1. Introduction
With significant improvements in medical care and quality of life, people around the world are living longer and longer. Today, the vast majority of people can hope to live into their 60s and beyond. The number of older people in most countries is increasing steadily. As a result, aging has become one of the most serious problems in the world. According to the World Health Organization (WHO), in 2020, there will be 1 billion people aged 60 and over, and that number is expected to rise to 1.4 billion. By 2050, the number of people aged 60 and over will have doubled to a staggering 2.1 billion. Between 2020 and 2050, the number of persons aged 80 and over is expected to more than triple to 426 million [1].
In addition, it is important to emphasize that aging is a key risk factor for most neurodegenerative diseases, including the most common ones, Alzheimer's disease (AD) and Parkinson's disease (PD). The risk of developing Alzheimer's disease increases exponentially with advancing age. More than 6 million Americans age 65 and older may be suffering from Alzheimer's disease. Even more people under the age of 65 are unfortunately suffering from the disease [2]. In 2018, as many as 5.7 million people in the United States were diagnosed with Alzheimer's disease, but unfortunately, there is still no proven cure for the disease, with some treatments only providing partial relief and limited ability to prevent the disease from progressing. Therefore, new AD-related therapies are essential for this situation [2]. Therefore, this paper analyzes one of the therapeutic modalities for Alzheimer's, stem cell therapy, based on the literature review at that time to understand the therapeutic principles and therapeutic effects of stem cell therapy. Alzheimer is considered as one of the untreatable diseases and as the frequency of the disease increases, more is known about the specific manifestations of the disease but not about its treatment options as the research in this paper can promote the popularization of Alzheimer's science.
2. Background
2.1. Alzheimer’s Disease
Alzheimer's disease (AD) is a chronic neurodegenerative disease first discovered and described in 1906 by German psychiatrist and neuropathologist Eros Alzheimer. It manifests itself primarily as progressive dementia, resulting in memory loss and cognitive impairment. Patients usually experience mild memory problems in the early stages, such as forgetting recent events and having difficulty remembering new information. As the disease progresses, symptoms such as language impairment, disorientation (e.g., getting lost), changes in mood and personality, and decreased judgment may gradually appear. In the advanced stage, patients may completely lose the ability to take care of themselves and need round-the-clock care from others [3].
It is caused by synaptic loss, the presence of amyloid-β (Aβ) plaques and tau junctions. Amyloid-β (Aβ) plaques are produced by the breakdown of amyloid precursor proteins by β-secretase and γ-secretase. Amyloid-β is toxic to neurons and increases free radicals in neurons. As a result, it leads to impaired mitochondrial redox activity as well as neuronal dysfunction [4]. Current treatment options mainly include the use of cholinesterase inhibitors (e.g., donepezil, carboplatin, etc.) and NMDA receptor antagonists (e.g., memantine) to ameliorate cognitive symptoms; the use of antidepressants and antipsychotics to treat symptomatic emotional and psychiatric symptoms; and new treatment methods such as immunotherapy and gene therapy are also being actively explored, such as mesenchymal stem cell. In addition, non-pharmacological treatments such as cognitive training, social activities, and healthy lifestyles (including balanced diet, moderate exercise, and adequate sleep) also play a role in slowing down the progression of the disease.
2.2. Mesenchymal Stem Cell
Neural stem cells (NSCs) are an approach that has received much attention and has significant research value today. NSCs are a class of cells with self-renewal ability and multidirectional differentiation potential, and they are capable of differentiating into various neuronal cell types such as neurons, astrocytes and oligodendrocytes. However, at present, neural stem cells mainly play the role of immunomodulators rather than direct neuronal replacement in practical applications. By immunomodulators, we mean drug therapies that can alter the body's immune response. In addition, Mesenchymal stem cells (MSCs) have an important role in therapy, but they do not integrate perfectly with host tissues and their therapeutic effect depends mainly on paracrine activity, which makes it necessary to explore new stem cells for the treatment of neurodegenerative diseases.
