1. Introduction
1.1. Background
Lipid droplet-accumulating microglia (LDAM), having distinct microglial states, are defined as having a striking buildup of lipid droplets [1]. Aging and other factors can lead to microglial transition to a persistent, detrimental, and harmful LDAM condition that is evolutionarily preserved. Specifically, microglial energetics and lipid processing are altered [6].
Lots of hypotheses including genetic factors related to lipid accumulation have been suggested as contributing factors to Alzheimer’s disease. Experiments and analyses of large scale genome-wide association have revealed APOE4 as the most potent genetic risk element for non-familial Alzheimer’s disease, while APOE2 stands out as the most robust genetic protective element [4].
Demyelination is a prevalent initiating factor in neurologic disorders, with microglia being instrumental in the clearance of lipid-laden myelin fragments subsequent to myelin damage. However, when there is an excessive breakdown of myelin, it may surpass the microglia's capacity to remove these substances, resulting in the accumulation of fatty acids and the buildup of pathogenic, lipid-enriched myelin remnants within the microglia [3].
1.2. Previous research
A recent study inspired by the original description of AD proved that lipid metabolism in brain tissue is influenced by APOE, a gene involved in the manufacture of proteins that help carry cholesterol and other types of fats in the bloodstream, and lipid-accumulating glia have negative impact on AD pathogenesis. It also raised the question that whether certain APOE variants act as inhibitors of transition to LDAM by curbing LD accumulation [2]. A growing amount of evidence shows that APOE2 shields against Alzheimer's disease (AD) via mechanisms that are both dependent and independent of amyloid-β (Aβ) [1].
In another study, characteristics of harmful LDAM have been identified, which encompass a decline in phagocytic capability, heightened production of reactive oxygen species, and the release of proinflammatory cytokines.
1.3. Outline of Experimentation
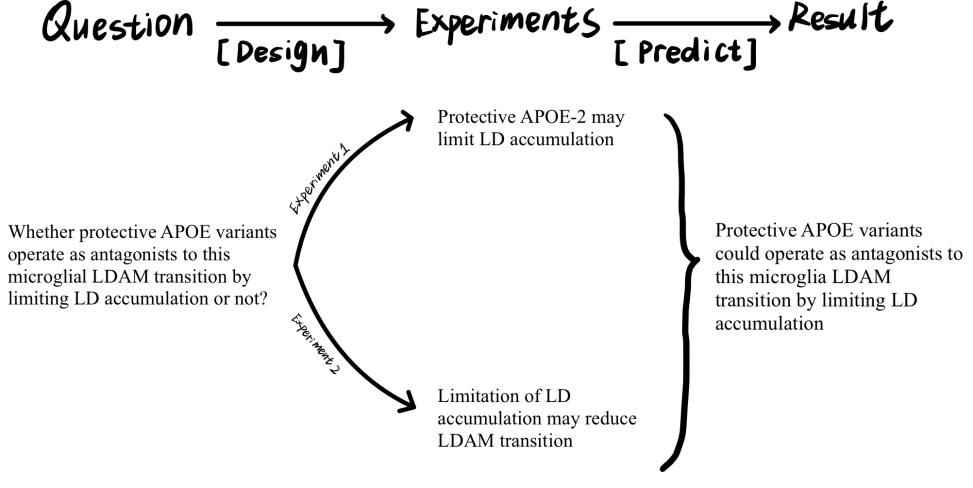
Figure 1. My research plan
This proposal will design experiments to solve this question in two steps based on previous research and explore the mechanism of it, informing potential therapeutic strategies for Alzheimer’s disease.
2. Methods
2.1. Western blotting
In the experiment outlined, the laser is set to a frequency of 2845 cm-1 to identify neutral lipids, which facilitates the visualization of CH2 symmetric stretching vibrations. The process involves the sequential capture of CARS and fluorescence/transmission images. For measuring CARS signal within microglia, a random sample of 20 microglia from the dentate gyrus of each specimen is imaged. The experiment then calculates the ratio of TMEM119+ microglia to CAS+ vesicles within the entire population of TMEM119+ microglia [1].
2.2. CARS Imaging
In this experiment mentioned below, the laser is tuned to 2845 cm−1 to identify neutral lipids, so that the symmetrical tensile oscillations of CH2 can be visualized. CARS and fluorescence/transmission images are obtained in order. Then randomly chosen microglia of each subject are then visualized to quantify the CARS signal in microglia. Finally, determine the proportion of TMEM119+ microglia containing CARS-positive vesicles relative to the overall count of TMEM119+ microglia [1].
