1. Introduction
Tetracycline antibiotics are named for their amine and tetracyclic core structure. Tetracycline is one of them, structurally being an amine and tetracyclic derivative, with a core made up of four interconnected rings: A, B, C, and D. The main functional groups include an amine group at the C2 position of ring A, a dimethylamino group at the C4 position, and a hydroxyl group at the C10 position of ring D. Its molecular formula is: C22H24N2O8. As a result of this conjugated configuration, it contains two chromophoric units within rings A, B, C, and the D aromatic ring, demonstrating considerable absorption in the ultraviolet spectrum ranging from 270 to 360 nm [1].Tetracycline is a broad-spectrum antibiotic commonly used to treat bacterial infections. The drug operates by selectively binding to the A site of the 30S subunit of bacterial ribosomes. This action obstructs the attachment of aminoacyl-tRNA at this site, which consequently hampers peptide chain elongation and disrupts the process of bacterial protein synthesis [2]. The specific structure of tetracycline consists of four six-membered rings, containing multiple hydroxyl, amide, and ketone groups [2]. China is a major producer, user, and seller of tetracycline antibiotics, and tetracyclines are the most widely used antibiotics in the livestock and poultry industry in China. Due to the poor volatility of tetracycline antibiotic residues, the main pathways of migration in the environment are through water bodies and soil. Whether from antibiotic industrial wastewater, medical antibiotics, or antibiotics used in the livestock industry, 25% to 75% of tetracycline is ultimately discharged into the environment in its original form or as metabolites, leading to varying degrees of pollution in environmental water bodies. As a result, the development of swift, straightforward, and exceptionally sensitive detection techniques is crucial for monitoring of pollution [3].
Enzyme-linked immunosorbent assay (ELISA) and colloidal gold immunochromatographic assay (GICA) are presently the predominant rapid detection techniques employed for the identification of tetracycline [4].The operational principle of the ELISA assay relies on a competitive interaction that encompasses the specific attachment of tetracycline to antibodies, subsequently leading to a chromogenic development reaction [5]. The operational mechanism of GICA relies on the precise interaction between antigen and antibody, coupled with the chromatic response produced by colloidal gold labeling [6]. Given this, this article will analyze the principles and differences of GICA and ELISA in detecting tetracycline.
2. Methodology
2.1. Indirect competitive Enzyme-Linked Immunosorbent Assay (ELISA)
The indirect competitive ELISA (Enzyme-Linked Immunosorbent Assay) is an immunoassay method used to detect specific antigens or antibodies. Its operational mechanism primarily relies on the specific interaction between the antigen and antibody, in conjunction with the enzymatic reaction that produces a measurable signal [7]. Below are the working steps and principles of the indirect competitive ELISA:
2.1.1. Antigen fixation
Known tetracycline antigens are pre-fixed to the bottom surface of the microplate wells. This is typically achieved through physical adsorption or chemical cross-linking.
2.1.2. Sample addition
The sample to be tested (which may contain tetracycline) is added to the microplate wells where the antigen is already fixed. Tetracycline in the sample will competitively bind with the specific antibody that will be added later, competing with the fixed antigen on the well bottom.
2.1.3. Addition of specific antibody
A known specific antibody labeled with an enzyme is added to each well. At this point, if tetracycline is present in the sample, it will competitively bind with the specific antibody, competing with the fixed antigen on the well bottom.
2.1.4. Washing
Unbound antibodies and other impurities are washed away, leaving only the antibodies bound to the antigen.
2.1.5. Color development
An appropriate enzyme substrate is introduced, resulting in the formation of a colored product due to the enzyme's catalytic action on the substrate. The intensity of the color is inversely proportional to the amount of tetracycline antigen bound to the microplate.
2.1.6. Result detection
The intensity of the chromogenic reaction is assessed through a colorimetric approach, typically employing a spectrophotometer, which enables the indirect quantification of the antigen or antibody concentration within the sample. The weaker the color, the higher the concentration of tetracycline in the sample, as more antibodies bind to the tetracycline in the sample rather than to the fixed antigen on the well bottom.
