1. Introduction
Breast cancer is the most commonly diagnosed cancer and the leading cause of cancer-related deaths among women worldwide, causing 670,000 deaths globally and accounting for 11.5% of all new cancer cases and 6.8% of all cancer deaths by 2022 [1]. Effective treatment for breast cancer is considered a combination of different approaches. In addition to the surgery and radiation therapy, adjuvant chemotherapy is also considered an important component [2] of curative treatment for many types of cancers, with the aim to improve the overall survival (OS) and disease-free survival (DFS) for patients undergoing pre-operative and post-operative care. Furthermore, biological characteristics vary from individual to individual, which has a high possibility [3] of influencing the final survival rate and curative effects.
Two common adjuvant chemotherapy regimens for breast cancer are the combination of doxorubicin plus cyclophosphamide (AC) [4, 6, 8], as well as single-agent paclitaxel (T) [4, 5]. While previous studies have examined the AC [6] and single-agent T [7] regimens individually, a direct comparative analysis of these two regimens is currently lacking. Notably, several studies have investigated the difference between the efficacy of AC and paclitaxel plus cyclophosphamide (TC), AC and doxorubicin plus paclitaxel (AT) [8, 9], but these comparisons do not directly show the curative effect of single-agent T since they do not precisely identify the source of the therapeutic effect. Furthermore, previous investigations into the efficacy of various chemotherapy regimens may not incorporate the most recent information and data, as the majority of the studies were based on datasets collected in the 1990s [8, 10].
Unlike earlier research, this study directly evaluates the efficacy of T in comparison to AC, using data from a clinical trial conducted between 2002 and 2010. This specific trial design made it possible to isolate the impact of T alone, avoiding potential confounding factors that could stem from AC treatments.
Previous investigations were typically smaller in scope, with sample sizes under 1000 participants [8, 11, 12], focusing on limited characteristics like the number of positive axillary nodes [11, 13]. Additionally, many studies restricted their analyses to fixed treatment durations [10, 11]. In contrast, the current study utilizes a far larger sample of 3,871 participants with a median follow-up of 6.1 years, allowing a more in-depth examination of treatment lengths—specifically, 4 versus 6 cycles. Furthermore, this dataset included a diverse range of tumor sizes, from less than 2 cm to over 5 cm, enabling a broader examination of patients.
This study also examines AC and single-agent T in terms of OS and DFS as well as how treatment duration (4 cycles vs. 6 cycles) and patient/tumor characteristics (race, dose density, menopause status, receptor status, tumor size, age category, histologic grade, HER2 status) influence the outcomes.
2. Method
2.1. Data Description
This research utilizes the rich dataset from the CALGB 40101 trial, providing a solid foundation to explore the comparative efficacy of different chemotherapy regimens and their interactions with patient factors concerning survival outcomes. CALGB 40101 was a phase III randomized study comparing the standard AC (Cyclophosphamide and Doxorubicin) with experimental Paclitaxel (T) as adjuvant therapies for breast cancer in women with 0-3 positive axillary lymph nodes. While AC served as the control group, T was the experimental treatment. The standard treatment duration comprised 4 cycles, whereas the experimental regimen was extended to 6 cycles.
2.2. Variable Description
Our research examined two primary sections of predictors: chemotherapy regimens(AC or T) and treatment durations (4 or 6 cycles). The primary objective was to assess the efficacy of different chemotherapy regimens while accounting for various patient factors in breast cancer. Thus, we focused on both overall survival (OS) and disease-free survival (DFS) as key outcome measures.
2.3. Collecting Methods
Patients were randomly assigned by computer to receive either 4 cycles (8 weeks) or 6 cycles (12 weeks) of AC or single-agent T. During the treatment period, patients were regularly monitored for survival status (alive or deceased). Overall survival (OS) was measured from study entry until death, with living patients censored at their last follow-up. Disease-free survival (DFS) was measured from study entry until the first relapse or death, with disease-free patients censored at their last known disease-free date.
2.4. Statistical Analysis
The analysis primarily compared the efficacy of the AC and T regimens, while also considering the role of patient characteristics on outcomes.
The chi-square test was used to explore the relationships between covariates and outcomes calculating 95% confidence intervals. Logistic regression models were employed to examine the effects of treatment on both survival and DFS.
The Kaplan-Meier method was employed to describe the distribution of overall survival time and disease-free survival time. The agent as well as the treatment duration were compared through a two-sided log-rank test with a 5% significance level.
Moreover, the Cox proportional hazards model adjusted for the agent, the length of treatment, age, race, receptor status, histologic grade, tumor size, prior hormonal therapy, and a sentinel node biopsy. Also, the model calculated the 95% confidence intervals and hazard ratio (HR) of the variables. To ensure the validity of the model, the assumption of the proportional hazard hypothesis was tested. At last, the Cox proportional hazards model was fitted to the subset to address the impact of treatment on the specific groups of patients.
3. Result
3871 participants were randomly divided into four groups (AC-4, 1142 patients; AC-6, 789 patients; T-4, 1151 patients; T-6, 789 patients). The characteristics of the patients are summarized in Table 1. The distribution of agent and duration was well balanced. Also, the tumor laterality was evenly distributed (left 50.45%, right 47.79%), with a small percentage having bilateral tumors (1.50%). Regarding menopause status, 39.81% of patients were pre-menopausal, while 60.19% were post-menopausal. 66.29% of patients were receptor-positive, while 33.48% and 66.29% were negative and positive for ER status, respectively. Histologic grade distribution suggested that 45.47% of patients had high-grade tumors, 39.40% intermediate, and 13.54% low-grade tumors. Survival status indicated that 93.13% of patients were alive at the time of data collection, with a mean survival of 68.02 months and a median of 71.1 months. DFS was observed in 88.71% of patients, with a mean of 63.37 months and a median of 64.03 months.
