transition to human-relevant models and eventual clinical trials will be key steps in translating these findings into viable treatments, potentially revolutionizing the management of neurodegenerative diseases.
References
[1]. Ising C., Venegas C., Zhang S., Scheiblich H., Schmidt S.V., Vieira-Saecker A., Schwartz S., Albasset S., McManus R.M., Tejera D., et al. (2019) NLRP3 inflammasome activation drives tau pathology. Nature. 575:669–673.
[2]. Nalivaeva, N. N., & Turner, A. J. (2019). Targeting amyloid clearance in Alzheimer's disease as a therapeutic strategy. British Journal of Pharmacology, 176(18), 3447–3463.
[3]. Hansen D.V., Hanson J.E., Sheng M. (2018) Microglia in Alzheimer’s disease. J. Cell Biol. 217:459–472. doi: 10.1083/jcb.201709069.
[4]. Deane R., Bell R.D., Sagare A., Zlokovic B.V. (2009) Clearance of amyloid-beta peptide across the blood-brain barrier: Implication for therapies in Alzheimer’s disease. CNS Neurol. Disord. Drug Targets. 8:16–30.
[5]. Chiu, C., Miller, M. C., Monahan, R., Osgood, D. P., Stopa, E. G., & Silverberg, G. D. (2015). P-glycoprotein expression and amyloid accumulation in human aging and Alzheimer's disease: preliminary observations. Neurobiology of aging, 36(9), 2475–2482.
[6]. Kuro-o, M., Matsumura, Y., Aizawa, H., Kawaguchi, H., Suga, T., Utsugi, T., Ohyama, Y., Kurabayashi, M., Kaname, T., Kume, E., Iwasaki, H., Iida, A., Shiraki-Iida, T., Nishikawa, S., Nagai, R., & Nabeshima, Y. I. (1997). Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature, 390(6655), 45–51.
[7]. Tan M.S., Yu J.T., Jiang T., Zhu X.C., Tan L. (2013) The NLRP3 inflammasome in Alzheimer’s disease. Mol. Neurobiol. 48:875–882.
[8]. Zhao, Y., Zeng, C. Y., Li, X. H., Yang, T. T., Kuang, X., & Du, J. R. (2020). Klotho overexpression improves amyloid-β clearance and cognition in the APP/PS1 mouse model of Alzheimer's disease. Aging cell, 19(10), e13239.
[9]. Greenberg, S. M., Bacskai, B. J., Hernandez-Guillamon, M., Pruzin, J., Sperling, R., & van Veluw, S. J. (2020). Cerebral amyloid angiopathy and Alzheimer disease – One peptide, two pathways. Nature Reviews Neurology, 16(1), 30–42.
[10]. Fung, T. Y., Iyaswamy, A., Sreenivasmurthy, S. G., Krishnamoorthi, S., Guan, X. J., Zhu, Z., Su, C. F., Liu, J., Kan, Y., Zhang, Y., Wong, H. L. X., & Li, M. (2022). Klotho an Autophagy Stimulator as a Potential Therapeutic Target for Alzheimer's Disease: A Review. Biomedicines, 10(3), 705.
[11]. Dubal, D. B., Zhu, L., Sanchez, P. E., Worden, K., Broestl, L., Johnson, E., Ho, K., Yu, G. Q., Kim, D., Betourne, A., Kuro-O, M., Masliah, E., Abraham, C. R., & Mucke, L. (2015). Life extension factor klotho prevents mortality and enhances cognition in hAPP transgenic mice. The Journal of neuroscience: the official journal of the Society for Neuroscience, 35(6), 2358–2371.
[12]. Massó, A., Sánchez, A., Gimenez-Llort, L., Lizcano, J. M., Cañete, M., García, B., Torres-Lista, V., Puig, M., Bosch, A., & Chillon, M. (2015). Secreted and Transmembrane αKlotho Isoforms Have Different Spatio-Temporal Profiles in the Brain during Aging and Alzheimer's Disease Progression. PloS one, 10(11), e0143623.
