1. Introduction
Diabetes mellitus, a chronic metabolic disease characterized by an inability to effectively control blood glucose levels, was first described in the 2nd century A.D. by Aretaeus of Cappadocia [1, 2]. Type 2 diabetes is the most common type, accounting for more than 90 per cent of diagnosed cases, causing more than 1.5 million deaths each year, and is expected to affect more than one billion people by 2050 [1, 3, 4]. The disease can damage the heart, eyes, kidneys, and nerves, eventually leading to death [5]. The main feature of diabetes is a deficiency of insulin, a hormone secreted by pancreatic β-cells that promotes cellular uptake of glucose [3, 6]. Despite the widespread use of insulin and its analogues, as well as glucagon-like peptide-1 receptor agonists (GLP-1 receptor agonists), the development of oral peptide drugs continues to present significant challenges. Increasing the dosage of insulin is an accepted treatment for diabetes, and insulin and its analogues and glucagon-like peptide-1 receptor agonists (GLP-1 receptor agonists) have become the most commonly used medications for the treatment of diabetes [7]. And GLP-1 is a peptide hormone present in the body that promotes insulin secretion [8], which was discovered later than insulin, due to studies that demonstrated that oral glucose promotes insulin release to a greater extent than intravenous glucose [9] GLP-1 receptor agonists interact with the GLP-1 receptor, thereby activating GLP-1 secretion [10]. Of the various insulin and analog preparations, all except Rybelsus are administered intravenously or subcutaneously [7]. Indeed, not only peptides used for the treatment of diabetes, but all peptides are difficult to design for oral administration [11]. Antidiabetic drugs make up the largest proportion of peptide drugs and have the highest need for developing oral delivery [12]. Studies have been trying various strategies to design oral peptide drugs such as lysine vasopressin intragastric delivery, nanoparticles, microneedles, self-emulsifying delivery systems, peptide conjugates and permeation enhancers among other technologies [12, 13]. Through an in-depth analysis of the existing literature, the paper explores strategies for the development of oral peptide drugs, focusing on various technologies such as nanoparticles, microneedles and permeation enhancers. In addition, the effectiveness and feasibility of different approaches are explored and their potential application in diabetes treatment is assessed. Therefore, the paper aims to investigate oral peptide drugs to meet the urgent needs of diabetes treatment. Oral drug delivery is favoured due to its high patient compliance and ease of production [14]. The successful development of oral peptide medications can improve the patient experience with medications and significantly improve diabetes outcomes, thus reducing the burden on society and the healthcare system.
2. The Structures of Insulin and Glp-1 Receptor Agonists
2.1. The Structure and Function of Insulin and Its Analogues
Insulin is a polypeptide consisting of 51 amino acids with 2 chains, 30 amino acids form B chain and 21 form A chain, and the two chains are connected by disulfide bridges [6]. For human, insulin have molecular mass with 5802 [6]. Also, Insulin is found in other mammals like pig or cow, and those insulin have 51 amino acids and 2 chains like human, but with some different particular amino acids [15]. Animals insulins can be also used in the early treatment of diabetes, and their effects of treatment do not have obvious difference with those of human insulin [15]. At present, the most common insulin drugs are analogues of human insulin [7]. And some amino acids are altered or additional functional groups are attached to amino acids by genetic engineering of specific bacteria such as E. coli and Saccharomyces cerevisiae [16,17]. By altering some amino acids, duration, stability or peak plasma concentration canl be improved [16]. For example, insulin Aspart, a short acting insulin, which replace the B28 amino acid proline with aspartic acid, has shorter time for absorbance and higher peak concentration [18]. Similarly, some insulin analogues like Glargine can acting longer time than human insulin, hence reduce the dosing frequency [16]. Figure 1 shows the common analogues of insulin.