2.2. Colloidal Gold Immunochromatographic Assay (GICA)
The colloidal gold immunochromatographic test strip is an efficient and straightforward immunoassay device commonly utilized for the detection of a range of small analytes, including drugs, toxins, hormones, and other substances [8]. Its working principle is based on the specific binding of antigen-antibody and the color reaction of colloidal gold labeling. Below is a detailed description of the steps and principles for detecting tetracycline:
2.2.1. Sample addition
The sample (tetracycline) is added to the sample well of the test strip. The sample liquid will flow down the test strip from the sample well due to capillary action.
2.2.2. Colloidal Gold-Labeled Antibody Binding
The initial area of the test strip (usually near the sample well) contains pre-fixed colloidal gold-labeled specific antibodies. In the event that the small molecular substance, specifically the antigen under investigation, exists within the sample, it will interact with the colloidal gold-conjugated antibodies, resulting in the formation of an antigen-antibody complex.
2.2.3. Chromatographic process
Under capillary action, the sample liquid and the already bound antigen-antibody complex continue to move forward along the test strip.
2.2.4. Control line display
The composition of the test strip in the detection reagent is shown in Figure 1.
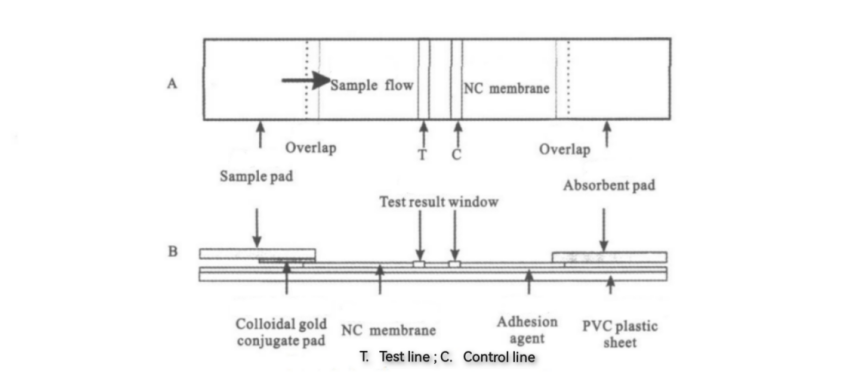
Figure 1: Top View(A) and Side View(B) of the Immunochromatographic Test Strip[1]
2.3. Test result and judgment
Due to capillary action, the liquid moves forward and reaches the T line. At the T line, the tetracycline present in the sample competes with the TC-BSA complex immobilized on the T line for binding to the anti-tetracycline monoclonal antibody-colloidal gold complex. The following situations may occur (see Figure 2)
(1) Negative : If the TC concentration in the sample is below 100 ng/ml or there is no TC, the gold-labeled antibody will flow to the T line with the TC-free sample liquid, bind with the TC fixed on the NC membrane through BSA, forming a "gold-labeled antibody-TC-BSA" complex, resulting in a red color reaction; the excess "gold-labeled antibody" continues to move backward and binds with the gold-labeled antibody at the C line (containing anti-gold-labeled antibody), forming a "gold-labeled antibody-anti-gold-labeled antibody" complex, showing red (T line red, C line red). The T line (test line, closer to the sample well) is darker or the same as the C line (control line).
(2) Positive : If the TC concentration ithe sample is above 100 ng/ml, the TC binds with the gold-labeled antibody, forming a "TC-gold-labeled antibody" complex. The T line contains LPS-BSA conjugates (TC is fixed on the NC membrane by conjugating with large molecules such as BSA), and the gold-labeled antibody is preferentially bound by the TC in the sample, so it cannot bind with the TC conjugated and fixed on the T line, thus inhibiting the binding reaction at the T line, resulting in no color reaction; the "TC-gold-labeled antibody" complex continues to move backward and binds with the gold-labeled antibody at the C line (containing anti-gold-labeled antibody), forming a "TC-gold-labeled antibody-anti-gold-labeled antibody" complex, showing red (T line colorless, C line red).
