1. Introduction
Hepatocellular carcinoma (HCC) is the most prevalent type of primary liver cancer with approximately 865,300 new individual cases diagnosed each year and leads to roughly 757,900 fatalities globally in recent years [1]. HCC represents the characteristic of heterogeneity and is associated with various etiological contributors, such as chronic infections with Hepatitis B Virus (HBV) and Hepatitis C Virus (HCV), along with alcohol consumption, exposure to aflatoxins, and fatty liver disease [2].
Surgery, radiotherapy, and chemotherapy are recognized and predominant worldwide as therapeutic modalities for cancer treatment. In the last few years, targeted therapy such as photothermal and photodynamic therapies has undergone significant enhancement and development [3]. Nonetheless, the individualized treatment of cancer remains in its nascent phase. The complexity of individual carcinogens leads to a diverse dynamic tumor microenvironment (TME) in which interaction with the cancer cells causes tumorigenesis, metastasis, and therapy resistance. Tracing back to the origin of human self defence system, immunotherapy provides an alternative direction of curing cancer by activating or enhancing the cytotoxicity of immune response against tumorigenesis [4]. Tyrosine kinase inhibitors (TKIs) are commonly applied to patients with resistance to chemotherapy as first-line treatments, but multiple clinical trials have demonstrated that the survival advantages conferred by second-line immune checkpoint inhibitor (ICI) treatments did not show improvement in HCC patients who had previously progressed on TKI therapies compared to those had not undergone previous treatment [5]. Gene screening and editing technology on the molecular level is desired to create remarkable approaches in new therapy development. Widely used genome editing technologies such as zinc-finger nucleases (ZFNs), transcription activator-like effector nucleases (TALENs) have made great progress in targeted therapy advancement. Despite gene editing technology in clinical applications remaining in experimental stages with many challenges, the latest clustered regularly interspaced short palindromic repeats associated nuclease 9 (CRISPR/Cas9) has been proverbially used in most of the biomedicine research laboratories for mechanism studies and medicine development. Previous research has proved that CRISPR/Cas9 allows simpler acquisition of homozygous mutants, simultaneous, and convenient introduction of multiple mutations at various sites with lower cytotoxicity than early generations of genome editing techniques [6]. The majority of trials focus on editing immune factors in immunotherapy not directly targeting the specific aberrant gene within tumor cells since large risks and absence of effectiveness are generated when operating on patients. Though the application of CRISPR/Cas9 therapies is less common in solid tumor trials than non-solid tumour such as blood cancer, many studies are continuously examining the potential therapeutic value of CIRSPR/Cas9 in common solid tumor such as liver, lung, intestinal and breast cancer [7]. This review presents evidence assessing the applicability and practicability of the CRISPR/Cas9 system in the development of immunotherapies for treating HCC both in animal models and human clinical trials.
2. Principle of Gene Editing via CRISPR-Cas9
2.1. Structure and Working Principle of CRISPR/Cas9
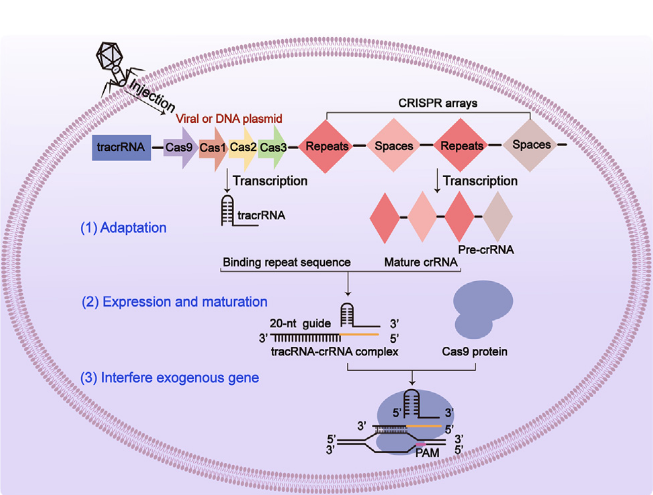
Figure 1: The schematic representation of the three-phase structure of CRISPR/Cas9 [8].