Mesenchymal stem cells (MSCs) are pluripotent stem cells found in adult bone marrow with strong differentiation ability, and their use in the treatment of AD and many central nervous system diseases is due to some of their own characteristics. MSCs have a wide range of origins, and they were initially isolated from the bone marrow and were thought to be bone marrow stromal cells. In subsequent studies, it was found that MSCs can also be derived from other tissues and can differentiate into various cell types such as osteoblasts, chondrocytes, adipocytes, muscle cells and neural cells under appropriate conditions [4]. MSCs have several significant advantages. First, MSCs are relatively easy to obtain and can be obtained from a variety of sources such as adipose tissue, bone marrow, umbilical cord blood and placenta [5]. In addition, MSCs have low expression of major histocompatibility complex (MHC) class I and class II molecules, which makes them less immunogenic and facilitates cell transplantation. While embryonic stem cells may trigger a strong immune response after transplantation and even risk tumor formation, MSCs are much less immunogenic and potentially risky, which improves safety to a certain extent. Meanwhile, MSCs also have neuroprotective effects, such as regulating ubiquitinated proteins and neuroinflammation, thus promoting neurogenesis and effectively improving neurological functional status.
3. Role of MSCs in Treating AD
According to the preclinical research. MSCs can improve several parts of the AD model. First, by reducing A𝛽 deposition and provoking its clearance in AD-treated animal models, the memory deficits can be alleviated. Similarly, MSCs can trigger autophagy which can decrease the levels of A𝛽 of pathological neurons in an AD mice model. In addition, MSCs can increase the level of acetylcholine which are essential neurotransmitter by regulating the expression of choline acetyltransferase and acetylcholinesterase. Microglial activation is a key element of neuroinflammation, it also plays a role in neuroinflammation which is the key pathogenesis of AD [6]. To be more specific, microglial processes have connections with neuronal synapses, prolonged activation of microglia related to AD induce synaptic toxicity, and accelerates neuronal loss. However, MSCs can reduce microglial activation by changing M1 phenotype, which will produce proinflammatory cytokine to M2 phenotype, which have an anti-inflammatory effect. This is proved in mouse mice cortexes. Finally, MSCs can upregulate the levels of neurotrophic factors, like the vascular endothelial growth factor (VEGF). When the brains of AD “patient” received MSCs, their neurons and neuronal integrity is improved.
Figure 1 shows in graphic form the steps of a multifunctional neural stem cell therapy for Alzheimer's disease. First, NSCs need to be genetically modified to stably express the key Aβ degrading protease NEP, which improves clearance and resistance. expression of NEP at the cell membrane consistently degrades Aβ in the brain, which improves the survival of NSCs in the AD microenvironment (blue arrows). Nanopreparations were then used to increase the efficiency of neuronal differentiation of NSC (red arrows) and to guide cell transplantation in vivo (black arrows). The use of SOX9 siRNA expression plasmid and retinoic acid (RA) enhances the efficiency of neuronal differentiation of NSC in pathological AD microenvironment by synergistically regulating Wnt/β-catenin and RA signaling pathways [7].
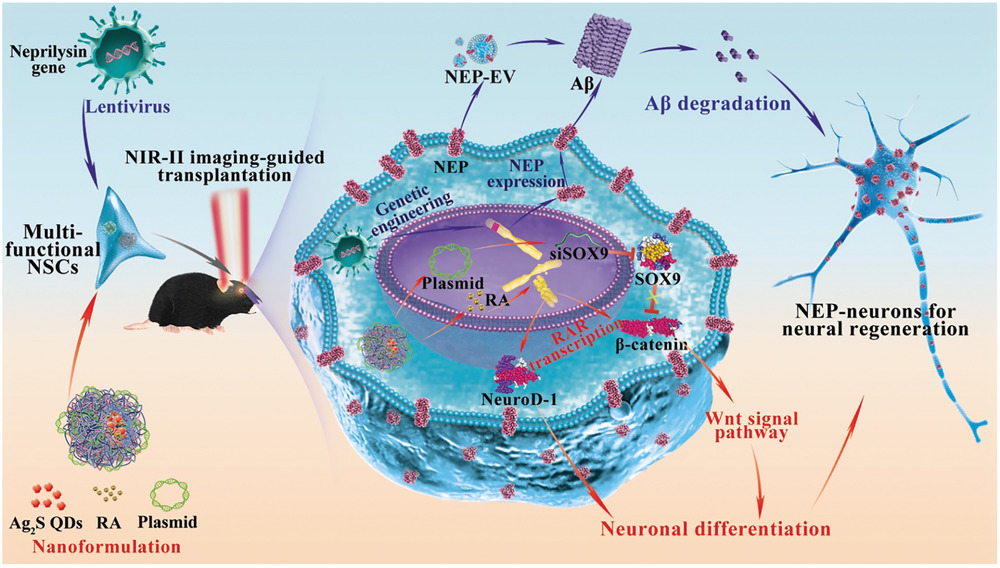
Figure 1. Multifunctional neural stem cell therapy for Alzheimer's disease [7]
3.1. Role of MSCs in Neuron Autophagy
By increasing the autophagy activity in neuron to increase the Aβ clearance is an indirect way to treat the AD, but that is what make it special. Autophagy is the main cellular mechanism for degrading and recycling intracellular proteins and organelles under different physiological and pathological conditions [8]. BECN1 and LC3-II play a key role in autophagy. It regulates the autophagy-promoting activity of PIK3C3/VPS34,16, but the level of this is reduced in AD patient. According to in vitro and in vivo model, fortunately, MSCs can upregulate the expression of LC3-II (By monitoring LC3 expression) and exert neuroprotective effects through enhancement of autophagy pathway-dependent Aβ clearance. Additionally, MSCs regulates BECN1 expression to regulate neuronal survival against Aβ toxicity [9].