(3) Invalid: The lack of the C line suggests a flawed operational procedure or that the test strip has deteriorated and is ineffective [2]..
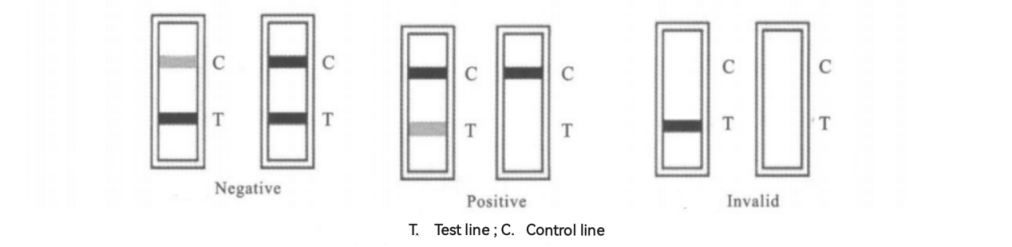
Figure 2: Positive and Negative Result Determination[1]
3. Results and discussion
Table 1: Detection limit and linear ranges of ELISA and GICA
Methods | Detection limit(ng/mL) | Linear range(ng/mL) | Derivation |
ELISA | 0.05 | 0.05-4.05 | Han Minqi. Determination of Tetracycline Concentration in Shanghai Raw Water by Enzyme-Linked Immunosorbent Assay. |
GICA | 100 | 100-500 | Tan Zunshe. Rapid Detection of Tetracycline Residues in Aquatic Products by Colloidal Gold Immunochromatographic Assay. |
According to Table 1, the detection limit of the enzyme-linked immunosorbent assay (ELISA) is 0.05 ng/mL, and the linear range is 0.05-4.05 ng/mL (data from Han Minqi. Determination of Tetracycline Concentration in Shanghai Raw Water by Enzyme-Linked Immunosorbent Assay). The detection limit of the colloidal gold immunochromatographic assay (GICA) is 100 ng/mL, and the linear range is 100-500 ng/mL (data from Tan Zunshe. Rapid Detection of Tetracycline Residues in Aquatic Products by Colloidal Gold Immunochromatographic Assay). Compared to GICA, ELISA has a lower detection limit but a smaller linear range. ELISA is a laboratory method with a high catalytic frequency, offering high sensitivity and specificity in detecting tetracycline, but it has complex detection steps, consumes more time, and requires a high level of expertise from the personnel conducting the tests. The colloidal gold method is a portable method. GICA uses immunocolloidal gold or rapid test methods for antibody detection. Through the rapid assessment of the colorimetric response between the test line and the control line, the concentration of the analyte can be determined, facilitating qualitative or semi-quantitative analysis. The reagents can be stored for a long time, the results are easy to interpret, the accuracy is high, and the requirements for operators are relatively low.
Enzyme-linked immunosorbent assay (ELISA) and gold immunochromatographic assay (GICA) are widely utilized immunoassay techniques. However, ELISA offers distinct advantages regarding detection sensitivity and analytical range. The reasons for this are as follows: (see Table 2)
Table 2: Comparison between ELISA and GICA
Comparison Item | ELISA (Enzyme-linked Immunosorbent Assay) | GICA (Colloidal Gold Immunochromatography Assay) |
Detection Sensitivity | Higher; can enhance sensitivity by extending reaction time and using high-sensitivity reagents | Relatively lower; mainly relies on visual observation, sensitivity is limited |
Detection Range | Broader; can adjust parameters to extend range and conduct quantitative analysis | Relatively limited; mainly used for qualitative or semi-quantitative analysis |
Signal Amplification | Better; uses enzymes to produce large amounts of products | Limited; mainly relies on colloidal gold particle aggregation |
Interference Factor Control | Better; multiple washes can remove non-specific binding | Worse; difficult to effectively remove interference in one step |
Detection Accuracy | Higher; quantitative detection using instruments, results are more accurate | Lower; mainly relies on visual judgment, easily influenced by subjective factors |
Operational Complexity | More complex; multi-step reactions | Lower; operation is simple and quick |
Suitable Scenarios | Laboratory precise quantitative analysis | On-site rapid detection |
In conclusion, ELISA's superior detection sensitivity and broader range compared to GICA can be attributed to its multi-step reaction processes, signal amplification mechanisms, and the use of instrumental detection techniques. Conversely, GICA's operational simplicity and speed render it more suitable for rapid on-site assessments. Each method possesses distinct characteristics and is appropriate for different application contexts.