Table 1. Patient and Disease Characteristics at Study Entry | ||
| n | proportion(%) |
Menopause status | ||
Pre-menopause | 1541 | 39.81 |
Post-menopause | 2330 | 60.19 |
Receptor status | ||
Recep+ & Unkown | 2628 | 67.89 |
Recep- | 1243 | 32.11 |
Receptor Status ER | ||
Negative | 1296 | 33.48 |
Positive | 2566 | 66.29 |
Histologic grade | ||
Low | 524 | 13.54 |
Intermediate | 1525 | 39.40 |
High | 1760 | 45.47 |
Treatment assigned | ||
AC-4 | 1142 | 29.50 |
AC-6 | 789 | 20.38 |
T-4 | 1151 | 29.73 |
T-6 | 789 | 20.38 |
Survival Months | ||
Mean | 68.02 | |
Median | 71.1 | |
Range | 0-123.43 | |
Disease Free Survival Months | ||
Mean | 63.37 | |
Median | 64.03 | |
Range | 0-120.71 | |
The treatment agent, duration, receptor status, and tumor size significantly impacted OS status by the logistic regression (p<0.001). The agent, the treatment duration, menopause status, receptor status, and tumor size also have a significant impact on DFS status (p<0.001).
OS and DFS distributions were described by the Kaplan-Meier method in Fig. 1 and grouped by receptor status ER, menopause status, and primary surgery. The log-rank test showed the treatment agent had a significant impact on OS (p = 0.0455). This suggests a significant difference in OS between patients treated with different agents. The agent also showed a significant effect (p = 0.0455) in the subgroup analysis stratified by treatment duration. Nevertheless, treatment duration and stratification by the agent did not show a significant difference associated with outcomes, as indicated by the log-rank test (p = 0.5).
Furthermore, the Cox proportional hazards model, which included both agent and treatment duration as predictors, indicates that the agent has a significant effect on survival (HR = 1.29, p = 0.047), while treatment duration did not show a significant influence on OS (HR = 1.089, p = 0.489). Additionally, including an interaction term of the agent and treatment duration did not show significant improvement, suggesting no evidence of an interaction effect of these two factors on OS (p = 0.2048). For the DFS, the survival difference by the agent (p = 0.01) is significant, while the difference by duration (p = 0.7) is not.
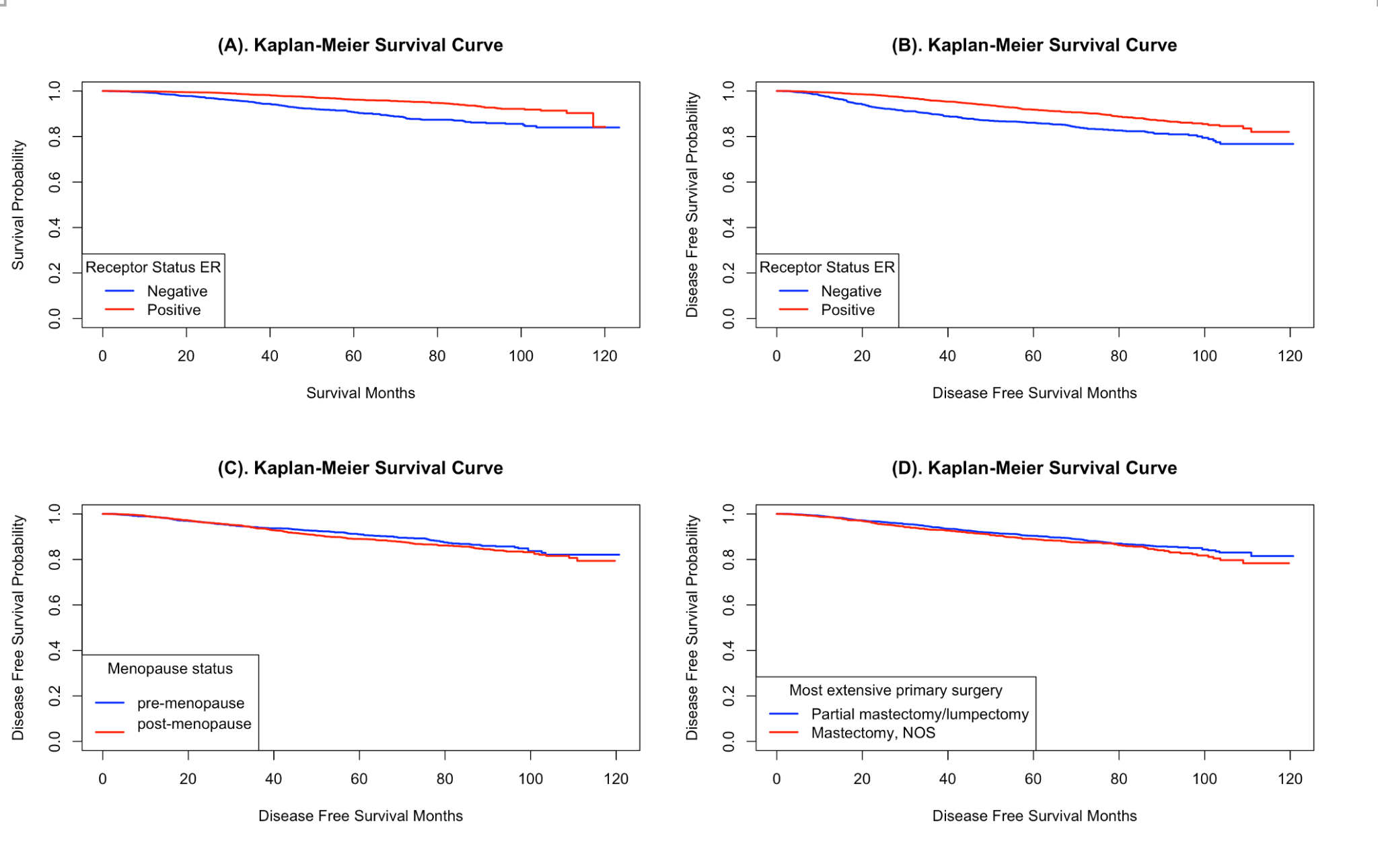
Figure 1. (A) OS for all patients grouped by receptor status ER. (B) DFS for all patients grouped by receptor status ER. (C) DFS for all patients grouped by their menopause status. (D) DFS for all patients grouped by primary surgery.