As shown in figure 1, the CRISPR/Cas9 system operates as a powerful mechanism incorporating genetic “scissors” that are capable of precise cleaving on double-stranded DNA (dsDNA). This system is formulated with a single guide RNA (sgRNA) and Cas9 protein that functions as an endonuclease. The sgRNA itself is consisted of CRISPR RNA (crRNA) and trans-activated crRNA (tracrRNA), which are linked through base pairing facilitated by the enzymatic action of RNase III. The primary role of the sgRNA is to direct the Cas9 protein to execute accurate incisions in the specified DNA sequence [9]. When external plasmid DNA is introduced into the host, it undergoes the first processing of adaption from intruding DNA to generate protospacers. The selection of these protospacers is determined by the adjacent motifs known as protospacer adjacent motifs (PAM). Subsequently, the protospacer is combined into a sequence associated with the invading plasmid or viral vectors, resulting in an updated spacer that retains specific memory. This updated spacer is then comprised into the CRISPR arrays. During the second phase of expression, a tracrRNA-crRNA complex is formed by integrating the pre-crRNA produced by the CRISPR array after transcription with the repetitive sequence of the tracrRNA. In the final stages of interference, the nuclease is activated when the tracrRNA-crRNA complex binds to the Cas9 protein. When exogenous genes introduce, sgRNA acts as a guide to accurately target the PAM. Upon recognition of the PAM, Cas9 cleaves the double-stranded DNA three nucleotides upstream of the PAM, a strategy that enhances the targeting of gene editing and helps protect the organism from foreign genomic invasions [10].
The CRISPR/Cas9 serves as a powerful biological tool for advancing biotechnology which utilizes the co-editing capabilities of sgRNA and Cas9 protein to modify DNA. However, a significant challenge lies in the inadequacy of delivery systems, which hampers its clinical application. Therefore, there are urgent needs to develop effective and precise delivery systems for cancer therapies. Previous studies have asserted that viral vectors are highly productive in delivering proteins by including plasmid-based CRISPR/Cas9 and ribonucleoprotein complexes. However, concerns regarding off-target effects and safety persist and present obstacles in clinical applications. For example, adenoviruses have been effectively utilized to target and edit Pten genes in animal models of non-alcoholic steatohepatitis in experimental settings, attributed to their capability to transport substantial genetic payloads and furnish supplementary nuclear localization signals. However, the use of adenovirus vectors haven been reported that can trigger inflammatory responses in vivo, potentially leading to hepatomegaly after four months. Similarly, while adeno-associated viruses can mitigate host immune responses and enhance delivery efficiency, the persistent expression of Cas9 nuclease may lead to significant off-target effects [8].
2.2. Combination of CRISPR/Cas9 with HCC Treatment
The CRISPR/Cas9 technology has been applied in clinical cancer treatment as a powerful genetic tool, aiding physicians in identifying and selecting genes involved in immune regulation at the whole-genome level. Over the past decade, cancer models have been reported to simulate liver cancer with higher efficiency and new experimental designs through the CRISPR/Cas9. Utilizing the CRISPR/Cas9 screening technology, with the aid of specialized sgRNA libraries, in vivo knockout screening of immune-regulatory genes has been achieved in mouse tumor models, thereby modulating the TME and optimizing the ability of immune cells to attack tumors. Existing data prove that a hypoxia environment is present in most solid tumor cases, including HCC [11]. Among these, Studies have shown that knocking out the Bclaf1 gene can inhibit the glycolytic pathway of Hypoxia-Inducible Factor-1 (HIF-1) in hypoxic conditions, thereby suppressing the occurrence and development of malignant tumors [9]. In addition, this method has been widely applied in various oncogenes and tumor suppressor gene identification and discover biomarker associated carcinogens for advanced therapeutic approaches.
3. Current Status of CRISPR/Cas9 and Targeted Therapy Applications
3.1. CIRSPR/Cas9 Application in ICI Therapy
Immunotherapy has recently demonstrated significant efficacy, specifically in cancer treatment. The fundamental mechanism of this therapeutic approach includes the genetic alteration of immune cells extracted from patients. These modified cells are subsequently reinfused to promote the targeted recognition and eradication of tumor cells. Over the past few years, the CRISPR/Cas9 methodology has become an effective genetic modification and has attracted considerable interest in tumor immunotherapy. As one of the consists of systemic therapy, immunotherapy with ICIs has shown potential for enhanced overall survival of HCC patients with primary target cell death protein-1 (PD-1), its ligand programmed cell death-ligand 1 (PD-L1) or proteins cytotoxic T lymphocyte antigen 4 (CTLA-4) [12].