3.2. Role of MSC-derived secretome and extracellular vehicles (EVs) in AD
MSC-derived secretome refers to the whole range of bioactive molecules secreted by MSCs, including proteins, growth factors, cytokines, chemokines, microRNAs (miRNAs), etc. These molcules can be encapsulated into EVs, which are small membrane structures secreted by cells, including exosomes and microvesicles. In this case MSCs act as “drug stores” that secrete neuroprotective agents which are the actual effectors of the therapeutic effects observed. EVs can deliver beneficial molecules directly to recipient cells to regulate cellular functions. Thus, researchers explored the amelioration in cognitive decline by observing the animal model through intracerebral, intravenous, or intranasal administration. Many studies reported reduced plaque deposition and Aβ levels. It can also regulate the inflammatory response which has discussed above.
4. Conclusion
In summary, there are plenty of in vitro and in vivo researches shows that MSCs is a potential therapy for AD, but it also faces some challenges. Although MSCs shows a clear influence on animal model, according to clinical experiment, there is no obvious improvement on the AD patient. However, these experiments indicated that MSCs are safe and feasible in clinical trials which is also an important factor in new therapy. For MSCs use as modulating the neural autophagy, the challenges may be to Develop drugs that specifically modulate autophagy. Since, it is various in different individuals, also the regulatory mechanism of autophagy is complex, it involved multiple signaling pathways. For the MSC-derived secretome and EVs, Standardized processes for MSC culture and EVs extraction need to be established to ensure consistency and reproducibility of therapeutic products. Also, the further clinical experiment needs to be carried to examine the effectiveness and safety of this therapy.
While MSCs present a safe and feasible treatment for AD, further investigation is required to optimize their therapeutic potential. The development of targeted drugs, standardized MSCs culture methods, and extensive clinical trials will be essential in harnessing the full therapeutic potential of MSCs in combating this debilitating disease.
However, the research in this paper has certain shortcomings, including the number of references cited, and the related experimental studies need to be further enhanced and optimized.
References
[1]. WHO, https://www.who.int/news-room/fact-sheets/detail/ageing-and-health
[2]. Hou, Y., Dan, X., Babbar, M., Wei, Y., Hasselbalch, S. G., Croteau, D. L., & Bohr, V. A. (2019). Ageing as a risk factor for neurodegenerative disease. Nature Reviews Neurology, 15(10), 565–581. https://doi.org/10.1038/s41582-019-0244-7
[3]. Andrzejewska, A., Dabrowska, S., Lukomska, B., & Janowski, M. (2021). Mesenchymal Stem Cells for Neurological Disorders. Advanced Science, 8(7), 2002944. https://doi.org/10.1002/advs.202002944
[4]. Shin, J. Y., Park, H. J., Kim, H. N., Oh, S. H., Bae, J.-S., Ha, H.-J., & Lee, P. H. (2014). Mesenchymal stem cells enhance autophagy and increase β-amyloid clearance in Alzheimer disease models. Autophagy, 10(1), 32–44. https://doi.org/10.4161/auto.26508
[5]. Zhu, Y., Ge, J., Huang, C., Liu, H., & Jiang, H. (2021). Application of mesenchymal stem cell therapy for aging frailty: From mechanisms to therapeutics. Theranostics, 11(12), 5675–5685. https://doi.org/10.7150/thno.46436
[6]. Leng, F., Edison, P. Neuroinflammation and microglial activation in Alzheimer disease: where do we go from here?. Nat Rev Neurol 17, 157–172 (2021). https://doi.org/10.1038/s41582-020-00435-y
[7]. Huang, D., Cao, Y., Yang, X., Liu, Y., Zhang, Y., Li, C., ... & Wang, Q. (2021). A nanoformulation‐mediated multifunctional stem cell therapy with improved beta‐amyloid clearance and neural regeneration for Alzheimer's disease. Advanced Materials, 33(13), 2006357.