2.3. Prefrontal cortex histochemical and fluorescent immunoassay techniques
The prefrontal cortex from each subject is extracted and immediately submerged in a 4% paraformaldehyde solution at 4°C for a duration of 24 hours. Subsequently, the samples are moved to a 30% sucrose solution prepared in 1× PBS and kept at 4°C until the tissues become fully submerged in their containers. The tissues are then embedded in OCT compound, preserved at −80°C, and sectioned using a cryostat. To prepare the free-floating 50-micrometer-thick sections for immunofluorescence, they are rinsed three times in PBST. Following this, they are blocked with a 5% donkey serum solution in PBST for 1 hour. The sections are then incubated in PBS for a period of 72 hours at 4°C. After the primary antibody incubation, the sections are rinsed again three times in PBST and subsequently incubated in PBS along with the appropriate secondary antibodies for 2 hours at room temperature. The sections are then treated with DAPI for 10 minutes, rinsed three more times with PBST, and finally mounted onto microscope slides using ProLong Glass mounting media. [2].
3. Experiments Design and Expected Results
3.1. Animals
The mice will be divided into 8 groups:
1) mice in early phases of Alzheimer’s disease carrying APOE2 genotypes(n = 5)
2) mice in early phases of Alzheimer’s disease carrying APOE3 genotypes (n = 5)
3) mice in early phases of Alzheimer’s disease carrying APOE4 genotypes (n = 5)
4) mice in advanced stage of Alzheimer’s disease with APOE2 genotypes(n = 5)
5) mice in advanced stage of Alzheimer’s disease with APOE3 genotypes(n = 5)
6) mice in advanced stage of Alzheimer’s disease with APOE4 genotypes (n = 10)
Groups 1-6 will be used to elaborate that APOE2 could limit lipid accumulation. Then fresh-frozen frontal cortex adjacent to the tissue are made to prepare for the following experiment to prove that limited lipid accumulation could reduce microglial LDAM transition.
3.2. APOE2 could limit lipid accumulation
3.2.1. APOE2 could efficiently clear myelin debris
Initially, we employ a model of demyelination triggered by toxin, specifically through the administration of cuprizone (CPZ). This method is chosen for its ability to effectively stimulate microglial activity and initiate reactivity without harming the blood-brain barrier. Experimental mice harboring APOE2,APOE3, and APOE4 are assigned to either a regular diet or one enriched with 0.2% CPZ over a four-week period to cause significant demyelination in the central nervous system. Following this, immunohistochemical staining is performed with a certain antibody for myelin basic protein (MBP). Consistent with previous findings, it is anticipated that the CPZ-treated mice will show a marked reduction within MBP immunoreactivity within corpus callosum, a region highly prone to demyelination, compared to the control group maintained on a standard diet. In the other group, which is fed a common diet, the level of myelination in the CC region is likely to be consistent across all APOE genotypes.
In order to evaluate the impact of APOE isoforms on the removal of damaged myelin, brain tissue pieces might be subjected to immunostaining using antibodies that recognize the breakdown products of myelin basic protein (dMBP). The absence of dMBP detection in control APOE-TR mice, coupled with the detection of minimal dMBP staining within the corpus callosum (CC) in APOE2-TR mice, suggests that the clearance of myelin debris has been effectively managed. Conversely, the presence of moderately increased dMBP immunoreactivity in APOE3 mice compared to the APOE2 group, and a significant buildup in APOE4 mice, indicates that the APOE isoforms contribute to myelin debris clearance following demyelination in a manner that is dependent on the specific isoform. [3].