The DFS was analyzed by the Cox proportional hazards model, incorporating various factors to evaluate their effect on the hazard (Table 2). The treatment with the agent increased the hazard by approximately 23.2% (HR = 1.2322, p = 0.0332), indicating a statistically significant effect, while the treatment duration did not show a significant impact (p = 0.8291). Tumor size was a significant predictor and larger tumor size increased the hazard (HR: 1.5652 and 2.0282, p: <0.001 and 0.019, respectively). Additionally, high histologic grade (HR = 1.82, p < 0.001) and age (HR = 2.19, p = 0.031) also significantly increased the hazard. Similarly, the agent, tumor size, sentinel node biopsy, age, and receptor status were significant factors influencing OS, and both increased the hazard. While race and ethnicity, as well as several other predictors like prior hormonal therapy, type biopsy, tumor laterality, and menopause status, did not show significant effects. At last, there was no evidence to reject the assumption of the proportional hazard hypothesis, which showed the validity of the models (p = 0.0696; p = 0.0716, respectively).
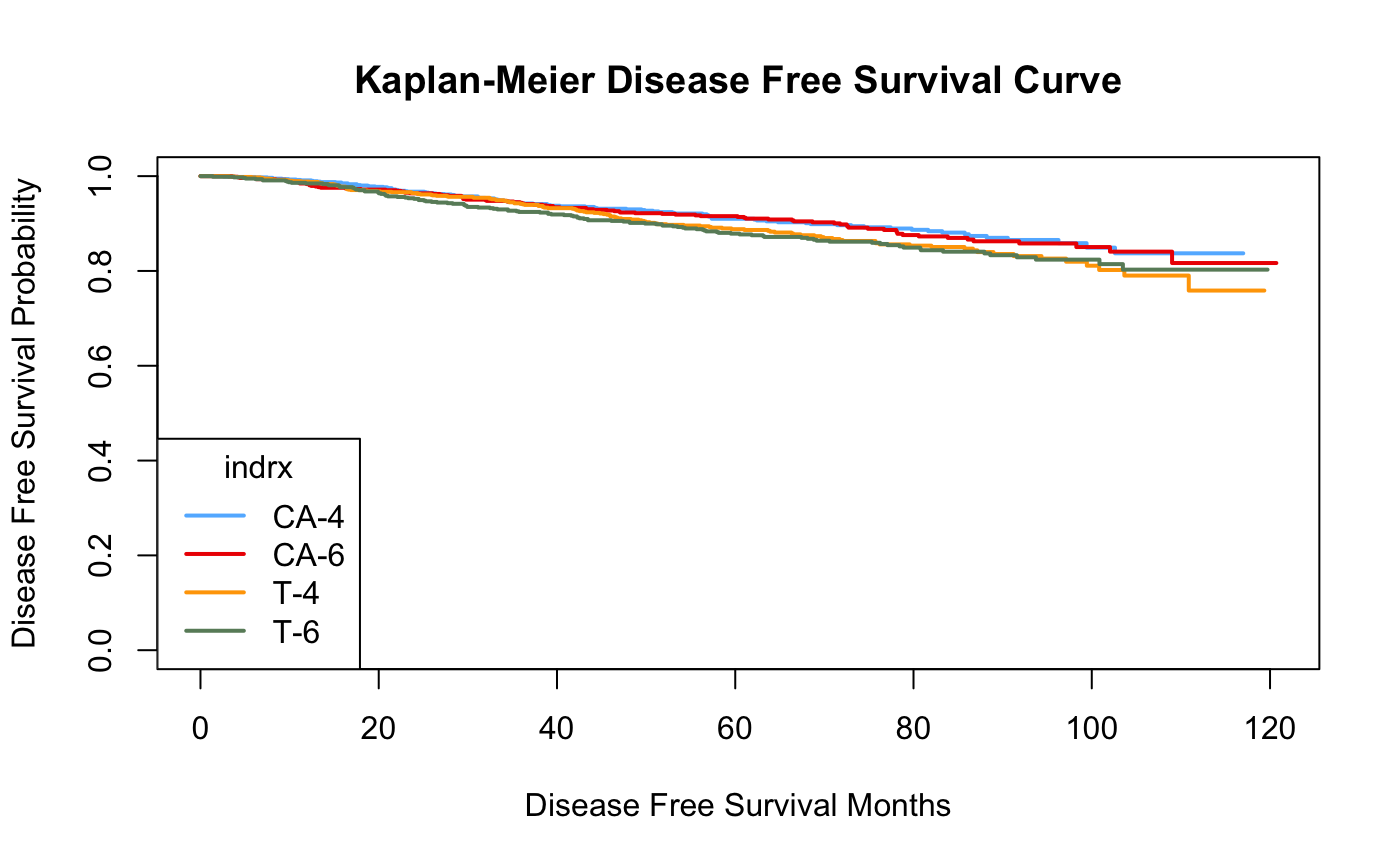
Figure 2. DFS for all patients grouped by the treatments
Table 2. Multivariable Proportional Hazards Models: Observed Effects on DFS and OS (n = 3,862) | |||||||
Factors | DFS | OS | |||||
HR | 95% CI* | P | HR | 95% CI* | P | ||
Agent (AC/T) | 1.23 | 1.02 to 1.49 | .033 | 1.29 | 1.01 to 1.65 | .044 | |
Tumor size was between 2 and 5 cm | 1.57 | 1.29 to 1.91 | < .0001 | 1.58 | 1.23 to 2.03 | .00038 | |
Tumor size was greater than 5cm | 2.03 | 1.12 to 3.66 | .019 | 3.22 | 1.67 to 6.21 | .00047 | |
Native Hawaiian or Pacific Islander or American Indian | 2.19 | 1.07 to 4.45 | .031 | 1.57 | 0.50 to 4.95 | .44 | |
Histologic grade (high) | 1.82 | 1.29 to 2.57 | .00068 | - | - | - | |
Sentinel node biopsy | 0.80 | 0.60 to 1.07 | .13 | 0.73 | 0.55 to 0.97 | .029 | |
Age | 1.02 | 1.00 to 1.03 | .024 | 1.04 | 1.02 to 1.05 | < .0001 | |
Receptor status | - | - | - | 2.19 | 1.71 to 2.82 | < .0001 | |
* There are two vacuums since the receptor status was not included in the first model and histologic grade (high) was not included in the second model. (1) the Cox proportional hazards model analyzed the DFS, adjusting for the agent, length, tumor size, sentinel node biopsy, age, race, histologic grade, menopause status, number of positive nodes, receptor status, and the primary surgery. (2) the Cox proportional hazards model analyzed the OS, adjusting for agent, length, tumor size, sentinel node biopsy, prior hormonal therapy, type biopsy, tumor laterality, race, ethnicity, age, receptor status, and menopause status.
