1. Introduction
Stress memory is based on sustained persistence of a primed condition in absence of stress. Three different types of memory are known to exist regarding the lifespan of plants: somatic, intergenerational, and transgenerational memory[1,2]. Priming usually takes place over a few days or weeks in somatic stress memory. After one generation without stress, the stress imprinted in intergenerational memory is still discernible. However, stress imprints in transgenerational memory remain visible and persistent even after two generations without abiotic stress[3].
Recent findings suggest that environmental cues can trigger events enabling information transfer to the subsequent generation without involving genes[1].Such forms of inter- or transgenerational inheritance have been described for aversive stimuli, such as chronic or early life stress that lead to altered response of the hypothalamic-pituitary-adrenal axis, increased anxiety and depressive-like behaviour in the following generations[4-8]. Behavioral dysregulation is also linked to several other neuropsychiatric disorders including ADHA and autism spectrum disorders.These disorders are caused by genetic factors, but they may also be affected by the environment.Thus, the roles of genetics and environment in determining vulnerability to psychiatric disorders have become more relevant to further analysis[9].
According to the current research, an environmental factor that was shown to lower the risk for various complex diseases, including those affecting the brain, is the combination of physical exercise and cognitive training, also called environmental enrichment[10]. Environmental enrichment(EE) has significant positive effect on LTP and cognitive ability. Environmental enrichment includes abundant social and physical environment which has been proved that could play a key role in memory enhancement. The EE male mice that underwent EE training and emotional expression training, for example, also showed significant enhanced cognitive abilities and synaptic plasticity in their offspring, although these F1 generation mice have never accept the similar EE training. This suggest that the effect caused by environmental enhancement is not only limited on the individual who directly accept the training, but also be passed on to their offspring through genetic transmission.
Studies have found that miR 212/132 in sperm is crucial in regulating this process according to other research.The miR 212/132 is a small non-coding RNA that is involved in the modulation of genes affecting memory and other cognitive processes. While these studies show that miR 212/132 plays a role in the effects of environmental enrichment, the current papers aim to identify and confirm the factors associated with the increase in cognitive levels.
Those approaches gives some insights, but also has drawback, because it is not combinatorial analysis of all genetic and environmental effects.
Therefore, it is the intention of this proposal to delve deeper into the antecedents of memory related to inheritance, more so the ones that maybe linked to the impacts of environmental enhancement. We will focus on the following aspects: Firstly, which specific RNA molecules or other molecules are implicated in the assumed influence of environmental enrichment on the transmission of memory? Second, how and why is the enhanced memory effect only for one generation but not across more generations? This may add the difficulty of genetic mechanisms or the impact of environmental factors on genes expression. Thus, we are planning to make experiments more systematic and comprehensive as to aim at answering these questions and pointing to new prospects and possibilities for research.
2. Methodology procedures and predicted results
2.1. Screening of the RNA
Neuroscience research greatly benefits from single-cell sequencing technologies, which can reveal transcriptional alterations on a cellular level[11]. RNA-seq methods are available for studying many different aspects of RNA biology, including single-cell gene expression, translation (the translatome) and RNA structure (the structurome). Exciting new applications are being explored, such as spatial transcriptomics (spatialomics). Together with new long-read and direct RNA-seq technologies and better computational tools for data analysis, innovations in RNA-seq are contributing to a fuller understanding of RNA biology, from questions such as when and where transcription occurs to the folding and intermolecular interactions that govern RNA function[12]. To perform an initial screening, we plan to conduct RNA sequencing (RNA-seq) on different generations of environmental enrichment (EE) mice, specifically including the F0 generation (i.e., the original mice subjected to environmental enrichment), the F1 generation (the offspring of the F0 generation), the F2 generation (the grandchildren of the F0 generation), and normal control mice (HC). RNA sequencing will allow us to followed RNA expression in different generations of mice that underwent EE intervention systematically to look for the gene expression changes.
Following the RNA sequencing, we shall filter out for RNAs that have increased levels in environmental enrichment mice (F0, F1, F2) as compared to normal control mice (HC). These RNAs may be categorized as being involved in a memory enhancing or cognitive function improving activity since their expression levels are elevated. Next, we will perform differential analysis on these RNAs, defining the differences as: D1 = F0–HC, D2 = F1–F0, and D3 = F2–F1. This approach will enable us to determine the relative amount of changes in the RNA levels of mice belonging to different generations.
Our selection criteria are set as follows: D1 > D2 > D3. This means that, we anticipate RNA levels to be considerably higher in the F0 generation compared to HC, lower in the F1 generation compared to F0, and even lower in the F2 generation as compared to F1. This decreasing pattern supports the hypothesis that the memory inheritance effect weakens gradually across generations. The RNAs that will be considered the potential target factors which might be involved in mediating the memory inheritance effects due to environmental enrichment are those that meet this criterion. However, various RNA effects can also be observed in experiments, so we have also developed corresponding predictive models, as shown in Figure 1.
Fig.1(a) is the expected model of decreasing RNA expression from generation to generation as a screening criterion. Irregular expression changes will also be obtained after RNA sequencing, as shown in Fig.1(b). According to the analysis, RNAs similar to this change are RNAs that are not relevant to this study. Fig.1(c) shows the expression results of RNA completely opposite to the standard. This kind of RNA is a factor that inhibits LTP level and also has genetic effect. The RNAs with a constant level of RNA expression and no genetic effect in the genetic aspect of neural memory (see Fig.1(d)).