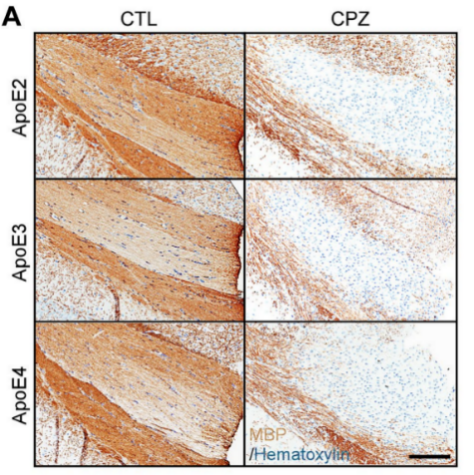
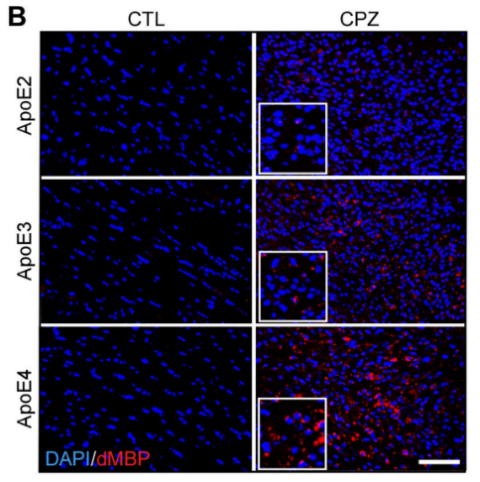
(a) Representative images of MBP (b) Representative images of dMBP
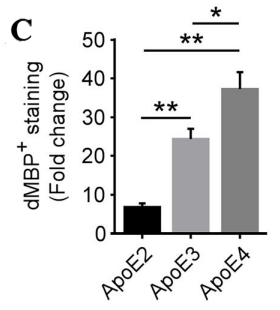
(c)Predicted figure of dMBP staining
Figure 2. Results in the demyelination model [3]
Figure 2 illustrates the dietary regimen of APOE-TR mice, which are either maintained on a standard diet (CTL group) or a diet supplemented with cuprizone (CPZ group) for a period of 4 weeks. (a) The preservation of myelin in the CC is assessed through immunostaining with an antibody against MBP. The figure displays sample images of this area. (b) The presence of myelin fragments is investigated through immunostaining using antibodies for dMBP. The figure presents sample images of dMBP staining, with dMBP depicted in red and DAPI in blue. c) The extent of dMBP immunoreactivity in the CC, as indicated by the percentage of area covered, is analyzed via immunofluorescence staining, while the proportional change between the CPZ and CTL groups is measured. [3].
3.2.2. Microgliosis is significantly increased in apoE2TR mice after sudden loss of myelin sheath
In order to evaluate the reaction of microglia to clopidogrel (CPZ) exposure, we could analyze ionized calcium-binding adapter molecule 1 (Iba1), in APOE-TR mice using immunohistochemical (IHC) staining and Western blotting techniques. It is possible that we will observe variations in the number of Iba1+ microglia among different APOE subtypes in mice that have been treated with CPZ. Specifically, a significant increase in Iba1+ immunoreactivity might be noted in APOE2-TR mice post-CPZ treatment, while a less pronounced enhancement could be seen in the APOE4-TR mice. Similarly, it seems that APOE2-TR mice exhibit increased Iba1 protein levels in comparison to those of APOE3-TR and APOE4-TR mice. There might be an inverse relationship between the intensity of microglial signals and the quantity of myelin debris in APOE-TR mice. This suggests that apoE2-expressing microglia are particularly reactive to sudden loss of myelin sheath, while apoE4-expressing microglia show a diminished capacity to respond to neuronal damage during CPZ-induced demyelination.
In summary, apoE2-expressing microglia exhibit a heightened capability to process myelin lipids and are more active and efficient in the clearance of lipid-rich myelin debris.
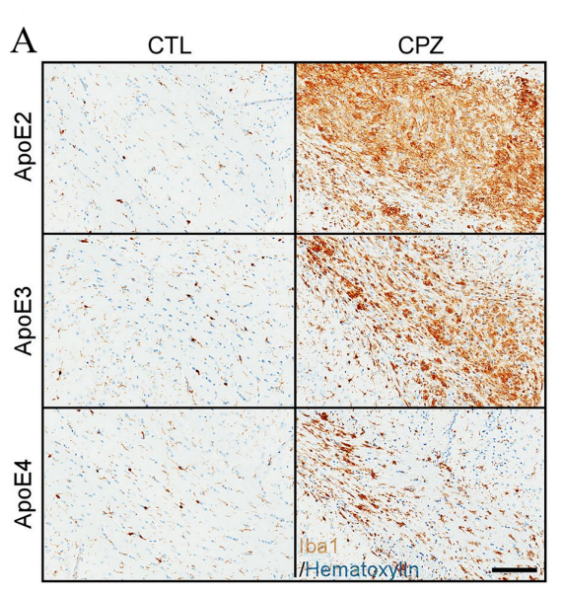
(a) Representative images of Iba1 immunoreactivity
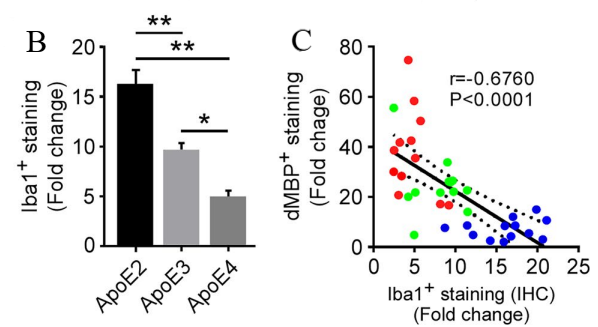
(b)Differences in Iba+ staining (c)The relationship between dMBP+ staining and Iba+ staining
Figure 3. Differences in Iba1+ immunoreactivity upon demyelination [3]
Figure 3 presents the following findings: (a) and (b) depict the examination of microglia within the corpus callosum (CC) of control and clopidogrel (CPZ)-treated apoE-TR mice through immunohistochemistry (IHC) for the Iba1 protein. The displayed images are representative of the Iba1 immunoreactivity observed. The fold change in Iba1+ staining (%area) is quantified and compared between CPZ-treated and control (CTL) groups. (c) Fold changes in Iba1+ microglia (IHCs) after CPZ treatment are found to be inversely correlated with fold changes in dMBP+ myelin sheath fragments. The data points are color-coded to represent different apoE isoforms: blue for apoE2, green for apoE3, and red for apoE4. [3].