Table 3. Comparative Outcomes on OS and DFS in different subgroups | |||
Factor | Subgroup | P value (OS) | P value (DFS) |
Menopause Status | Pre-menopause | 0.5 | 0.4 |
Post-menopause | 0.06 | 0.01 | |
Hormone Receptor Status | Positive, Unknown | 0.2 | 0.09 |
Negative | 0.12 | 0.06 | |
Her2-neu Status | Positive | 0.2 | 0.2 |
Negative | 0.7 | 0.6 | |
Unknown | 0.03 | 0.02 | |
Receptor Status ER | ER-negative | 0.03 | 0.02 |
ER-positive | 0.5 | 0.2 | |
Most Extensive Primary Surgery | Mastectomy | 0.4 | 0.3 |
Partial mastectomy/lumpectomy | 0.06 | 0.03 | |
Table 3 summarizes the results of log-rank tests comparing OS and DFS between treatment regimens (AC vs. T) across various subgroups. Significant differences are noted for p < 0.05.
4. Discussion
The treatment duration did not show significant impact on OS and DFS, which might be contrary to our initial expectations. One critical aspect to consider in this finding is the dose intensity, which refers to the amount of chemotherapy delivered per unit time. Research has shown that dose density plays a critical role in treatment efficacy. Reductions in dose intensity may weaken the effects of chemotherapy agents, leading to poorer survival outcomes. Studies have indicated that maintaining dose intensity, rather than extending the number of cycles, was vital for achieving better clinical outcomes, suggesting that the total dose delivered over a given period may be more important than the actual number of cycles [36]. In our study, the amount of drugs delivered per unit of time was the identical across different treatment durations, maintaining a consistent dose intensity. Consequently, the consistent dose intensity in different treatments could contribute to the lack of significant differences in OS and DFS observed between the 4-cycle and 6-cycle groups. Furthermore, the relatively small difference in treatment length between different cycles made it harder to observe the significant difference.
Age has consistently been a crucial factor when considering different cancer treatments. Aging leads to changes in the immune system known as immunosenescence [15]. With aging, the thymus, a primary lymphoid organ responsible for T-cell development, undergoes progressive atrophy [14, 34]. This results in a decline in the production of T cells [35], which are essential for generating effective immune responses to new antigens, including tumors. Other immune organs and functions, such as telomere shortening, have also undergone certain degrees of senescence and impairment, reducing immune responsiveness and the ability to fight infections and cancer. This would increase the risk of tumor metastasis and recurrence, which is consistent with our finding that one year older increased the hazard of relapse by approximately 2% and an increased hazard of death by approximately 4%.
Larger tumor sizes are consistently associated with poorer DFS and OS in breast cancer patients. Larger tumors typically indicate a greater number of tumor cells and a higher grade of malignancy [16], increasing the risk of metastasis and recurrence, thereby significantly reducing the patient's OS and DFS. Larger tumors generally have more aggressive and metastatic potential [17], as the tumor cells have more opportunities to enter the blood vessels and lymphatic system, increasing the likelihood of distant metastasis. Additionally, larger tumors may be more challenging to completely remove through surgery or effectively target with local radiotherapy [18]. The inability to thoroughly eradicate the primary tumor can increase the risk of recurrence, further compromising DFS. Similar to our findings, it was discovered that tumors with a size greater than 5 cm increased the hazard of relapse by approximately 103% and the hazard of death by approximately 222%.
The AC adjuvant chemotherapy agent primarily acts by directly killing tumor cells and inhibiting tumor growth and metastasis [19, 20]. In contrast, T mainly works by modulating the dynamics of microtubules through the regulation of estrogen signaling, thereby inhibiting tumor cell growth [21, 22]. However, in postmenopausal women, the decline in estrogen levels reduces the regulatory effect on microtubule stability [23, 24], which diminishes the efficacy of paclitaxel. This highlights the advantage of AC's direct targeting effect on cancer cells, which is consistent with our finding that patients receiving T had a 31% higher risk of recurrence compared to the AC group. Apart from DFS, the curative effectiveness of the agents has no significant difference on OS. For postmenopausal women, once disease recurrence occurs, OS may be impacted by other factors such as subsequent treatment regimens and comorbidities, such as variable metastasis and lymphedema, which could reduce the differences in DFS between AC and T.
The histologic grade serves as a crucial and practical standard for grading breast cancer, offering a direct, cost-effective, and highly accurate means of assessing tumor biological characteristics [37]. Patients with a high histologic grade face an elevated risk of mortality and are more prone to relapse [38], aligning with our finding that a high histologic grade raises the relapse hazard by approximately 82% compared to a low histologic grade.
Receptor status is an important factor to be considered when treating cancer since the treatments have various effects on different receptor statuses. Receptors, proteins located on the surface of breast cells, transmit signals to the cells to initiate growth and division upon hormone binding. The Hormonal therapy has a better performance when the tumor is ER+ and/or PgR- [28], while dehydroepiandrosterone (DHEA) and its sulfate inhibit the growth of ER- cancer more efficiently [39]. Moreover, according to previous studies, hormone receptor-negative breast cancers are inclined to grow faster than receptor-positive ones. Women with ER- and Pgr- tumors have a higher risk of death [25, 26, 27]. Our research also discovered that the negative receptor status increases the hazard of relapse by about 119%, which strongly affects the DFS.