[8]. Ceccariglia, S., Cargnoni, A., Silini, A. R., & Parolini, O. (2020). Autophagy: A potential key contributor to the therapeutic action of mesenchymal stem cells. Autophagy, 16(1), 28–37. https://doi.org/10.1080/15548627.2019.1630223
[9]. Giovannelli, L. (2023). Mesenchymal stem cell secretome and extracellular vesicles for neurodegenerative diseases: Risk-benefit profile and next steps for the market access. Bioactive Materials.
Cite this article
Xu,Q. (2025). Analysis of mesenchymal stem cells in Alzheimer’s disease. Theoretical and Natural Science,76,90-94.
Data availability
The datasets used and/or analyzed during the current study will be available from the authors upon reasonable request.
Disclaimer/Publisher's Note
The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of EWA Publishing and/or the editor(s). EWA Publishing and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
About volume
Volume title: Proceedings of the 4th International Conference on Biological Engineering and Medical Science
© 2024 by the author(s). Licensee EWA Publishing, Oxford, UK. This article is an open access article distributed under the terms and
conditions of the Creative Commons Attribution (CC BY) license. Authors who
publish this series agree to the following terms:
1. Authors retain copyright and grant the series right of first publication with the work simultaneously licensed under a Creative Commons
Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this
series.
2. Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the series's published
version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial
publication in this series.
3. Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and
during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See
Open access policy for details).
References
[1]. WHO, https://www.who.int/news-room/fact-sheets/detail/ageing-and-health
[2]. Hou, Y., Dan, X., Babbar, M., Wei, Y., Hasselbalch, S. G., Croteau, D. L., & Bohr, V. A. (2019). Ageing as a risk factor for neurodegenerative disease. Nature Reviews Neurology, 15(10), 565–581. https://doi.org/10.1038/s41582-019-0244-7
[3]. Andrzejewska, A., Dabrowska, S., Lukomska, B., & Janowski, M. (2021). Mesenchymal Stem Cells for Neurological Disorders. Advanced Science, 8(7), 2002944. https://doi.org/10.1002/advs.202002944
[4]. Shin, J. Y., Park, H. J., Kim, H. N., Oh, S. H., Bae, J.-S., Ha, H.-J., & Lee, P. H. (2014). Mesenchymal stem cells enhance autophagy and increase β-amyloid clearance in Alzheimer disease models. Autophagy, 10(1), 32–44. https://doi.org/10.4161/auto.26508
[5]. Zhu, Y., Ge, J., Huang, C., Liu, H., & Jiang, H. (2021). Application of mesenchymal stem cell therapy for aging frailty: From mechanisms to therapeutics. Theranostics, 11(12), 5675–5685. https://doi.org/10.7150/thno.46436
[6]. Leng, F., Edison, P. Neuroinflammation and microglial activation in Alzheimer disease: where do we go from here?. Nat Rev Neurol 17, 157–172 (2021). https://doi.org/10.1038/s41582-020-00435-y
[7]. Huang, D., Cao, Y., Yang, X., Liu, Y., Zhang, Y., Li, C., ... & Wang, Q. (2021). A nanoformulation‐mediated multifunctional stem cell therapy with improved beta‐amyloid clearance and neural regeneration for Alzheimer's disease. Advanced Materials, 33(13), 2006357.
[8]. Ceccariglia, S., Cargnoni, A., Silini, A. R., & Parolini, O. (2020). Autophagy: A potential key contributor to the therapeutic action of mesenchymal stem cells. Autophagy, 16(1), 28–37. https://doi.org/10.1080/15548627.2019.1630223
[9]. Giovannelli, L. (2023). Mesenchymal stem cell secretome and extracellular vesicles for neurodegenerative diseases: Risk-benefit profile and next steps for the market access. Bioactive Materials.