3.3. Limited lipid accumulation could reduce microglial LDAM transition
The experiment will use fresh-frozen frontal cortex beside the tissue from mice in early stages of Alzheimer’s disease and age and sex-matched mice with advanced Alzheimer’s disease, both with APOE2, APOE3 or APOE4 genotypes.
To ensure that differences in the content of lipid droplets accumulation exist between two experimental groups, we will isolate microglia with CD11b+CD45lo from mice hippocampi and then perform CARS laser-scanning microscopy at a frequency of 2,845 cm−1, which is specific to the frequency of CH2 for neutral lipids and effectively recognizes lipid droplets.
In order to assess the variations in phagocytic capacity, reactive oxygen species (ROS) generation, and immune response signaling between two study groups, we conduct the experiment in the following manner. Initially, we expose them to pHrodo red Zymosan particles. A lower proportion of microglia with Zymosan+ BODIPY+ compared to microglia with Zymosan+ BODIPY- microglia suggests that LDAM exhibits significant impairments in the phagocytosis of Zymosan. Additionally, we utilize a constitutively fluorescent dye (A555) to tag myelin debris and observe that microglia with reduced lipid accumulation engulf fewer A555+ myelin particles in comparison to those with increased lipid accumulation.
Subsequently, we apply CellROX to the microglia, a dye which remains non-luminescent in a decreased environment and brightly fluoresces once oxidized by reactive oxygen species (ROS) in aged hippocampal microglia. In our prediction, higher fluorescence will be shown in microglia with higher lipid accumulation than the other group.
Ultimately, we determine the levels of cytokines present in the supernatant by employing a multiplex array technique, 8 hours post the application of LPS or saline for control purposes. It is anticipated that, under standard conditions (with saline treatment), microglia exhibiting greater lipid accumulation will discharge higher quantities of various cytokines, such as CCL3, CXCL10, and IL-6, when contrasted with the alternative group. [1].
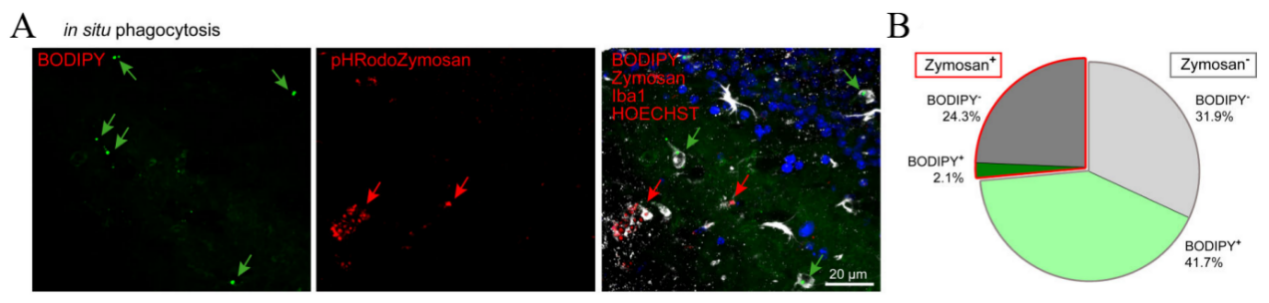
(a) (b) the proportions of Iba1+ cells that contain Zymosan, categorized by BODIPY- and BODIPY+positive.