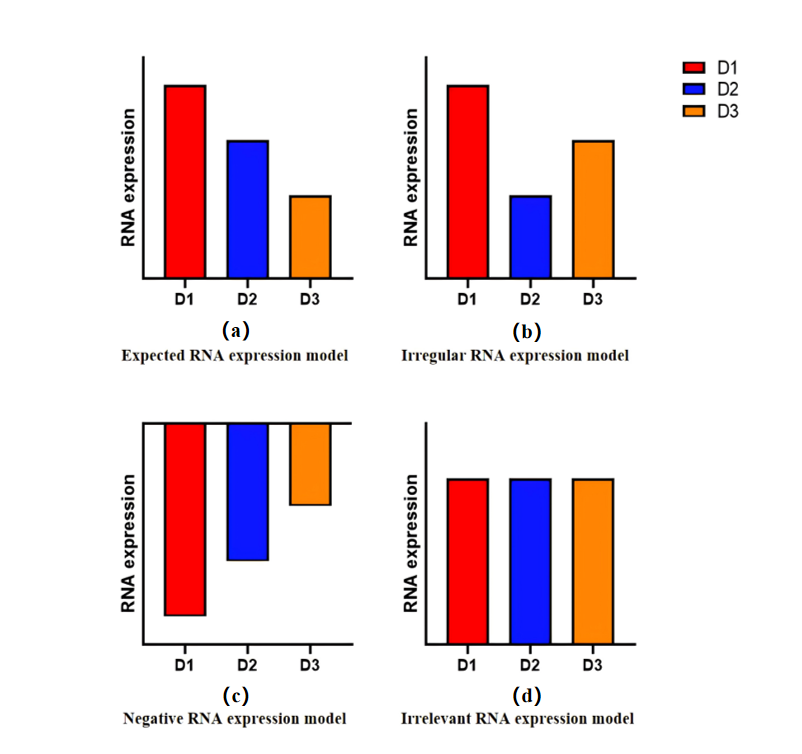
Figure 1. Possible RNA Expression Results
After that, we will proceed with the second step of the screening, divided into two experimental groups:
First Group: Firstly, the ordinary control male mice (HC) will be trained for ten weeks so as to become environmental enrichment mice (EE). These EE mice will be crossed with ordinary control female mice to generate the F1 generation of mice. After this the F1 generation mice will be crossed with ordinary control female mice to obtained the F2 generation. The purpose of this group is to investigate the long-term effects of environmental enrichment on memory and cognitive function using genetic transmittance techniques.
Second Group: Ordinary control mice (HC) will be used as F0 generation and will be mated to generate F1 and F2 generation. This group of mice will therefore be used to compare the differences that will be observed in the first group of mice.
The F2 generation mice from both the experimental groups will be subjected to the two weeks of environmental enrichment training. It should be noted that, according to existing literature, environmental enrichments training that lasts for only two weeks is typically inadequate to induce significant memory improvement; therefore, we adopt this brief training regimen as a true test of the experimental hypothesis.
The predicted model was shown in Figure 2. This diagram demonstrated that two weeks of EE training activated RNA-related expression levels in F2 generations of EE mice. Previous studies have shown that 2 weeks of training does not directly increase LTP or memory, so the cause of this effect should be genetic, as we predicted.
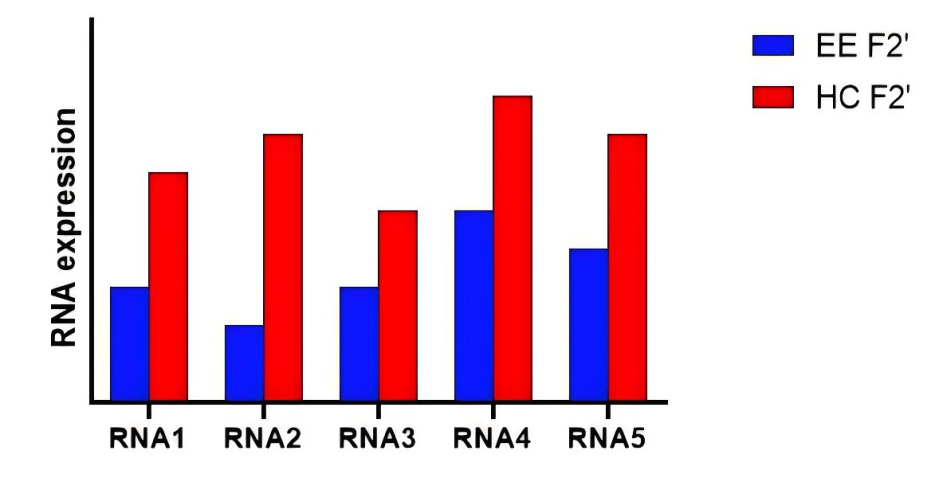
Figure 2. RNA Expression Level Prediction Model
After the training, we will perform the long-term potentiation (LTP) level tests in the F2 generation mice, and apply the Morris Water Maze (MWM) and Fear Conditioning (FC) tests to evaluate their cognitive function and memory respectively. The memory inheritance effects will be confirmed or rejected depending with the scores from these tests. If F2 generation mice from the first group give significantly better results in these tests than the F2 generation mice of the second group, it can be concluded that although the F2 generation mice were not directly trained in the environmental enrichment training, they have inherited some of the memory enhancement effects of the training and they also possess superior cognitive abilities as compared to the ordinary HC mice. This result would support our hypothesis of the memory inheritance effects.