The Native Hawaiian or Pacific Islander or American Indian had a higher hazard of relapse and their risk of reoccurring breast cancer was 2.19 times of the whites. The relatively high recurrence rate could be attributed to their living conditions and socioeconomic, biological, and behavioral factors. Studies have shown that Native Hawaiian, Pacific Islander, and American Indian populations often face greater socioeconomic disparities [32], including lower incomes, higher poverty rates, and reduced access to quality healthcare [33]. In addition, most native Hawaiian women have low or no participation in routine due to the Hawaiian culture and religious customs. As a result, native Hawaiian women may have poorer health conditions, which leads to a higher hazard of relapse.
5. Conclusion
The efficacy of AC and T had significant differences when treating breast cancer as adjuvant therapy, while the treatment duration did not show a significant impact. Also, age, tumor size, receptor status, and histologic grade significantly affect OS or DFS.
Acknowledgment
We wanted to extend our gratitude to the Project Data Sphere (PDS) and the Alliance for Clinical Trials in Oncology for granting us access to their data, which has been invaluable to our study.
References
[1]. Ferlay J, Ervik M, Lam F, Laversanne M, Colombet M, Mery L, Piñeros M, Znaor A, Soerjomataram I, Bray F (2024). Global Cancer Observatory: Cancer Today. Lyon, France: International Agency for Research on Cancer. Available from: https://gco.iarc.who.int/today, accessed [21/07/2024].
[2]. Early Breast Cancer Trialists' Collaborative Group (EBCTCG) (2005). Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet (London, England), 365(9472), 1687–1717.
[3]. Cardoso, F., van't Veer, L. J., Bogaerts, J., Slaets, L., Viale, G., Delaloge, S., Pierga, J. Y., Brain, E., Causeret, S., DeLorenzi, M., Glas, A. M., Golfinopoulos, V., Goulioti, T., Knox, S., Matos, E., Meulemans, B., Neijenhuis, P. A., Nitz, U., Passalacqua, R., Ravdin, P., … MINDACT Investigators (2016). 70-Gene Signature as an Aid to Treatment Decisions in Early-Stage Breast Cancer. The New England journal of medicine, 375(8), 717–729.
[4]. Clifford A. Hudis, Luca Gianni, Triple‐Negative Breast Cancer: An Unmet Medical Need, The Oncologist, Volume 16, Issue S1, January 2011, Pages 1–11
[5]. Abu Samaan, T. M., Samec, M., Liskova, A., Kubatka, P., & Büsselberg, D. (2019). Paclitaxel’s mechanistic and clinical effects on breast cancer. Biomolecules, 9(12), 789.
[6]. R Buzzoni et al., Adjuvant chemotherapy with doxorubicin plus cyclophosphamide, methotrexate, and fluorouracil in the treatment of resectable breast cancer with more than three positive axillary nodes.. JCO 9, 2134-40(1991).
[7]. Kimura, M., Sano, M., Fujimori, M., Nakagomi, H., Negishi, T., Yanagita, Y., & Sato, N. (2008). Neoadjuvant paclitaxel for operable breast cancer: multicenter phase II trial with clinical outcomes. Anticancer research, 28(2B), 1239–1244.
[8]. Biganzoli, L., Cufer, T., Bruning, P., Coleman, R., Duchateau, L., Calvert, A. H., Gamucci, T., Twelves, C., Fargeot, P., Epelbaum, R., Lohrisch, C., & Piccart, M. J. (2002). Doxorubicin and paclitaxel versus doxorubicin and cyclophosphamide as first-line chemotherapy in metastatic breast cancer: The European Organization for Research and Treatment of Cancer 10961 Multicenter Phase III Trial. Journal of clinical oncology: official journal of the American Society of Clinical Oncology, 20(14), 3114–3121.
[9]. Diéras, V., Fumoleau, P., Romieu, G., Tubiana-Hulin, M., Namer, M., Mauriac, L., Guastalla, J. P., Pujade-Lauraine, E., Kerbrat, P., Maillart, P., Pénault-Llorca, F., Buyse, M., & Pouillart, P. (2004). Randomized parallel study of doxorubicin plus paclitaxel and doxorubicin plus cyclophosphamide as neoadjuvant treatment of patients with breast cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology, 22(24), 4958–4965.
[10]. Jones, S. E., Savin, M. A., Holmes, F. A., O'Shaughnessy, J. A., Blum, J. L., Vukelja, S., McIntyre, K. J., Pippen, J. E., Bordelon, J. H., Kirby, R., Sandbach, J., Hyman, W. J., Khandelwal, P., Negron, A. G., Richards, D. A., Anthony, S. P., Mennel, R. G., Boehm, K. A., Meyer, W. G., & Asmar, L. (2006). Phase III trial comparing doxorubicin plus cyclophosphamide with docetaxel plus cyclophosphamide as adjuvant therapy for operable breast cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology, 24(34), 5381–5387.
[11]. Saura, C., Tseng, L. M., Chan, S., Chacko, R. T., Campone, M., Manikhas, A., Nag, S. M., Leichman, C. G., Dasappa, L., Fasching, P. A., Hurtado de Mendoza, F., Symmans, W. F., Liu, D., Mukhopadhyay, P., Horak, C., Xing, G., & Pusztai, L. (2013). Neoadjuvant doxorubicin/cyclophosphamide followed by ixabepilone or paclitaxel in early stage breast cancer and evaluation of βIII-tubulin expression as a predictive marker. The oncologist, 18(7), 787–794. https://doi.org/10.1634/theoncologist.2013-0075
[12]. Rau, K. M., Lin, Y. C., Chen, Y. Y., Chen, J. S., Lee, K. D., Wang, C. H., & Chang, H. K. (2015). Pegylated liposomal doxorubicin (Lipo-Dox®) combined with cyclophosphamide and 5-fluorouracil is effective and safe as salvage chemotherapy in taxane-treated metastatic breast cancer: an open-label, multi-center, non-comparative phase II study. BMC cancer, 15, 423. https://doi.org/10.1186/s12885-015-1433-4
[13]. Citron, M. L., Berry, D. A., Cirrincione, C., Hudis, C., Winer, E. P., Gradishar, W. J., Davidson, N. E., Martino, S., Livingston, R., Ingle, J. N., Perez, E. A., Carpenter, J., Hurd, D., Holland, J. F., Smith, B. L., Sartor, C. I., Leung, E. H., Abrams, J., Schilsky, R. L., Muss, H. B., … Norton, L. (2003). Randomized trial of dose-dense versus conventionally scheduled and sequential versus concurrent combination chemotherapy as postoperative adjuvant treatment of node-positive primary breast cancer: first report of Intergroup Trial C9741/Cancer and Leukemia Group B Trial 9741. Journal of clinical oncology : official journal of the American Society of Clinical Oncology, 21(8), 1431–1439.