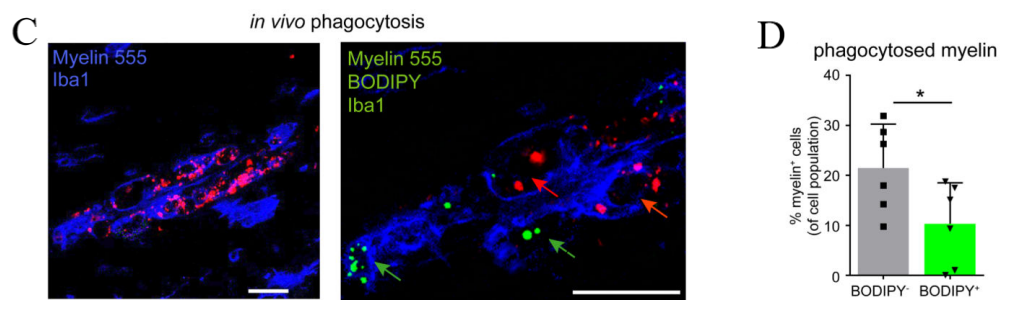
(c) Representative images of myelin (d) Measurement of the amount of myelin that cells have taken in.
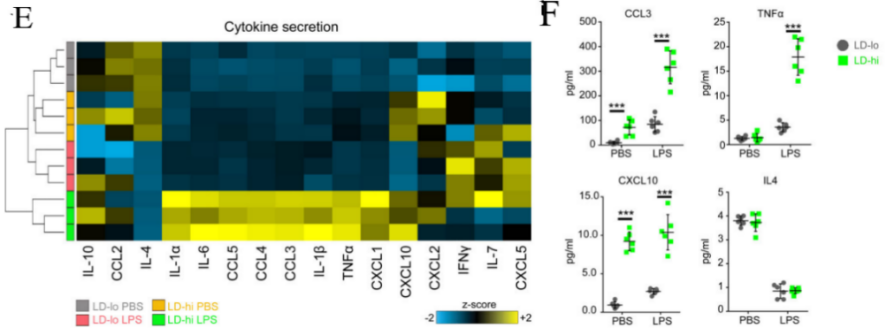
(e) Heatmap of changes in cytokine secretion (f) Graphical representation and measurement of CellROX fluorescence levels
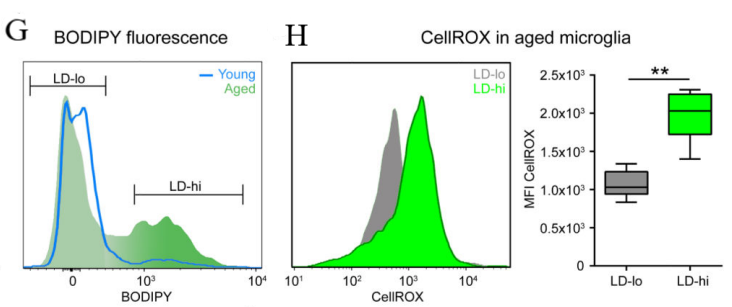
(g) a classification strategy for low lipid (LD-lo) and high lipid (LD-hi) microglia (h)Histogram and quantification of CellROX fluorescence
Figure 4. the proportions of Iba1+ cells that contain Zymosan and are either BODIPY- or BODIPY+. [1]
Figure 4 demonstrates that LPS (100ng/ml) is used to treat isolated microglia with LD-lo and LD-hi lipid content derived from aged mice for 8 hours, and multiplex array is used to measure cytokine concentrations in the medium. Then perform 3 (BV2 cells) or 2 (primary cells) experiments, in triplicate. The figure includes: (g) a gating strategy for distinguishing BODIPYlo (LD-lo) and BODIPYhi (LD-hi) microglia from aged mice, and (h) a histogram along with quantification data for CellROX fluorescence intensity in both LD-lo and LD-hi microglia populations. [1].
An additional experiment could be performed to better prove this prediction. ACSL1, crucial for lipid droplet (LD) formation, serves as a significant biomarker for identifying human astrocytes rich in lipids (LDAM). So an experiment is designed with two fresh-frozen cortex with AD APOE-2. The age, sex and so on are all the same in these cortex. We then add different amount of LD into the two brain tissues. By using the immunofluorescence image, we may probably see the differences between less LD and more LD. If the red ACSL1 is less significant in brain tissue with less LD, it can show that limit LD accumulation could reduce the amount of ACSL1. Then, it causes a reduction in LDAM [2].