The predicted results are shown in Fig.3. Direct measurements of LTP levels were used to demonstrate that the above elevated RNA levels were associated with elevated LTP levels.
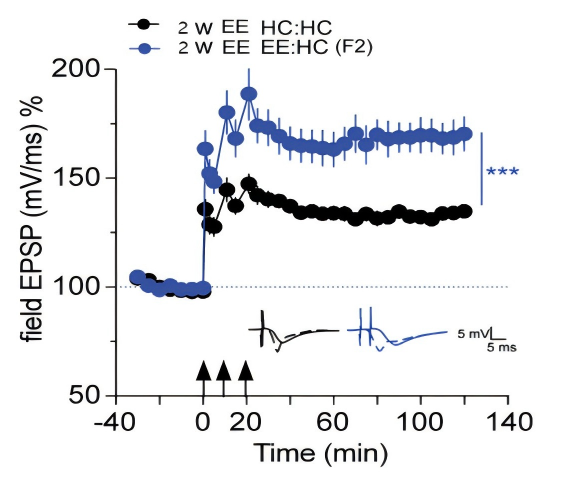
Figure 3. LTP Enhancement Model[10]
The cognitive ability was calculated using linear mixed models to account for batch and litter effects (see Experimental Procedures). *p < 0.05 (t-value=2.80, df=10). HC: HC: n = 29, N = 6; EE: HC: n = 32, N = 7 (n represents number of mice, N represents number in litter). And the expected result is showed in Fig.4(a). Fig.4(b) illustrating the magnitude of change of each individual parameter that went into the cognitive score calculation.
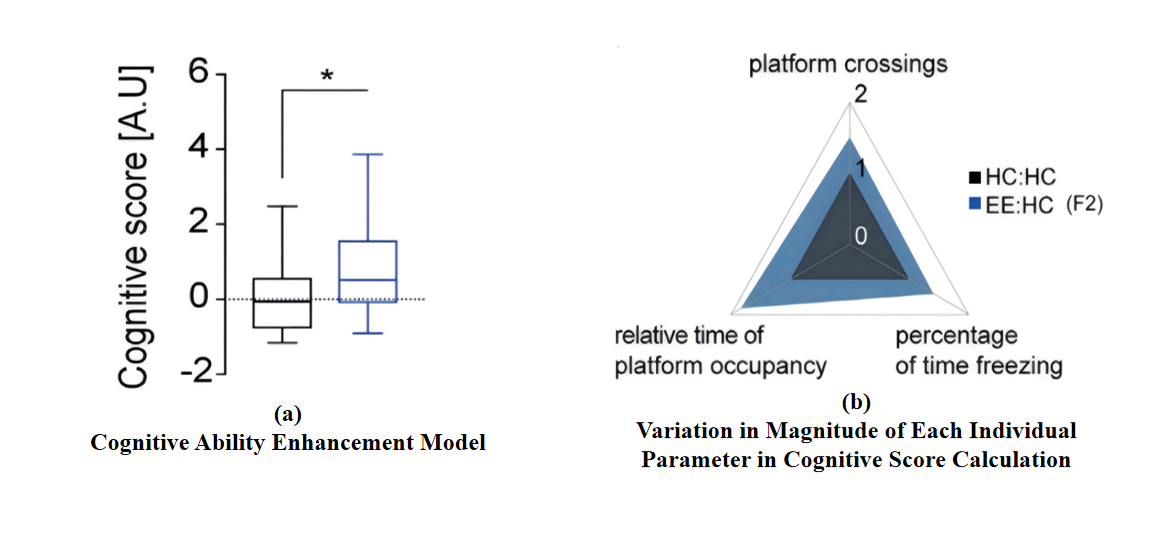
Figure 4. Cognitive Ability Evaluation Model[10]
Last, we will carry out RNA sequencing (RNA-seq) for the F2 generation mice from both experimental groups to determine the overrepresented RNAs as compared to the HC mice. These RNAs will be compared with the target factors as obtained in the first step of screening so that we can see if there are any similarities between the two. This process will help us confirm or rejected the previously proposed hypothesis and, in addition, determine which RNAs are involved in the inheritance of effects of environmental enrichment in memory. The predicted results in this section are shown in Figure 5.