3.1.1. PD-1/PD-L1 Locus for CAR-T Therapy
Many ICIs have been discovered and designed as monotherapy to target the PD-1. Meanwhile, chimeric antigen receptor T (CAR T) cells have been engineered to express specific receptors that allow them to recognize and activate against tumor cells to induce a targeted immune response. PD-1 is responsible for activation and regulatory T cells, and its ligand PD-L1 is expressed in most tumor cell types. Research has demonstrated that disruption of the native PD-1 locus can adequately enhance the tumoricidal capacity of CAR T cell therapy. Significant approaches were achieved in 2016 to effectively knock down the PD-1 molecule on T cells using CRISPR/Cas9 technology to treat metastatic non-small cell lung carcinoma, encouraging promising outcomes in clinical trials. The disrupted PD-1 protein typically inhibits cells' immune response, and thus, cancer exploits this function to proliferate. Therefore, knocking out PD-1 can reactivate the cellular immune response. Researchers extracted immune cells from the subject's blood and then used CRISPR-Cas9 to deactivate the gene encoding the PD-1 protein. CRISPR-Cas9 combined a DNA-cutting enzyme with a molecular guide that could be programmed to instruct the enzyme precisely where to cut [13]. Recent studies have shown that modifying the PD-1 site on immune checkpoints using CRISPR/Cas9 can significantly enhance the effectiveness of CAR-T therapy in treating HCC. This discovery underscores the potential of CRISPR/Cas9 in improving the outcomes of cancer immunotherapy. This strategy exceeds the limitations encountered with CAR T therapy due to PD-L1 expression on tumor cells. It indicates that precise alterations at the PD-1 locus may support the effectiveness of CAR T cells in HCC treatment [14].
3.1.2. Combination Approach of PD-1/PD-L1 and Anti-VEGF Therapy Via CIRSPR/Csas9 Based HIF-1α
HIF-1α is an oxygen-dependent transcription factor induced under hypoxic conditions. It plays a critical role in the progression, vascularization, chemoresistance, and maintenance of liver cancer stem cells in HCC. HIF-1α stimulates hypoxic pathways that result in the elevated present of vascular endothelial growth factor (VEGF). The VEGF triggers the epithelial-to-mesenchymal transition, enhances tumor invasion, and promotes metastasis through alternating substances in the surrounding matrix. In the current investigation, a lentivirus-mediated CRISPR/Cas9 system directly targeting the human HIF-1α gene was involved in elucidating its function in the human liver cancer cell line. The packaging vector disrupted HIF-1α by reducing cellular proliferation, migration, and invasiveness, and it also triggered apoptosis under hypoxic conditions. More recent publications claim that a combination of PD-1/PD-L1 inhibitors and anti-VEGF agents can significantly improve the therapy efficacy in advanced HCC patients while the evaluation of immune-related toxicity from these treatments is ongoing [15].
3.2. CRISPR/Cas9 is A New Strategy to Prevent Viral Hepatitis from Progressing to HCC
Due to the inability to completely eliminate covalently closed circular DNA (cccDNA) caused by HBV infection, current treatments can only alleviate the hepatitis infection but cannot cure it completely [16]. The CRISPR/Cas9 technology offers a new direction for therapeutic approaches. CRISPR/Cas9 can enhance experimental outcomes by targeting HBV cccDNA. Kostyusheva et.al used cell line of HepG2 human hepatoma cells demonstrated that small molecular inhibitors such as RI-1 and NU7026 can effectively inhibit homologous recombination (HR) and non-homologous end joining (NHEJ), thereby increasing the effects induced by CRISPR/Cas9 to reduce the levels of cccDNA in HBV leading HCC [17].
HCV infection is also one of the causes leading to HCC, and current studies have shown that experiments involving CRISPR/Cas9 can efficiently suppress the expression of HCV. Price et al. designed an rgRNA similar to the tracrRNA of Francisella novicida (FnCas9) and utilized the characteristic that Cas9 derived from FnCas9 can be edited and applied on specific RNA substrates [18]. After transfecting this rgRNA into human hepatocellular carcinoma cells and co-culturing with a fluorescent recombinant virus, they observed that the expression of FnCas9 reduced the levels of viral protein expression.
4. Conclusion
This review integrates recent CRISPR/Cas9 research related to the development of HCC and lists several potential therapeutic targets identified in different malignancies using CRISPR/Cas9 genome editing technology. Over the years, this system has been refined to extend its applications to a broader range of screenings, such as base editor and epigenetic screening. However, improving CRISPR/Cas9 as a cancer therapy choice involves several issues, such as the epigenetic linkage of pathogenic locus, immune responses to Cas9, verification of therapeutic targets in animal models, and optimizing delivery methods while minimizing off-target effects, all of which must be addressed before proceeding to clinical application. Despite all these challenges, CRISPR/Cas9 offers valuable opportunities in cancer gene therapy and may take a crucial part in cancer treatment.
References
[1]. Bray, F., Ferlay, J., Soerjomataram, I., Siegel, R. L., Torre, L. A., & Jemal, A. (2024). Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: A Cancer Journal for Clinicians, 74(3), 229-263.