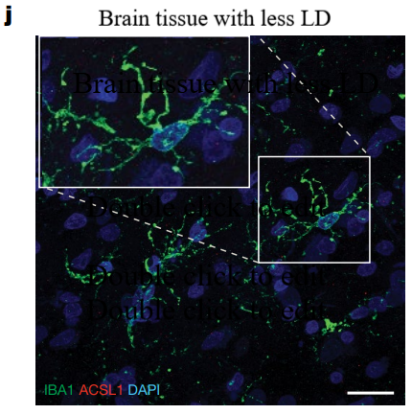
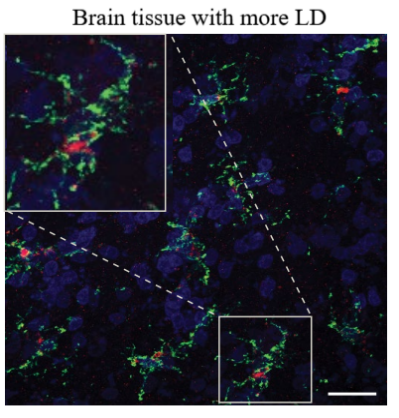
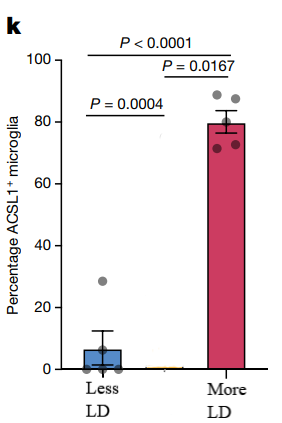
Figure 5. Predicted representative immunofluorescence images and percentage of IBA1+ microglia
As depicted in Figure 5, brain tissues with higher (left side) and lower (right side) lipid droplet (LD) content are subjected to staining for microglial markers: IBA1 is stained green, ACSL1 is stained red, and DAPI is stained blue. (j) Presented are illustrative immunofluorescence photographs of the mouse frontal cortex, which is contiguous with the tissues used in the snRNA-seq study. (k) The figure also includes a quantification of the proportion of ACSL1-positive IBA1+ microglia. The sample size for this analysis is five per group, with each dot in the graph representing data from an individual subject.
4. Discussion
Here I would discuss some related potential therapeutic strategies for Alzheimer’s disease if the conclusion is right.
Initially, a study proposed that molecules capable of engaging with lipid droplets (LDs) as well as the essential autophagosome component LC3 could potentially boost the autophagic breakdown of LDs. Researchers engineered and synthesized these molecules by attaching LC3-binding elements to LD-binding agents through a linking structure. These newly created compounds were found to be highly effective in clearing LDs. Consequently, it is hypothesized that these chimeric autophagy-tethering compounds could be a potential therapeutic approach for treating Alzheimer’s disease by restricting lipid accumulation [5].
Conversely, miR-223 is considered a pivotal regulator of lipid phagocytosis during demyelination. Recent findings reveal that Cathepsin B (CTSB) interacts with miR-223 during autophagy, linking databases of miRNA, mRNA, and autophagy-related genes. Laboratory experiments indicate that miR-223 boosts CTSB levels in BV2 cells, thereby promoting autophagy, diminishing lipid droplet buildup, and decreasing IL-1β levels, a significant pro-inflammatory factor. By suppressing the activity of CTSB, miR-223 promotes the targeted breakdown of lipid droplets. [7].
5. Conclusion
In the proposal, we divide the illustration into two parts: APOE2 could limit lipid accumulation and limited lipid accumulation could lead to reduced LDAM transition. First, we try to prove the first statement by proving that individuals with APOE2 genotypes could efficiently clear myelin debris and microgliosis is dramatically increased upon acute demyelination. Then, we elaborate the second statement in two ways: proving that microglia with less lipid accumulation not exhibiting impairments in phagocytic activity, generating lower amounts of reactive oxygen species (ROS), and emitting reduced quantities of pro-inflammatory cytokines., or directly measuring the amount of LDAM.
References
[1]. Marschallinger J., Iram T., Zardeneta M., Lee S.E., Lehallier B., Haney M.S., Pluvinage J.V., Mathur V., Hahn O., Morgens D.W., Kim J., Tevini J., Felder T.K., Wolinski H., Bertozzi C.R., Bassik M.C., Aigner L.,& Wyss-Coray T.(2020)Lipid droplet accumulating microglia represent a dysfunctional and pro-inflammatory state in the aging brain. Nat Neurosci,23(2):194-208.
[2]. Haney M.S., Pálovics R., Munson C.N., Long C., Johansson P.K., Yip O., Dong W., Rawat E., West E., Schlachetzki J.C.M., Tsai A., Guldner I.H., Lamichhane B.S., Smith A., Schaum N., Calcuttawala K., Shin A., Wang Y.H., Wang C., Koutsodendris N., Serrano G.E., Beach T.G., Reiman E.M., Glass C.K.., Abu-Remaileh M., Enejder A, Huang Y., &Wyss-Coray T.(2024)APOE4/4 is linked to damaging lipid droplets in Alzheimer’s disease microglia. Nature, 628(8006):154-161.