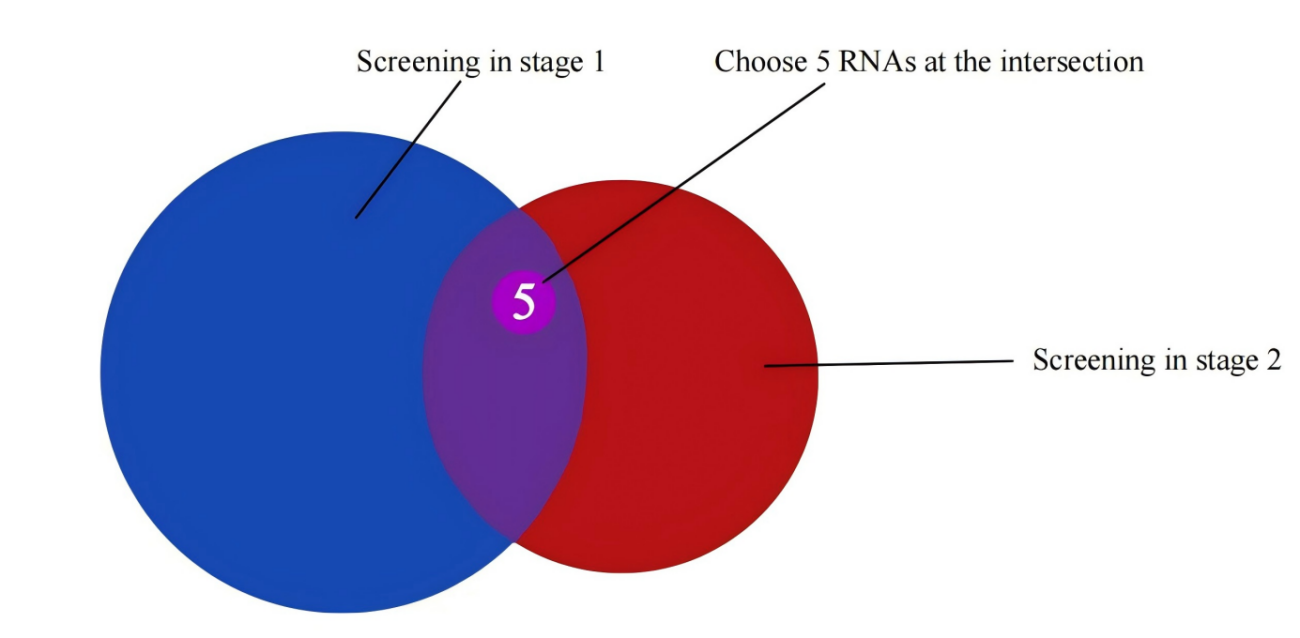
Figure 5. Final Target Selection Model
2.2. Confirmatory experiment
From the previous screening, several RNAs which conform with the initial hypotheses have been found. We will select five of the RNAs. Here in this experimental set up the lineage cultivation will be done on the male mice belonging to the EE (experimental group) and HC (control group) with the restriction of different experimental factors. The general design of the experiment consists of three main phases, of which each has the corresponding treatment group.
Group 1: We will conduct lineage cultivation on EE male mice, and treat F2 generation fertilized eggs, and let them grow. For this group, only interference RNA will be injected into the F2 fertilized eggs to eliminate other interferences.
Group 2: Similar lineage cultivation will also be done on EE male mice. Following the derivation of F2 generation fertilized eggs, we will inject five RNAs that earlier discovered in these experiments, one at a time, into the eggs. These RNAs will not be mixed but injected separately into F2 fertilized eggs in order to determine the contribution of each RNA.
Group 3: The same treatment will be applied to HC male mice as is applied on the rats of the previous groups. The F2 generation fertilized eggs will be injected with the same 5 different RNAs that were injected to the fertilized eggs in Group 2. This group is designated in order to compare with the results of Group 2, to determine whether RNA injection has effects on fertilized eggs from different types of mice.
Subsequently, the fertilized eggs will be grown into individuals, and their cognitive abilitie will be evaluated by MWM (Morris Water Maze) and FC (Fear Conditioning) tests. Levels of LTP (Long-Term Potentiation) will be measured by LTP recording. The outcomes will be compared in all the three groups.
The experimental workflow is shown in Figure 6.
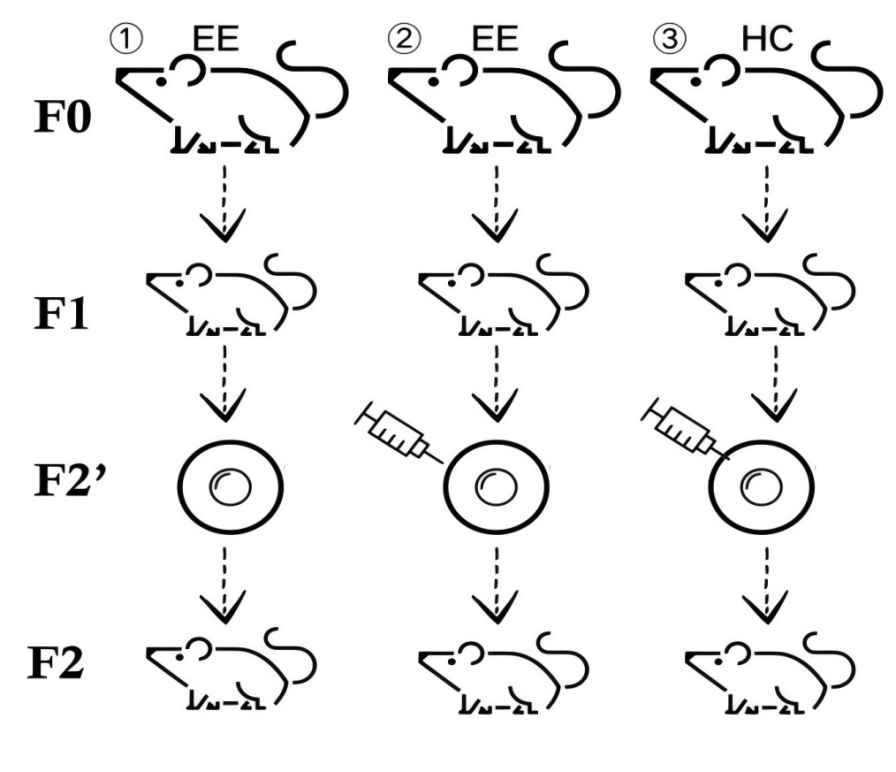
Figure 6. Experimental Procedure
For the injection volume, to get the results which are closer to our experimental objectives, we propose to inject a small volume into fertilized eggs. The data on the specific injection dosage will be collected in advance with the help of preliminary experiments.