4. Conclusion
This research investigates the contamination of tetracycline antibiotics within China's livestock and poultry industries, comparing two rapid detection methodologies: Enzyme-Linked Immunosorbent Assay (ELISA) and Colloidal Gold Immunochromatographic Assay (GICA). The findings indicate that ELISA outperforms GICA in terms of detection sensitivity and linear range, rendering it more suitable for high-precision laboratory environments. In contrast, GICA's ease of use and rapidity present significant advantages for field assessments, particularly for individuals lacking expertise. Each technique possesses unique strengths and weaknesses, highlighting the necessity for environmental monitoring and pollution mitigation.
Nevertheless, the study is not without its limitations. The sample size and diversity were constrained; future investigations should aim to incorporate a wider array of samples to enhance generalizability. Furthermore, while the operational methodologies were delineated, an evaluation of economic feasibility and cost-effectiveness was absent, which is crucial for real-world applications. A more comprehensive literature review could also bolster the research's contextual framework and depth.
Subsequent studies could investigate innovative detection technologies, such as nanotechnology or biosensors, to improve sensitivity and specificity. Research focusing on the co-detection of multiple antibiotics could address issues of compound pollution [9]. Additionally, investigations into the long-term impacts of tetracycline on environmental and public health are imperative for guiding policy formulation [10].
References
[1]. Liu Jintao. (2013). Research on Tetracycline Enzyme-Linked Immunosorbent Assay (Master's thesis, Tianjin University of Science and Technology). Master’s.
[2]. Tan Zunshe, Lu Heng, Shao Wei, & Zhang Shaoen. (2010). Rapid Detection of Tetracycline Residues in Aquatic Products by Colloidal Gold Immunochromatography. Journal of Northwest Agriculture (08), 32-37.
[3]. Sun Xuemei. (2017). Electrochemical Analysis Method for Tetracycline in Environmental Water Samples (Master's thesis, Chengdu University of Technology). Master’s.
[4]. Li Yingying, Xue Yuming, Yan Qingli, Cheng Yan, Pang Yixiu, Yu Hao, & Yang Pengfei. (2024). Comparison of HIV Antibody Detection Results by Enzyme-Linked Immunosorbent Assay and Colloidal Gold Chromatography. Jiangsu Health Care (02), 163-164.
[5]. Han Minqi, Jiang Zenghui, Lu Zhihui, & Cao Xiani. (2015). Determination of Tetracycline Concentration in Shanghai Raw Water by Enzyme-Linked Immunosorbent Assay. Water Supply Technology (03), 57-59.
[6]. Xu Ying. (2021). Research on Three Rapid Immunochromatographic Detection Technologies for Tetracycline Residues in Milk (Master's thesis, China Jiliang University). Master’s. Retrieved from
[7]. Li Yalin, Zhang Junsheng, & Wang Qinhui. (2005). Determination of Tetracycline Residues in Milk by Enzyme-Linked Immunosorbent Assay. Yunnan Animal Husbandry and Veterinary Medicine (02), 27.
[8]. Ngom, B., Guo, Y., Wang, X., & Bi, D. (2010). Development and application of lateral flow test strip technology for detection of infectious agents and chemical contaminants: a review. Analytical and bioanalytical chemistry, 397, 1113-1135.