[3]. Wang N., Wang M., Jeevaratnam S., Rosenberg C., Ikezu T.C., Shue F., Doss S.V., Alnobani A., Martens Y.A., Wren M., Asmann Y.W., Zhang B.., Bu G,& Liu C.C.(2022)Opposing effects of apoE2 and apoE4 on microglial activation and lipid metabolism in response to demyelination. Mol Neurodegener,17(1):75.
[4]. Serrano-Pozo A., Das S.,& Hyman B.T.(2021)APOE and Alzheimer’s Disease: Advances in Genetics, Pathophysiology, and Therapeutic Approaches. Lancet Neurol,20(1):68-80.
[5]. Fu Y., Chen N., Wang Z., Luo S., Ding Y., &Lu B.(2021)Degradation of lipid droplets by chimeric autophagy-tethering compounds. Cell Res,31(9):965-979.
[6]. Victor M.B., Leary N., Luna X., Meharena H.S., Scannail A.N., Bozzelli P.L., Samaan G., Murdock M.H., von Maydell D., Effenberger A.H., Cerit O., Wen H.L., Liu L.., Welch G, Bonner M., &Tsai L.H.(2022)Lipid accumulation induced by APOE4 impairs microglial surveillance of neuronal-network activity. Cell Stem Cell,29(8):1197-1212.e8.
[7]. Ma H., Ou Z.L., Alaeiilkhchi N., Cheng Y.Q., Chen K., Chen J.Y., Guo R.Q., He M.Y., Tang S.Y., Zhang X., Huang Z.P., Liu J., Liu J., Zhu Q.A., Huang Z.C., &Jiang H.(2024)MiR-223 enhances lipophagy by suppressing CTSB in microglia following lysolecithin induced demyelination in mice. Lipids Health Dis,23(1):194.
[8]. Jung E.S., &Mook-Jung I.(2020)New Microglia on the Block. Cell Metab, 31(4),664-666.
[9]. Simpson D.S.A., &Oliver P.L.(2020)ROS Generation in Microglia: Understanding Oxidative Stress and Inflammation in Neurodegenerative Disease. Antioxidants (Basel),9(8),743.
[10]. Andrews S.J., Fulton-Howard B., &Goate A.(2019)Protective Variants in Alzheimer’s Disease. Curr Genet Med Rep, 7(1):1-12.
[11]. Li Z., Shue F., Zhao N., Shinohara M.,& Bu G. (2020)APOE2: protective mechanism and therapeutic implications for Alzheimer's disease. Mol Neurodegener,15(1):63.
[12]. Lanfranco M.F., Sepulveda J., Kopetsky G., &Rebeck G.W.(2021)Expression and secretion of apoE isoforms in astrocytes and microglia during inflammation. Glia, 69(6),1478-1493.
[13]. Jackson R.J., Keiser M..S, Meltzer J..C., Fykstra DP.., Dierksmeier SE., Melloni A., Nakajima T., Tecedor L., Ranum P..T, Carrell E., Chen Y., Holtzman D..M., Davidson B.L., Hyman B.T.(2023)APOE2 gene therapy reduces amyloid deposition and improves markers of neuroinflammation and neurodegeneration in a mouse model of Alzheimer disease. bioRxiv [Preprint]. 2023 Aug 16:2023.08.14.552850.
[14]. Huang Y.A., Zhou B., Nabet A.M., Wernig M., Südhof T.C.(2019)Differential Signaling Mediated by ApoE2, ApoE3, and ApoE4 in Human Neurons Parallels Alzheimer's Disease Risk. Neurosci, 39(37):7408-7427.
Cite this article
Zhou,Z. (2025). APOE2 act as inhibitors to microglial LDAM transition by restricting LD accumulation. Theoretical and Natural Science,78,76-84.
Data availability
The datasets used and/or analyzed during the current study will be available from the authors upon reasonable request.
Disclaimer/Publisher's Note
The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of EWA Publishing and/or the editor(s). EWA Publishing and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
About volume
Volume title: Proceedings of the 4th International Conference on Biological Engineering and Medical Science
© 2024 by the author(s). Licensee EWA Publishing, Oxford, UK. This article is an open access article distributed under the terms and
conditions of the Creative Commons Attribution (CC BY) license. Authors who
publish this series agree to the following terms:
1. Authors retain copyright and grant the series right of first publication with the work simultaneously licensed under a Creative Commons
Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this
series.
2. Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the series's published
version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial
publication in this series.
3. Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and
during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See
Open access policy for details).