Fig.7 shows the predicted results of this set of experiments. The five EE sperm RNA selected were injected into fertilized oocytes in small amounts to provide cognition for the offspring advantage, which is reflected in a significant increase in cognitive scores which was showed in Fig.7 (a). Fig.7(b) will further demonstrate, through three evaluation dimensions of cognitive ability, that the selected RNA acts as an activator of memory inheritance.
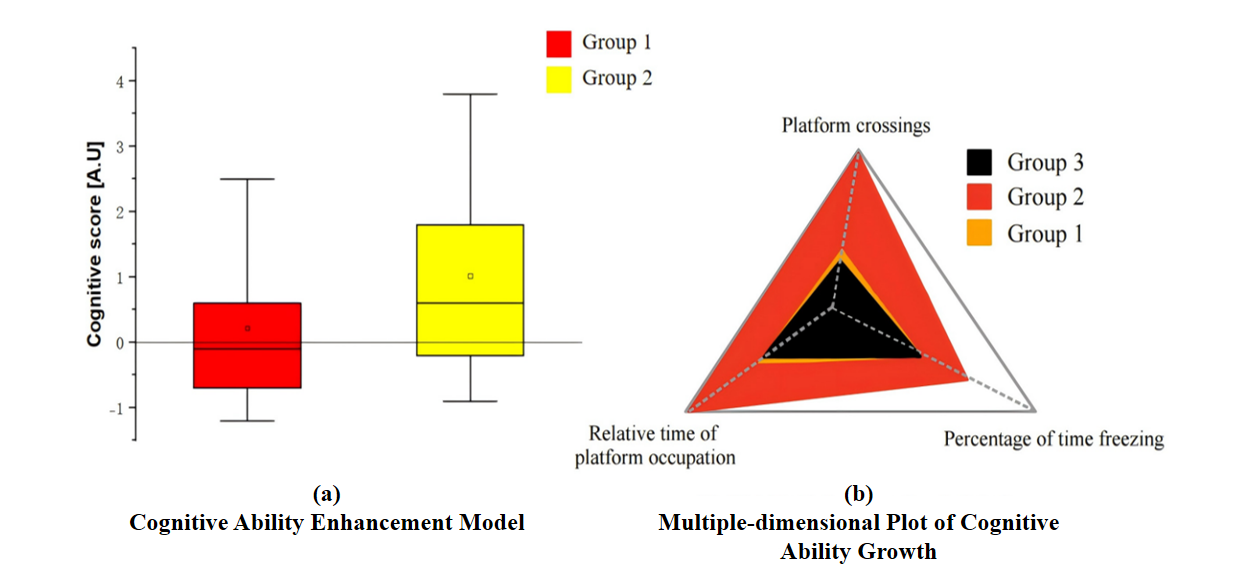
Figure 7. Cognitive Ability Comparison
The Fig. 8 was designed to demonstrate that RNA injection is directly related to LTP growth.
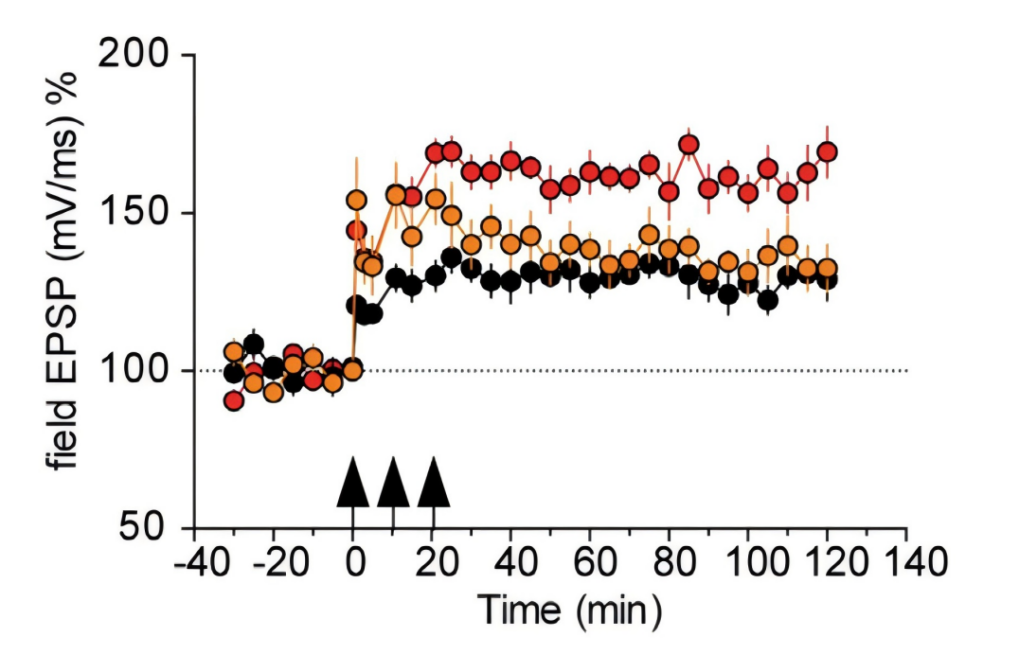
Figure 8. LTP Enhancement Model[10]
Thus, the distinctive experimental treatments of the three groups will allow us to reasonably confirm or reject the proposed hypotheses as well as collect the necessary experimental data. This forms the entire process of this experiment.