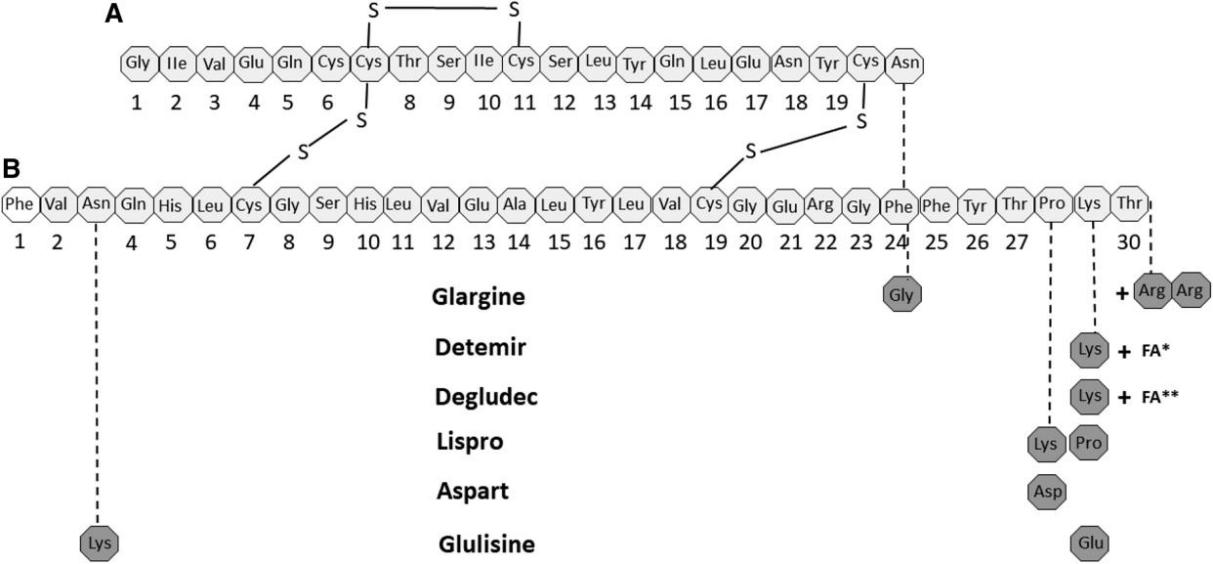
Figure 1: Structures of Human Insulin and Analogue s[16]
2.2. The Structural Feature of GLP-1 Receptor Agonists
GLP-1 consists of 30 or 31 amino acids in 1 chain [19]. In humans, GLP-1 consists of 31 amino acids [20]. Despite the fact that a number of animals are capable of secreting GLP-1, only a limited number of studies have concentrated on its structural composition and its effects on humans [21]. Similar to insulin, the common GLP-1 diabetes mellitus drugs are GLP-1 analogues, professionally known as GLP-1 receptor agonists [7]. As with insulin, changing some amino acids and adding some functional groups to increase the potency or stability are the most common methods to make analogues [22]. For example, semaglutide, which changed 3 amino acids and attached with a C18 di-acid, can prolong the duration of action and thus reduce the number of administrations compared to native human GLP-1 [23, 24]. Figure 2 and Figure 3 show the structure of human GLP-1 and the GLP-1 analogue - semaglutide.
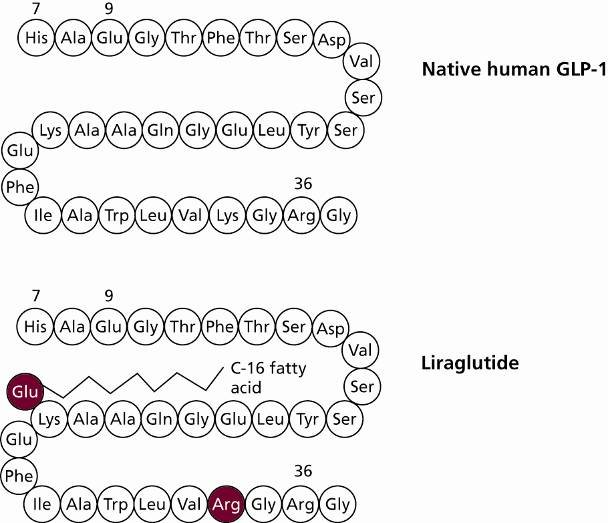
Figure 2: Structure of Human GLP-1 [20]
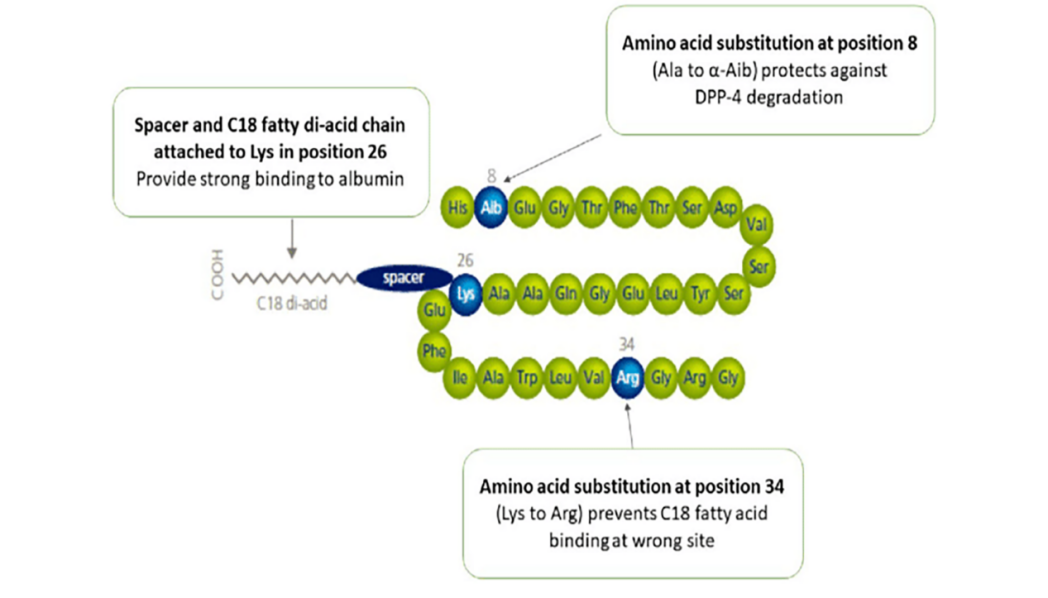
Figure 3: Structure of Semaglutide [23]
3. The Challenges and Barriers of Oral Administration
Though oral delivery of drug is the most popular way of administering drug,but for insulin and GLP-1 receptor, there are some barriers prevent the appearance of oral insulin or oral GLP-1 receptor agonist [11,14]. Oral peptides usually require a stable and predictable environment to assess absorption, distribution, metabolism, and excretion (ADME) in patients due to the peptides’ enzyme sensitivity and PH [25, 26].