References
[1]. Marschallinger J., Iram T., Zardeneta M., Lee S.E., Lehallier B., Haney M.S., Pluvinage J.V., Mathur V., Hahn O., Morgens D.W., Kim J., Tevini J., Felder T.K., Wolinski H., Bertozzi C.R., Bassik M.C., Aigner L.,& Wyss-Coray T.(2020)Lipid droplet accumulating microglia represent a dysfunctional and pro-inflammatory state in the aging brain. Nat Neurosci,23(2):194-208.
[2]. Haney M.S., Pálovics R., Munson C.N., Long C., Johansson P.K., Yip O., Dong W., Rawat E., West E., Schlachetzki J.C.M., Tsai A., Guldner I.H., Lamichhane B.S., Smith A., Schaum N., Calcuttawala K., Shin A., Wang Y.H., Wang C., Koutsodendris N., Serrano G.E., Beach T.G., Reiman E.M., Glass C.K.., Abu-Remaileh M., Enejder A, Huang Y., &Wyss-Coray T.(2024)APOE4/4 is linked to damaging lipid droplets in Alzheimer’s disease microglia. Nature, 628(8006):154-161.
[3]. Wang N., Wang M., Jeevaratnam S., Rosenberg C., Ikezu T.C., Shue F., Doss S.V., Alnobani A., Martens Y.A., Wren M., Asmann Y.W., Zhang B.., Bu G,& Liu C.C.(2022)Opposing effects of apoE2 and apoE4 on microglial activation and lipid metabolism in response to demyelination. Mol Neurodegener,17(1):75.
[4]. Serrano-Pozo A., Das S.,& Hyman B.T.(2021)APOE and Alzheimer’s Disease: Advances in Genetics, Pathophysiology, and Therapeutic Approaches. Lancet Neurol,20(1):68-80.
[5]. Fu Y., Chen N., Wang Z., Luo S., Ding Y., &Lu B.(2021)Degradation of lipid droplets by chimeric autophagy-tethering compounds. Cell Res,31(9):965-979.
[6]. Victor M.B., Leary N., Luna X., Meharena H.S., Scannail A.N., Bozzelli P.L., Samaan G., Murdock M.H., von Maydell D., Effenberger A.H., Cerit O., Wen H.L., Liu L.., Welch G, Bonner M., &Tsai L.H.(2022)Lipid accumulation induced by APOE4 impairs microglial surveillance of neuronal-network activity. Cell Stem Cell,29(8):1197-1212.e8.
[7]. Ma H., Ou Z.L., Alaeiilkhchi N., Cheng Y.Q., Chen K., Chen J.Y., Guo R.Q., He M.Y., Tang S.Y., Zhang X., Huang Z.P., Liu J., Liu J., Zhu Q.A., Huang Z.C., &Jiang H.(2024)MiR-223 enhances lipophagy by suppressing CTSB in microglia following lysolecithin induced demyelination in mice. Lipids Health Dis,23(1):194.
[8]. Jung E.S., &Mook-Jung I.(2020)New Microglia on the Block. Cell Metab, 31(4),664-666.
[9]. Simpson D.S.A., &Oliver P.L.(2020)ROS Generation in Microglia: Understanding Oxidative Stress and Inflammation in Neurodegenerative Disease. Antioxidants (Basel),9(8),743.
[10]. Andrews S.J., Fulton-Howard B., &Goate A.(2019)Protective Variants in Alzheimer’s Disease. Curr Genet Med Rep, 7(1):1-12.
[11]. Li Z., Shue F., Zhao N., Shinohara M.,& Bu G. (2020)APOE2: protective mechanism and therapeutic implications for Alzheimer's disease. Mol Neurodegener,15(1):63.
[12]. Lanfranco M.F., Sepulveda J., Kopetsky G., &Rebeck G.W.(2021)Expression and secretion of apoE isoforms in astrocytes and microglia during inflammation. Glia, 69(6),1478-1493.
[13]. Jackson R.J., Keiser M..S, Meltzer J..C., Fykstra DP.., Dierksmeier SE., Melloni A., Nakajima T., Tecedor L., Ranum P..T, Carrell E., Chen Y., Holtzman D..M., Davidson B.L., Hyman B.T.(2023)APOE2 gene therapy reduces amyloid deposition and improves markers of neuroinflammation and neurodegeneration in a mouse model of Alzheimer disease. bioRxiv [Preprint]. 2023 Aug 16:2023.08.14.552850.
[14]. Huang Y.A., Zhou B., Nabet A.M., Wernig M., Südhof T.C.(2019)Differential Signaling Mediated by ApoE2, ApoE3, and ApoE4 in Human Neurons Parallels Alzheimer's Disease Risk. Neurosci, 39(37):7408-7427.