[14]. Gui, J., Mustachio, L. M., Su, D. M., & Craig, R. W. (2012). Thymus Size and Age-related Thymic Involution: Early Programming, Sexual Dimorphism, Progenitors and Stroma. Aging and disease, 3(3), 280–290.
[15]. Fulop, T., Witkowski, J. M., Olivieri, F., & Larbi, A. (2018, December). The integration of inflammaging in age-related diseases. In Seminars in immunology (Vol. 40, pp. 17-35). Academic Press.
[16]. Michaelson, J. S., Silverstein, M., Sgroi, D., Cheongsiatmoy, J. A., Taghian, A., Powell, S., ... & Smith, B. (2003). The effect of tumor size and lymph node status on breast carcinoma lethality. Cancer: Interdisciplinary International Journal of the American Cancer Society, 98(10), 2133-2143.
[17]. Holliday, D. L., & Speirs, V. (2011). Choosing the right cell line for breast cancer research. Breast cancer research, 13, 1-7.
[18]. Kuo, H. T., Que, J., Lin, L. C., Yang, C. C., Koay, L. B., & Lin, C. H. (2017). Impact of tumor size on outcome after stereotactic body radiation therapy for inoperable hepatocellular carcinoma. Medicine, 96(50), e9249.
[19]. Kümler, I., Christiansen, O. G., & Nielsen, D. L. (2014). A systematic review of bevacizumab efficacy in breast cancer. Cancer treatment reviews, 40(8), 960-973.
[20]. Tewey, K. M., Rowe, T. C., Yang, L., Halligan, B. D., & Liu, L. F. (1984). Adriamycin-induced DNA damage mediated by mammalian DNA topoisomerase II. Science, 226(4673), 466-468.
[21]. Weaver, B. A. (2014). How Taxol/paclitaxel kills cancer cells. Molecular biology of the cell, 25(18), 2677-2681
[22]. Abal, M., Andreu, J. M., & Barasoain, I. (2003). Taxanes: microtubule and centrosome targets, and cell cycle dependent mechanisms of action. Current cancer drug targets, 3(3), 193-203.
[23]. Gérard, C., & Goldbeter, A. (2014). The balance between cell cycle arrest and cell proliferation: control by the extracellular matrix and by contact inhibition. Interface focus, 4(3), 20130075.
[24]. Stanton, R. A., Gernert, K. M., Nettles, J. H., & Aneja, R. (2011). Drugs that target dynamic microtubules: a new molecular perspective. Medicinal research reviews, 31(3), 443-481.
[25]. Dunnwald, Lisa K., Mary Anne Rossing, and Christopher I. Li. "Hormone receptor status, tumor characteristics, and prognosis: a prospective cohort of breast cancer patients." Breast cancer research 9 (2007): 1-10.
[26]. Bardou, Valerie-Jeanne, et al. "Progesterone receptor status significantly improves outcome prediction over estrogen receptor status alone for adjuvant endocrine therapy in two large breast cancer databases." Journal of clinical oncology21.10 (2003): 1973-1979.
[27]. Lower, Elyse E., et al. "Impact of metastatic estrogen receptor and progesterone receptor status on survival." Breast cancer research and treatment 90 (2005): 65-70.
[28]. Fan W, Chang J, Fu P. Endocrine therapy resistance in breast cancer: current status, possible mechanisms and overcoming strategies. Future Med Chem. 2015;7(12):1511–1519. doi:10.4155/fmc.15.93
[29]. Nahleh, Z. (2008). Androgen Receptor As A Target For the Treatment of Hormone Receptor-Negative Breast Cancer: An Unchartered Territory. Future Oncology, 4(1), 15–21.
[30]. Cooper GM. The Cell: A Molecular Approach. 2nd edition. Sunderland, MA: Sinauer Associates; 2000.
[31]. Slamon, D. J., Godolphin, W., Jones, L. A., Holt, J. A., Wong, S. G., Keith, D. E., Levin, W. J., Stuart, S. G., Udove, J., & Ullrich, A. (1989). Studies of the HER-2/neu proto-oncogene in human breast and ovarian cancer. Science (New York, N.Y.), 244(4905), 707–712.
[32]. Native Hawaiian women and the experience of breast cancer.Lau, B., Tominez, P., Shing, J. Z., Vo, J. B., Pollom, E., & Taparra, K. (2023). Racial disparities among Asian American, Native Hawaiian, and other Pacific Islander Patients with cancer who refuse recommended radiation therapy or surgery. Cancers, 15(13), 3358.)
[33]. Eide P. (2006). Native Hawaiian women and the experience of breast cancer. Women & health, 44(4), 41–59.
[34]. Palmer, D. B. (2013). The effect of age on thymic function. Frontiers in Immunology, 4, 316.
[35]. Aubert, G., & Lansdorp, P. M. (2008). Telomeres and aging. Physiological reviews, 88(2), 557–579.
[36]. Gunasekaran, G.H., Hassali, M.A.B.A., Sabri, W.M.A.B.W. et al. Impact of chemotherapy schedule modification on breast cancer patients: a single-centre retrospective study. Int J Clin Pharm 42, 642–651 (2020).
[37]. Rakha, Emad A., et al. "Breast cancer prognostic classification in the molecular era: the role of histological grade." Breast cancer research 12 (2010): 1-12.
[38]. Henson, Donald Earl, et al. "Relationship among outcome, stage of disease, and histologic grade for 22,616 cases of breast cancer. The basis for a prognostic index." Cancer 68.10 (1991): 2142-2149.