[13]. Zeng, C. Y., Yang, T. T., Zhou, H. J., Zhao, Y., Kuang, X., Duan, W., & Du, J. R. (2019). Lentiviral vector-mediated overexpression of Klotho in the brain improves Alzheimer's disease-like pathology and cognitive deficits in mice. Neurobiology of Aging, 78, 18–28.
[14]. Castner, S. A., Gupta, S., Wang, D., Moreno, A. J., Park, C., Chen, C., Poon, Y., Groen, A., Greenberg, K., David, N., Boone, T., Baxter, M. G., Williams, G. V., & Dubal, D. B. (2023). Longevity factor klotho enhances cognition in aged nonhuman primates. Nature aging, 3(8), 931–937.
[15]. Aykac, A., & Sehirli, A. Ö. (2021). The Function and Expression of ATP-Binding Cassette Transporters Proteins in the Alzheimer's Disease. Global medical genetics, 8(4), 149–155.
[16]. Wahrle S.E., Jiang H., Parsadanian M., Kim J., Li A., Knoten A., Jain S., Hirsch-Reinshagen V., Wellington C.L., Bales K.R., et al. (2008) Overexpression of ABCA1 reduces amyloid deposition in the PDAPP mouse model of Alzheimer disease. J. Clin. Investig. 118:671–682.
[17]. Hanson, K., Fisher, K., & Hooper, N. M. (2021). Exploiting the neuroprotective effects of α-klotho to tackle ageing- and neurodegeneration-related cognitive dysfunction. Neuronal signaling, 5(2), NS20200101.
Cite this article
Chen,E.Y.;Zhu,S. (2025). Klotho in Alzheimer's Disease: Evaluating Therapeutic Potential and Addressing Experimental Challenges. Theoretical and Natural Science,78,60-66.
Data availability
The datasets used and/or analyzed during the current study will be available from the authors upon reasonable request.
Disclaimer/Publisher's Note
The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of EWA Publishing and/or the editor(s). EWA Publishing and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
About volume
Volume title: Proceedings of the 4th International Conference on Biological Engineering and Medical Science
© 2024 by the author(s). Licensee EWA Publishing, Oxford, UK. This article is an open access article distributed under the terms and
conditions of the Creative Commons Attribution (CC BY) license. Authors who
publish this series agree to the following terms:
1. Authors retain copyright and grant the series right of first publication with the work simultaneously licensed under a Creative Commons
Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this
series.
2. Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the series's published
version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial
publication in this series.
3. Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and
during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See
Open access policy for details).
References
[1]. Ising C., Venegas C., Zhang S., Scheiblich H., Schmidt S.V., Vieira-Saecker A., Schwartz S., Albasset S., McManus R.M., Tejera D., et al. (2019) NLRP3 inflammasome activation drives tau pathology. Nature. 575:669–673.
[2]. Nalivaeva, N. N., & Turner, A. J. (2019). Targeting amyloid clearance in Alzheimer's disease as a therapeutic strategy. British Journal of Pharmacology, 176(18), 3447–3463.
[3]. Hansen D.V., Hanson J.E., Sheng M. (2018) Microglia in Alzheimer’s disease. J. Cell Biol. 217:459–472. doi: 10.1083/jcb.201709069.
[4]. Deane R., Bell R.D., Sagare A., Zlokovic B.V. (2009) Clearance of amyloid-beta peptide across the blood-brain barrier: Implication for therapies in Alzheimer’s disease. CNS Neurol. Disord. Drug Targets. 8:16–30.
[5]. Chiu, C., Miller, M. C., Monahan, R., Osgood, D. P., Stopa, E. G., & Silverberg, G. D. (2015). P-glycoprotein expression and amyloid accumulation in human aging and Alzheimer's disease: preliminary observations. Neurobiology of aging, 36(9), 2475–2482.