3. Discussion
The analysis of existing work by other authors and results of our experiments indicates that both motor activity and physical cognition can increase synaptic plasticity, and lead to the improvement of learning and memory functions, and also decrease risks of multi-faceted processes, including Alzheimer’s disease.AD is chronic and multifactorial in its development and elaboration of the progression mechanism of the disease is a challenge.The knowledge that women are at a higher risk for Alzheimer disease may help continue advancement of additional knowledge of these complicated processes and future investigation concerning the progression of the disease in males and females[13].Thus, sperm of male mice was chosen as the object of study in the present work. Several prior works have established the link between the miR-137-5p and the neurotoxicity of beta-amyloid 1-42[14].A study done on adult male mice has shown that enriching the environment can increase synaptic plasticity and cognitive function in offspring. This impact is controlled by RNA in sperm in specific; a chief representant of RNA is miR-212/132.However, there are some problems and open issues in the current quantitative studies, and the purpose of this paper is to provide the experimental treatments and data for these problems.
Although this article cannot provide specific data as the experiments have not yet been conducted, our proposed predictive model helps to conclude which RNAs mediate the transgenerational effects of environmental enrichment on mouse memory. This memory genetic effect is gradually diluted through generations and that is why we might not directly see the memory improvement by the F2 generation because their expression levels might be relatively low. However, this effect can be activated by the addition of a small amount of related factors, resulting in observable memory growth.
In addition, there are certain limitations in our experimental design:
The sequence of our experimental steps is rather tightly connected. In the Experiment 1, we employ RNA-Seq technology for the identification and screening of the RNAs, which are expressed at less generation as compared to other ones. But if such RNAs are not identified through this screening, then it would mean that this intergenerational genetic phenomenon may not exist, which would hinder to the continuation of further experiments.
That is, we have neglected some inhibitory factors and other variables. In memory regulation, there can be some factors which are negatively related to the regulation of memory. However, this experiment only contained the discussion on the positive factors and did not bother to explain the roles of these negative factors.
Further, some of the factors that could be indicated may be intermediates that are not directly involved in regulation of memory.
In addition, we did not explore the specific molecular mechanisms which ensure these factors affect the intergenerational transfer of enhanced synaptic plasticity and improved cognitive ability. The overall experiment has not sought to understand how these factors enrich information processing capabilities by modulating synaptic plasticity or dig deeper into their functions in relation to epigenetic inheritance.
Specifically, it is still unclear understood how these factors affect the synaptic plasticity and improvement of cognitive ability and how their genetic inheritance at the molecular level is regulated.
4. Conclusion
The working flowchart of my proposal is showed in Fig.9.