[2]. Sayiner, M., Golabi, P., & Younossi, Z. M. (2019). Disease burden of hepatocellular carcinoma: A global perspective. Digestive Diseases and Sciences, 64(4), 910-917.
[3]. Li, X., Lovell, J. F., Yoon, J., & Chen, X. (2020). Clinical development and potential of photothermal and photodynamic therapies for cancer. Nature Reviews Clinical Oncology, 17(11), 657-674.
[4]. Nagarsheth, N., Wicha, M. S., & Zou, W. (2017). Chemokines in the cancer microenvironment and their relevance in cancer immunotherapy. Nature Reviews Immunology, 17(9), 559-572.
[5]. Xie, Y., et al. (2024). Targeting AXL induces tumor-intrinsic immunogenic response in tyrosine kinase inhibitor-resistant liver cancer. Cell Death & Disease, 15(2), 110. https://doi.org/10.1038/s41419-024-06493-0
[6]. Jiang, C., Meng, L., Yang, B., & Luo, X. (2020). Application of CRISPR/Cas9 gene editing technique in the study of cancer treatment. Clinical Genetics, 97(1), 73-88.
[7]. Liu, B., Saber, A., & Haisma, H. J. (2019). CRISPR/Cas9: A powerful tool for identification of new targets for cancer treatment. Drug Discovery Today, 24(4), 955-970. https://doi.org/10.1016/j.drudis.2019.02.011
[8]. Yu, S., et al. (2023). Research progress and application of the CRISPR/Cas9 gene-editing technology based on hepatocellular carcinoma. Asian Journal of Pharmaceutical Sciences, 18(4), 100828.
[9]. Feng, X., Li, Z., Liu, Y., Chen, D., & Zhou, Z. (2024). CRISPR/Cas9 technology for advancements in cancer immunotherapy: From uncovering regulatory mechanisms to therapeutic applications. Experimental Hematology & Oncology, 13(1), 102.
[10]. Makarova, K. S., et al. (2011). Evolution and classification of the CRISPR–Cas systems. Nature Reviews Microbiology, 9(6), 467-477.
[11]. Ogunwobi, O. O., et al. (2019). Mechanisms of hepatocellular carcinoma progression. World Journal of Gastroenterology, 25(19), 2279-2293.
[12]. Van Doorn, D. J., Takkenberg, R. B., & Klümpen, H. J. (2020). Immune checkpoint inhibitors in hepatocellular carcinoma: An overview. Pharmaceuticals (Basel), 14(1).
[13]. Cyranoski, D. (2016). CRISPR gene-editing tested in a person for the first time. Nature, 539(7630), 479.
[14]. Xu, Y., Chen, C., Guo, Y., Hu, S., & Sun, Z. (2022). Effect of CRISPR/Cas9-edited PD-1/PD-L1 on tumor immunity and immunotherapy. Frontiers in Immunology, 13, 848327.
[15]. Feng, Z., Rong, P., & Wang, W. (2020). Meta-analysis of the efficacy and safety of PD-1/PD-L1 inhibitors administered alone or in combination with anti-VEGF agents in advanced hepatocellular carcinoma. Gut, 69(10), 1904-1906.
[16]. Bartosh, U. I., Dome, A. S., Zhukova, N. V., et al. (2023). CRISPR/Cas9 as a new antiviral strategy for treating hepatitis viral infections. International Journal of Molecular Sciences, 25(1), 334.
[17]. Kostyusheva, A. P., Kostyushev, D. S., Brezgin, S. A., Zarifyan, D. N., Volchkova, E. V., & Chulanov, V. P. (2019). Small molecular inhibitors of DNA double strand break repair pathways increase the anti-HBV activity of CRISPR/Cas9. Molecular Biology, 53(2), 274-285.
[18]. Price, A. A., Sampson, T. R., Ratner, H. K., Grakoui, A., & Weiss, D. S. (2015). Cas9-mediated targeting of viral RNA in eukaryotic cells. Proceedings of the National Academy of Sciences, 112(19), 6164-6169.
Cite this article
Wei,Y. (2025). Assessment of the Viability of CRISPR/Cas9 Application in Immunotherapy for Hepatocellular. Theoretical and Natural Science,90,31-36.
Data availability
The datasets used and/or analyzed during the current study will be available from the authors upon reasonable request.
Disclaimer/Publisher's Note
The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of EWA Publishing and/or the editor(s). EWA Publishing and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
About volume
Volume title: Proceedings of ICMMGH 2025 Workshop: Computational Modelling in Biology and Medicine
© 2024 by the author(s). Licensee EWA Publishing, Oxford, UK. This article is an open access article distributed under the terms and
conditions of the Creative Commons Attribution (CC BY) license. Authors who
publish this series agree to the following terms:
1. Authors retain copyright and grant the series right of first publication with the work simultaneously licensed under a Creative Commons
Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this
series.
2. Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the series's published
version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial
publication in this series.
3. Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and
during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See
Open access policy for details).
References
[1]. Bray, F., Ferlay, J., Soerjomataram, I., Siegel, R. L., Torre, L. A., & Jemal, A. (2024). Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: A Cancer Journal for Clinicians, 74(3), 229-263.
[2]. Sayiner, M., Golabi, P., & Younossi, Z. M. (2019). Disease burden of hepatocellular carcinoma: A global perspective. Digestive Diseases and Sciences, 64(4), 910-917.
[3]. Li, X., Lovell, J. F., Yoon, J., & Chen, X. (2020). Clinical development and potential of photothermal and photodynamic therapies for cancer. Nature Reviews Clinical Oncology, 17(11), 657-674.
[4]. Nagarsheth, N., Wicha, M. S., & Zou, W. (2017). Chemokines in the cancer microenvironment and their relevance in cancer immunotherapy. Nature Reviews Immunology, 17(9), 559-572.
[5]. Xie, Y., et al. (2024). Targeting AXL induces tumor-intrinsic immunogenic response in tyrosine kinase inhibitor-resistant liver cancer. Cell Death & Disease, 15(2), 110. https://doi.org/10.1038/s41419-024-06493-0
[6]. Jiang, C., Meng, L., Yang, B., & Luo, X. (2020). Application of CRISPR/Cas9 gene editing technique in the study of cancer treatment. Clinical Genetics, 97(1), 73-88.
[7]. Liu, B., Saber, A., & Haisma, H. J. (2019). CRISPR/Cas9: A powerful tool for identification of new targets for cancer treatment. Drug Discovery Today, 24(4), 955-970. https://doi.org/10.1016/j.drudis.2019.02.011
[8]. Yu, S., et al. (2023). Research progress and application of the CRISPR/Cas9 gene-editing technology based on hepatocellular carcinoma. Asian Journal of Pharmaceutical Sciences, 18(4), 100828.
[9]. Feng, X., Li, Z., Liu, Y., Chen, D., & Zhou, Z. (2024). CRISPR/Cas9 technology for advancements in cancer immunotherapy: From uncovering regulatory mechanisms to therapeutic applications. Experimental Hematology & Oncology, 13(1), 102.
[10]. Makarova, K. S., et al. (2011). Evolution and classification of the CRISPR–Cas systems. Nature Reviews Microbiology, 9(6), 467-477.
[11]. Ogunwobi, O. O., et al. (2019). Mechanisms of hepatocellular carcinoma progression. World Journal of Gastroenterology, 25(19), 2279-2293.
[12]. Van Doorn, D. J., Takkenberg, R. B., & Klümpen, H. J. (2020). Immune checkpoint inhibitors in hepatocellular carcinoma: An overview. Pharmaceuticals (Basel), 14(1).
[13]. Cyranoski, D. (2016). CRISPR gene-editing tested in a person for the first time. Nature, 539(7630), 479.
[14]. Xu, Y., Chen, C., Guo, Y., Hu, S., & Sun, Z. (2022). Effect of CRISPR/Cas9-edited PD-1/PD-L1 on tumor immunity and immunotherapy. Frontiers in Immunology, 13, 848327.
[15]. Feng, Z., Rong, P., & Wang, W. (2020). Meta-analysis of the efficacy and safety of PD-1/PD-L1 inhibitors administered alone or in combination with anti-VEGF agents in advanced hepatocellular carcinoma. Gut, 69(10), 1904-1906.
[16]. Bartosh, U. I., Dome, A. S., Zhukova, N. V., et al. (2023). CRISPR/Cas9 as a new antiviral strategy for treating hepatitis viral infections. International Journal of Molecular Sciences, 25(1), 334.
[17]. Kostyusheva, A. P., Kostyushev, D. S., Brezgin, S. A., Zarifyan, D. N., Volchkova, E. V., & Chulanov, V. P. (2019). Small molecular inhibitors of DNA double strand break repair pathways increase the anti-HBV activity of CRISPR/Cas9. Molecular Biology, 53(2), 274-285.
[18]. Price, A. A., Sampson, T. R., Ratner, H. K., Grakoui, A., & Weiss, D. S. (2015). Cas9-mediated targeting of viral RNA in eukaryotic cells. Proceedings of the National Academy of Sciences, 112(19), 6164-6169.