[6]. Kuro-o, M., Matsumura, Y., Aizawa, H., Kawaguchi, H., Suga, T., Utsugi, T., Ohyama, Y., Kurabayashi, M., Kaname, T., Kume, E., Iwasaki, H., Iida, A., Shiraki-Iida, T., Nishikawa, S., Nagai, R., & Nabeshima, Y. I. (1997). Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature, 390(6655), 45–51.
[7]. Tan M.S., Yu J.T., Jiang T., Zhu X.C., Tan L. (2013) The NLRP3 inflammasome in Alzheimer’s disease. Mol. Neurobiol. 48:875–882.
[8]. Zhao, Y., Zeng, C. Y., Li, X. H., Yang, T. T., Kuang, X., & Du, J. R. (2020). Klotho overexpression improves amyloid-β clearance and cognition in the APP/PS1 mouse model of Alzheimer's disease. Aging cell, 19(10), e13239.
[9]. Greenberg, S. M., Bacskai, B. J., Hernandez-Guillamon, M., Pruzin, J., Sperling, R., & van Veluw, S. J. (2020). Cerebral amyloid angiopathy and Alzheimer disease – One peptide, two pathways. Nature Reviews Neurology, 16(1), 30–42.
[10]. Fung, T. Y., Iyaswamy, A., Sreenivasmurthy, S. G., Krishnamoorthi, S., Guan, X. J., Zhu, Z., Su, C. F., Liu, J., Kan, Y., Zhang, Y., Wong, H. L. X., & Li, M. (2022). Klotho an Autophagy Stimulator as a Potential Therapeutic Target for Alzheimer's Disease: A Review. Biomedicines, 10(3), 705.
[11]. Dubal, D. B., Zhu, L., Sanchez, P. E., Worden, K., Broestl, L., Johnson, E., Ho, K., Yu, G. Q., Kim, D., Betourne, A., Kuro-O, M., Masliah, E., Abraham, C. R., & Mucke, L. (2015). Life extension factor klotho prevents mortality and enhances cognition in hAPP transgenic mice. The Journal of neuroscience: the official journal of the Society for Neuroscience, 35(6), 2358–2371.
[12]. Massó, A., Sánchez, A., Gimenez-Llort, L., Lizcano, J. M., Cañete, M., García, B., Torres-Lista, V., Puig, M., Bosch, A., & Chillon, M. (2015). Secreted and Transmembrane αKlotho Isoforms Have Different Spatio-Temporal Profiles in the Brain during Aging and Alzheimer's Disease Progression. PloS one, 10(11), e0143623.
[13]. Zeng, C. Y., Yang, T. T., Zhou, H. J., Zhao, Y., Kuang, X., Duan, W., & Du, J. R. (2019). Lentiviral vector-mediated overexpression of Klotho in the brain improves Alzheimer's disease-like pathology and cognitive deficits in mice. Neurobiology of Aging, 78, 18–28.
[14]. Castner, S. A., Gupta, S., Wang, D., Moreno, A. J., Park, C., Chen, C., Poon, Y., Groen, A., Greenberg, K., David, N., Boone, T., Baxter, M. G., Williams, G. V., & Dubal, D. B. (2023). Longevity factor klotho enhances cognition in aged nonhuman primates. Nature aging, 3(8), 931–937.
[15]. Aykac, A., & Sehirli, A. Ö. (2021). The Function and Expression of ATP-Binding Cassette Transporters Proteins in the Alzheimer's Disease. Global medical genetics, 8(4), 149–155.
[16]. Wahrle S.E., Jiang H., Parsadanian M., Kim J., Li A., Knoten A., Jain S., Hirsch-Reinshagen V., Wellington C.L., Bales K.R., et al. (2008) Overexpression of ABCA1 reduces amyloid deposition in the PDAPP mouse model of Alzheimer disease. J. Clin. Investig. 118:671–682.
[17]. Hanson, K., Fisher, K., & Hooper, N. M. (2021). Exploiting the neuroprotective effects of α-klotho to tackle ageing- and neurodegeneration-related cognitive dysfunction. Neuronal signaling, 5(2), NS20200101.