[9]. James, C. A., Miller-Schulze, J. P., Ultican, S., Gipe, A. D., & Baker, J. E. (2016). Evaluating contaminants of emerging concern as tracers of wastewater from septic systems. Water research, 101, 241-251.
[10]. Granados-Chinchilla, F., & Rodríguez, C. (2017). Tetracyclines in food and feedingstuffs: from regulation to analytical methods, bacterial resistance, and environmental and health implications. Journal of analytical methods in chemistry, 2017(1), 1315497.
Cite this article
Wei,M. (2025). Comparison of Enzyme-Linked Immunosorbent Assay (ELISA) and Colloidal Gold Immunochromatographic Assay (GICA) for Tetracycline Detection. Theoretical and Natural Science,85,18-23.
Data availability
The datasets used and/or analyzed during the current study will be available from the authors upon reasonable request.
Disclaimer/Publisher's Note
The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of EWA Publishing and/or the editor(s). EWA Publishing and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
About volume
Volume title: Proceedings of the 3rd International Conference on Modern Medicine and Global Health
© 2024 by the author(s). Licensee EWA Publishing, Oxford, UK. This article is an open access article distributed under the terms and
conditions of the Creative Commons Attribution (CC BY) license. Authors who
publish this series agree to the following terms:
1. Authors retain copyright and grant the series right of first publication with the work simultaneously licensed under a Creative Commons
Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this
series.
2. Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the series's published
version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial
publication in this series.
3. Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and
during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See
Open access policy for details).
References
[1]. Liu Jintao. (2013). Research on Tetracycline Enzyme-Linked Immunosorbent Assay (Master's thesis, Tianjin University of Science and Technology). Master’s.
[2]. Tan Zunshe, Lu Heng, Shao Wei, & Zhang Shaoen. (2010). Rapid Detection of Tetracycline Residues in Aquatic Products by Colloidal Gold Immunochromatography. Journal of Northwest Agriculture (08), 32-37.
[3]. Sun Xuemei. (2017). Electrochemical Analysis Method for Tetracycline in Environmental Water Samples (Master's thesis, Chengdu University of Technology). Master’s.
[4]. Li Yingying, Xue Yuming, Yan Qingli, Cheng Yan, Pang Yixiu, Yu Hao, & Yang Pengfei. (2024). Comparison of HIV Antibody Detection Results by Enzyme-Linked Immunosorbent Assay and Colloidal Gold Chromatography. Jiangsu Health Care (02), 163-164.
[5]. Han Minqi, Jiang Zenghui, Lu Zhihui, & Cao Xiani. (2015). Determination of Tetracycline Concentration in Shanghai Raw Water by Enzyme-Linked Immunosorbent Assay. Water Supply Technology (03), 57-59.
[6]. Xu Ying. (2021). Research on Three Rapid Immunochromatographic Detection Technologies for Tetracycline Residues in Milk (Master's thesis, China Jiliang University). Master’s. Retrieved from
[7]. Li Yalin, Zhang Junsheng, & Wang Qinhui. (2005). Determination of Tetracycline Residues in Milk by Enzyme-Linked Immunosorbent Assay. Yunnan Animal Husbandry and Veterinary Medicine (02), 27.
[8]. Ngom, B., Guo, Y., Wang, X., & Bi, D. (2010). Development and application of lateral flow test strip technology for detection of infectious agents and chemical contaminants: a review. Analytical and bioanalytical chemistry, 397, 1113-1135.
[9]. James, C. A., Miller-Schulze, J. P., Ultican, S., Gipe, A. D., & Baker, J. E. (2016). Evaluating contaminants of emerging concern as tracers of wastewater from septic systems. Water research, 101, 241-251.
[10]. Granados-Chinchilla, F., & Rodríguez, C. (2017). Tetracyclines in food and feedingstuffs: from regulation to analytical methods, bacterial resistance, and environmental and health implications. Journal of analytical methods in chemistry, 2017(1), 1315497.