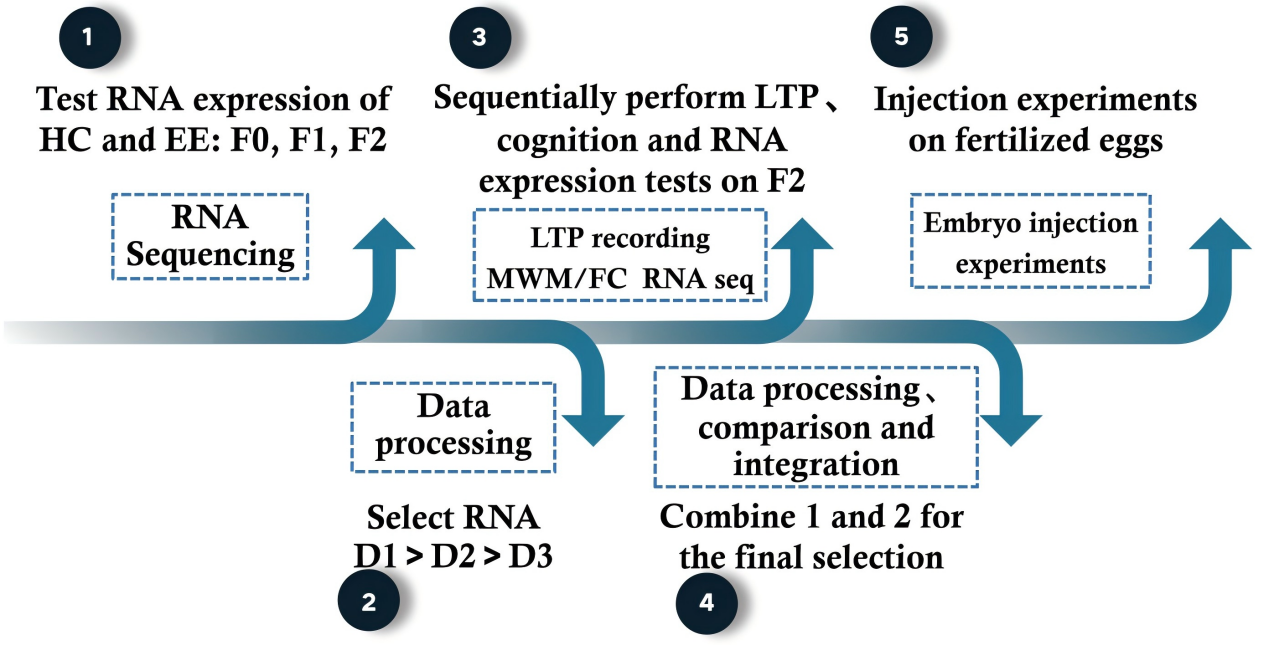
Figure 9. Working flowchart
From those procedure, we can draw several conclusions:
(1) Enriched environment (EE) training can directly enhance mice’s cognitive abilities and synaptic plasticity, and this effect is heritable but weakens with each generation.
(2) From the above experiments, the researchers can determine some of the RNA molecules that influence synaptic plasticity and cognitive capacities.
(3) After EE training, comparing the two groups of mice reveals a small amount of genetic inheritance.
(4) The selected RNA is similar to substances like RNA activators. Their addition can only enhance the LTP levels and cognitive abilities of the offspring of the EE mice.
Acknowledgments
First of all, I would like to thank Professor Samuel Kunes and the two teaching assistants for their guidance to our group these days. Thank my mother for her constant companionship and care. I would like to thank Chang Donglin, Zhao Mengyang and Wang Renren for their cooperation in this project. Finally, special thanks to my friend Wang Yutong for his help in drawing.
References
[1]. Hua W, Han X, Li F, Lu L, Sun Y, Hassanian-Moghaddam H, Tian M, Lu Y, Huang Q. Transgenerational Effects of Arsenic Exposure on Learning and Memory in Rats: Crosstalk between Arsenic Methylation, Hippocampal Metabolism, and Histone Modifications. Environ Sci Technol. 2024 Apr 16;58(15):6475-6486. doi: 10.1021/acs.est.3c07989. Epub 2024 Apr 5. PMID: 38578163.
[2]. Gallusci P, Agius DR, Moschou PN, Dobránszki J, Kaiserli E, Martinelli F (2023) Deep inside the epigenetic memories of stressed plants. Trend Plant Sci 28(2):142–153. https://doi.org/10.1016/j. tplants.2022.09.004
[3]. Zhang Q, Tian Y (2022) Molecular insights into the transgenerational inheritance of stress memory. J Genet Genom 49(2):89–95
[4]. Epigenetic and transgenerational reprogramming of brain development.Nat. Rev. Neurosci. 2015; 16: 332-344
[5]. Implication of sperm RNAs in transgenerational inheritance of the effects of early trauma in mice. Nat. Neurosci. 2014; 17: 667-669
[6]. Potential of environmental enrichment to prevent transgenerational effects of paternal trauma. Neuropsychopharmacology. 2016; 41: 2749-2758
[7]. Epigenetic transmission of the impact of early stress across generations. Biol. Psychiatry. 2010; 68: 408-415
[8]. Paternal stress exposure alters sperm microRNA content and reprograms offspring HPA stress axis regulation. J. Neurosci. 2013; 33: 9003-9012
[9]. Ishiwari K, King CP, Martin CD, Tripi JA, George AM, Lamparelli AC, Chitre AS, Polesskaya O, Richards JB, Solberg Woods LC, Gancarz AM, Palmer AA, Dietz DM, Mitchell SH, Meyer PJ. Environmental enrichment promotes adaptive responding during tests of behavioral regulation in male heterogeneous stock rats. Sci Rep. 2024 Feb 20;14(1):4182. doi: 10.1038/s41598-024-53943-y. PMID: 38378969; PMCID: PMC10879139.
[10]. Benito et al., 2018, Cell Reports 23, 546–554 April 10, 2018 ª 2018 The Authors. https://doi.org/10.1016/j.celrep.2018.03.059
[11]. Waag R, Bohacek J. Single-Nucleus RNA-Sequencing in Brain Tissue. Curr Protoc. 2023 Nov;3(11):e919. doi: 10.1002/cpz1.919. PMID: 37987152.
[12]. Stark, R., Grzelak, M. & Hadfield, J. RNA sequencing: the teenage years. Nat Rev Genet 20, 631–656 (2019). https://doi.org/10.1038/s41576-019-0150-2
[13]. Lopez-Lee C, Torres ERS, Carling G, Gan L. Mechanisms of sex differences in Alzheimer's disease. Neuron. 2024 Apr 17;112(8):1208-1221. doi: 10.1016/j.neuron.2024.01.024. Epub 2024 Feb 22. PMID: 38402606; PMCID: PMC11076015.