Cite this article
Zhang,J.;Zhou,X.;Sun,R. (2025). Comparative Efficacy of Adjuvant Chemotherapy Regimens for Breast Cancer: A Study on Cyclophosphamide and Doxorubicin (AC) Versus Paclitaxel (T) and the Impact of Biological Characteristics. Theoretical and Natural Science,78,235-244.
Data availability
The datasets used and/or analyzed during the current study will be available from the authors upon reasonable request.
Disclaimer/Publisher's Note
The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of EWA Publishing and/or the editor(s). EWA Publishing and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
About volume
Volume title: Proceedings of the 4th International Conference on Biological Engineering and Medical Science
© 2024 by the author(s). Licensee EWA Publishing, Oxford, UK. This article is an open access article distributed under the terms and
conditions of the Creative Commons Attribution (CC BY) license. Authors who
publish this series agree to the following terms:
1. Authors retain copyright and grant the series right of first publication with the work simultaneously licensed under a Creative Commons
Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this
series.
2. Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the series's published
version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial
publication in this series.
3. Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and
during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See
Open access policy for details).
References
[1]. Ferlay J, Ervik M, Lam F, Laversanne M, Colombet M, Mery L, Piñeros M, Znaor A, Soerjomataram I, Bray F (2024). Global Cancer Observatory: Cancer Today. Lyon, France: International Agency for Research on Cancer. Available from: https://gco.iarc.who.int/today, accessed [21/07/2024].
[2]. Early Breast Cancer Trialists' Collaborative Group (EBCTCG) (2005). Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet (London, England), 365(9472), 1687–1717.
[3]. Cardoso, F., van't Veer, L. J., Bogaerts, J., Slaets, L., Viale, G., Delaloge, S., Pierga, J. Y., Brain, E., Causeret, S., DeLorenzi, M., Glas, A. M., Golfinopoulos, V., Goulioti, T., Knox, S., Matos, E., Meulemans, B., Neijenhuis, P. A., Nitz, U., Passalacqua, R., Ravdin, P., … MINDACT Investigators (2016). 70-Gene Signature as an Aid to Treatment Decisions in Early-Stage Breast Cancer. The New England journal of medicine, 375(8), 717–729.
[4]. Clifford A. Hudis, Luca Gianni, Triple‐Negative Breast Cancer: An Unmet Medical Need, The Oncologist, Volume 16, Issue S1, January 2011, Pages 1–11
[5]. Abu Samaan, T. M., Samec, M., Liskova, A., Kubatka, P., & Büsselberg, D. (2019). Paclitaxel’s mechanistic and clinical effects on breast cancer. Biomolecules, 9(12), 789.
[6]. R Buzzoni et al., Adjuvant chemotherapy with doxorubicin plus cyclophosphamide, methotrexate, and fluorouracil in the treatment of resectable breast cancer with more than three positive axillary nodes.. JCO 9, 2134-40(1991).
[7]. Kimura, M., Sano, M., Fujimori, M., Nakagomi, H., Negishi, T., Yanagita, Y., & Sato, N. (2008). Neoadjuvant paclitaxel for operable breast cancer: multicenter phase II trial with clinical outcomes. Anticancer research, 28(2B), 1239–1244.
[8]. Biganzoli, L., Cufer, T., Bruning, P., Coleman, R., Duchateau, L., Calvert, A. H., Gamucci, T., Twelves, C., Fargeot, P., Epelbaum, R., Lohrisch, C., & Piccart, M. J. (2002). Doxorubicin and paclitaxel versus doxorubicin and cyclophosphamide as first-line chemotherapy in metastatic breast cancer: The European Organization for Research and Treatment of Cancer 10961 Multicenter Phase III Trial. Journal of clinical oncology: official journal of the American Society of Clinical Oncology, 20(14), 3114–3121.
[9]. Diéras, V., Fumoleau, P., Romieu, G., Tubiana-Hulin, M., Namer, M., Mauriac, L., Guastalla, J. P., Pujade-Lauraine, E., Kerbrat, P., Maillart, P., Pénault-Llorca, F., Buyse, M., & Pouillart, P. (2004). Randomized parallel study of doxorubicin plus paclitaxel and doxorubicin plus cyclophosphamide as neoadjuvant treatment of patients with breast cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology, 22(24), 4958–4965.
[10]. Jones, S. E., Savin, M. A., Holmes, F. A., O'Shaughnessy, J. A., Blum, J. L., Vukelja, S., McIntyre, K. J., Pippen, J. E., Bordelon, J. H., Kirby, R., Sandbach, J., Hyman, W. J., Khandelwal, P., Negron, A. G., Richards, D. A., Anthony, S. P., Mennel, R. G., Boehm, K. A., Meyer, W. G., & Asmar, L. (2006). Phase III trial comparing doxorubicin plus cyclophosphamide with docetaxel plus cyclophosphamide as adjuvant therapy for operable breast cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology, 24(34), 5381–5387.
[11]. Saura, C., Tseng, L. M., Chan, S., Chacko, R. T., Campone, M., Manikhas, A., Nag, S. M., Leichman, C. G., Dasappa, L., Fasching, P. A., Hurtado de Mendoza, F., Symmans, W. F., Liu, D., Mukhopadhyay, P., Horak, C., Xing, G., & Pusztai, L. (2013). Neoadjuvant doxorubicin/cyclophosphamide followed by ixabepilone or paclitaxel in early stage breast cancer and evaluation of βIII-tubulin expression as a predictive marker. The oncologist, 18(7), 787–794. https://doi.org/10.1634/theoncologist.2013-0075
[12]. Rau, K. M., Lin, Y. C., Chen, Y. Y., Chen, J. S., Lee, K. D., Wang, C. H., & Chang, H. K. (2015). Pegylated liposomal doxorubicin (Lipo-Dox®) combined with cyclophosphamide and 5-fluorouracil is effective and safe as salvage chemotherapy in taxane-treated metastatic breast cancer: an open-label, multi-center, non-comparative phase II study. BMC cancer, 15, 423. https://doi.org/10.1186/s12885-015-1433-4
[13]. Citron, M. L., Berry, D. A., Cirrincione, C., Hudis, C., Winer, E. P., Gradishar, W. J., Davidson, N. E., Martino, S., Livingston, R., Ingle, J. N., Perez, E. A., Carpenter, J., Hurd, D., Holland, J. F., Smith, B. L., Sartor, C. I., Leung, E. H., Abrams, J., Schilsky, R. L., Muss, H. B., … Norton, L. (2003). Randomized trial of dose-dense versus conventionally scheduled and sequential versus concurrent combination chemotherapy as postoperative adjuvant treatment of node-positive primary breast cancer: first report of Intergroup Trial C9741/Cancer and Leukemia Group B Trial 9741. Journal of clinical oncology : official journal of the American Society of Clinical Oncology, 21(8), 1431–1439.
[14]. Gui, J., Mustachio, L. M., Su, D. M., & Craig, R. W. (2012). Thymus Size and Age-related Thymic Involution: Early Programming, Sexual Dimorphism, Progenitors and Stroma. Aging and disease, 3(3), 280–290.
[15]. Fulop, T., Witkowski, J. M., Olivieri, F., & Larbi, A. (2018, December). The integration of inflammaging in age-related diseases. In Seminars in immunology (Vol. 40, pp. 17-35). Academic Press.
[16]. Michaelson, J. S., Silverstein, M., Sgroi, D., Cheongsiatmoy, J. A., Taghian, A., Powell, S., ... & Smith, B. (2003). The effect of tumor size and lymph node status on breast carcinoma lethality. Cancer: Interdisciplinary International Journal of the American Cancer Society, 98(10), 2133-2143.
[17]. Holliday, D. L., & Speirs, V. (2011). Choosing the right cell line for breast cancer research. Breast cancer research, 13, 1-7.
[18]. Kuo, H. T., Que, J., Lin, L. C., Yang, C. C., Koay, L. B., & Lin, C. H. (2017). Impact of tumor size on outcome after stereotactic body radiation therapy for inoperable hepatocellular carcinoma. Medicine, 96(50), e9249.
[19]. Kümler, I., Christiansen, O. G., & Nielsen, D. L. (2014). A systematic review of bevacizumab efficacy in breast cancer. Cancer treatment reviews, 40(8), 960-973.
[20]. Tewey, K. M., Rowe, T. C., Yang, L., Halligan, B. D., & Liu, L. F. (1984). Adriamycin-induced DNA damage mediated by mammalian DNA topoisomerase II. Science, 226(4673), 466-468.
[21]. Weaver, B. A. (2014). How Taxol/paclitaxel kills cancer cells. Molecular biology of the cell, 25(18), 2677-2681
[22]. Abal, M., Andreu, J. M., & Barasoain, I. (2003). Taxanes: microtubule and centrosome targets, and cell cycle dependent mechanisms of action. Current cancer drug targets, 3(3), 193-203.
[23]. Gérard, C., & Goldbeter, A. (2014). The balance between cell cycle arrest and cell proliferation: control by the extracellular matrix and by contact inhibition. Interface focus, 4(3), 20130075.
[24]. Stanton, R. A., Gernert, K. M., Nettles, J. H., & Aneja, R. (2011). Drugs that target dynamic microtubules: a new molecular perspective. Medicinal research reviews, 31(3), 443-481.
[25]. Dunnwald, Lisa K., Mary Anne Rossing, and Christopher I. Li. "Hormone receptor status, tumor characteristics, and prognosis: a prospective cohort of breast cancer patients." Breast cancer research 9 (2007): 1-10.
[26]. Bardou, Valerie-Jeanne, et al. "Progesterone receptor status significantly improves outcome prediction over estrogen receptor status alone for adjuvant endocrine therapy in two large breast cancer databases." Journal of clinical oncology21.10 (2003): 1973-1979.
[27]. Lower, Elyse E., et al. "Impact of metastatic estrogen receptor and progesterone receptor status on survival." Breast cancer research and treatment 90 (2005): 65-70.
[28]. Fan W, Chang J, Fu P. Endocrine therapy resistance in breast cancer: current status, possible mechanisms and overcoming strategies. Future Med Chem. 2015;7(12):1511–1519. doi:10.4155/fmc.15.93
[29]. Nahleh, Z. (2008). Androgen Receptor As A Target For the Treatment of Hormone Receptor-Negative Breast Cancer: An Unchartered Territory. Future Oncology, 4(1), 15–21.
[30]. Cooper GM. The Cell: A Molecular Approach. 2nd edition. Sunderland, MA: Sinauer Associates; 2000.
[31]. Slamon, D. J., Godolphin, W., Jones, L. A., Holt, J. A., Wong, S. G., Keith, D. E., Levin, W. J., Stuart, S. G., Udove, J., & Ullrich, A. (1989). Studies of the HER-2/neu proto-oncogene in human breast and ovarian cancer. Science (New York, N.Y.), 244(4905), 707–712.
[32]. Native Hawaiian women and the experience of breast cancer.Lau, B., Tominez, P., Shing, J. Z., Vo, J. B., Pollom, E., & Taparra, K. (2023). Racial disparities among Asian American, Native Hawaiian, and other Pacific Islander Patients with cancer who refuse recommended radiation therapy or surgery. Cancers, 15(13), 3358.)
[33]. Eide P. (2006). Native Hawaiian women and the experience of breast cancer. Women & health, 44(4), 41–59.
[34]. Palmer, D. B. (2013). The effect of age on thymic function. Frontiers in Immunology, 4, 316.
[35]. Aubert, G., & Lansdorp, P. M. (2008). Telomeres and aging. Physiological reviews, 88(2), 557–579.
[36]. Gunasekaran, G.H., Hassali, M.A.B.A., Sabri, W.M.A.B.W. et al. Impact of chemotherapy schedule modification on breast cancer patients: a single-centre retrospective study. Int J Clin Pharm 42, 642–651 (2020).
[37]. Rakha, Emad A., et al. "Breast cancer prognostic classification in the molecular era: the role of histological grade." Breast cancer research 12 (2010): 1-12.
[38]. Henson, Donald Earl, et al. "Relationship among outcome, stage of disease, and histologic grade for 22,616 cases of breast cancer. The basis for a prognostic index." Cancer 68.10 (1991): 2142-2149.