[14]. Jiang Y, Bian W, Chen J, Cao X, Dong C, Xiao Y, Xu B, Sun X. miRNA-137-5p improves spatial memory and cognition in Alzheimer's mice by targeting ubiquitin-specific peptidase 30. Animal Model Exp Med. 2023 Dec;6(6):526-536. doi: 10.1002/ame2.12368. Epub 2023 Dec 18. PMID: 38111333; PMCID: PMC10757218.
Cite this article
Lin,S. (2025). The Mechanism of RNA-Dependent Intergenerational Inheritance and Factors Affecting Its Transition to Transgenerational Inheritance. Theoretical and Natural Science,76,124-133.
Data availability
The datasets used and/or analyzed during the current study will be available from the authors upon reasonable request.
Disclaimer/Publisher's Note
The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of EWA Publishing and/or the editor(s). EWA Publishing and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
About volume
Volume title: Proceedings of the 4th International Conference on Biological Engineering and Medical Science
© 2024 by the author(s). Licensee EWA Publishing, Oxford, UK. This article is an open access article distributed under the terms and
conditions of the Creative Commons Attribution (CC BY) license. Authors who
publish this series agree to the following terms:
1. Authors retain copyright and grant the series right of first publication with the work simultaneously licensed under a Creative Commons
Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this
series.
2. Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the series's published
version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial
publication in this series.
3. Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and
during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See
Open access policy for details).
References
[1]. Hua W, Han X, Li F, Lu L, Sun Y, Hassanian-Moghaddam H, Tian M, Lu Y, Huang Q. Transgenerational Effects of Arsenic Exposure on Learning and Memory in Rats: Crosstalk between Arsenic Methylation, Hippocampal Metabolism, and Histone Modifications. Environ Sci Technol. 2024 Apr 16;58(15):6475-6486. doi: 10.1021/acs.est.3c07989. Epub 2024 Apr 5. PMID: 38578163.
[2]. Gallusci P, Agius DR, Moschou PN, Dobránszki J, Kaiserli E, Martinelli F (2023) Deep inside the epigenetic memories of stressed plants. Trend Plant Sci 28(2):142–153. https://doi.org/10.1016/j. tplants.2022.09.004
[3]. Zhang Q, Tian Y (2022) Molecular insights into the transgenerational inheritance of stress memory. J Genet Genom 49(2):89–95
[4]. Epigenetic and transgenerational reprogramming of brain development.Nat. Rev. Neurosci. 2015; 16: 332-344
[5]. Implication of sperm RNAs in transgenerational inheritance of the effects of early trauma in mice. Nat. Neurosci. 2014; 17: 667-669
[6]. Potential of environmental enrichment to prevent transgenerational effects of paternal trauma. Neuropsychopharmacology. 2016; 41: 2749-2758
[7]. Epigenetic transmission of the impact of early stress across generations. Biol. Psychiatry. 2010; 68: 408-415
[8]. Paternal stress exposure alters sperm microRNA content and reprograms offspring HPA stress axis regulation. J. Neurosci. 2013; 33: 9003-9012
[9]. Ishiwari K, King CP, Martin CD, Tripi JA, George AM, Lamparelli AC, Chitre AS, Polesskaya O, Richards JB, Solberg Woods LC, Gancarz AM, Palmer AA, Dietz DM, Mitchell SH, Meyer PJ. Environmental enrichment promotes adaptive responding during tests of behavioral regulation in male heterogeneous stock rats. Sci Rep. 2024 Feb 20;14(1):4182. doi: 10.1038/s41598-024-53943-y. PMID: 38378969; PMCID: PMC10879139.
[10]. Benito et al., 2018, Cell Reports 23, 546–554 April 10, 2018 ª 2018 The Authors. https://doi.org/10.1016/j.celrep.2018.03.059
[11]. Waag R, Bohacek J. Single-Nucleus RNA-Sequencing in Brain Tissue. Curr Protoc. 2023 Nov;3(11):e919. doi: 10.1002/cpz1.919. PMID: 37987152.
[12]. Stark, R., Grzelak, M. & Hadfield, J. RNA sequencing: the teenage years. Nat Rev Genet 20, 631–656 (2019). https://doi.org/10.1038/s41576-019-0150-2
[13]. Lopez-Lee C, Torres ERS, Carling G, Gan L. Mechanisms of sex differences in Alzheimer's disease. Neuron. 2024 Apr 17;112(8):1208-1221. doi: 10.1016/j.neuron.2024.01.024. Epub 2024 Feb 22. PMID: 38402606; PMCID: PMC11076015.
[14]. Jiang Y, Bian W, Chen J, Cao X, Dong C, Xiao Y, Xu B, Sun X. miRNA-137-5p improves spatial memory and cognition in Alzheimer's mice by targeting ubiquitin-specific peptidase 30. Animal Model Exp Med. 2023 Dec;6(6):526-536. doi: 10.1002/ame2.12368. Epub 2023 Dec 18. PMID: 38111333; PMCID: PMC10757218.