3.1. Complex Environment of Gastrointestinal Tract
The internal environment of the gastrointestinal tract varies considerably for different patients and can also be affected by behaviors such as eating or posture [11]. The PH value indicates the acidity or alkalinity of a fluid [27]. False PH can lead the oral drug release the API in a wrong environment hence cause the unexpected biological response of patients [28]. PH values of oral medications varied significantly from 1.0 to 7.5 [29]. In addition, some diseases can also change the PH value of stomach and small intestine, like cystic fibrosis can lower the PH of duodenum than 5.5 [11]. Moreover, a study conducted by Tanya L. Russell et al. showed that patients had different PH values before and after meals, and that for older adults, postprandial gastric and duodenal PH values take longer than for younger adults to return to fasting state levels [30]. Though postures of patients are often overlooked, but it indeed affect the ADME of drugs, for example, sitting, standing and recumbent right can help the absorbance of oral drug [31].
3.2. Special Properties of Peptides
Compared with traditional small molecular drugs, biopharmaceuticals commonly have complex and huge structures, which means that biopharmaceuticals like peptide need to overcome barriers than small molecular drugs. In particular, peptides are highly unstable, and due to the complex and accurate structures of biopharmaceuticals, any biodegradation, temperature, or light changes may lead to changes in their structure, which can lead to incorrect folding or unfolding, thus resulting in incorrect interactions or immune responses, and as a result, unexpected biological reactions can occur [32]. For peptide, the presence of many enzymes in the gastrointestinal tract, such as pepsin, trypsin, chymotrypsin, carboxypeptidase, pancreatic amylase, and pancreatic lipase, which catalyze peptide degradation, implies that peptides must be well protected [33]. In addition, due to the larger structure and higher molecular mass of biopharmaceuticals, membrane permeability is poorer than that of small molecule drugs [34].
3.3. Cost Matters
While the majority of peptide drugs are not suitable for oral administration, the exception is Rybelsus®, which is an oral semaglutide developed by Novo Nordisk. The bioavailability of Rybelsus® is only 0.4-1%, which means that by the oral route, only a very small amount of the active ingredient is able to enter the body's circulation under the oral route, resulting in less efficient drug utilization. As a result, despite the convenience this drug offers for oral formulation, its production cost is significantly higher than that of Ozempic®, another injectable semaglutide drug developed by Novo Nordisk [35,36]. This cost difference is partly attributed to complex environmental factors in the gastrointestinal tract, such as different pH values, enzymatic degradation and first-pass effects, which all impair the absorption efficiency of orally administered drugs [37]. In order to compensate for this absorption barrier, more Semaglutide needs to be added to the formulation of Rybelsus®, resulting in a significant increase in the amount of API used. Although the cost of the formulation of Rybelsus® (e.g., preparation and packaging) is lower than that of the injectable Ozempic®, the overall cost is still higher than that of Ozempic® due to the large amount of API required, and this disparity is even more pronounced in large-scale production [35]. Figure 4 provides a detailed comparison of the differences in production costs between Rybelsus® and Ozempic®.
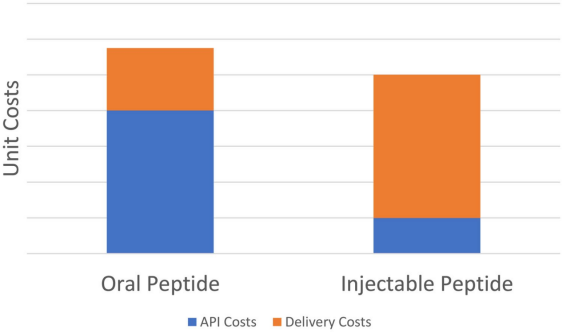
Figure 4: Cost Difference between Rybelsus® and Ozempic® [35]
4. Potential Technologies for Oral Peptide Drugs
Although developing oral peptide drugs face several difficulties, there are still some technologies which have potential to solve some problems.
4.1. Nanoparticles
Nanoparticles are particles with diameters between 1 nm and 100 nm, which can exhibit high stability and a strong drug-carrying capacity for drug delivery [38]. Also, they are able to carry both hydrophilic and hydrophobic substances, which makes them a preferred choice for the design of orally administered peptides [39]. Transmembrane transport of nanoparticles in the gastrointestines relies on four main mechanisms: transmembrane transport, receptor-mediated transport, carrier-mediated transport, and M-cell transport. M-cells are located in the intestinal collecting lymph nodes and are capable of absorbing nanoparticles and inducing an immune response [40]. Nanoparticles overcome the barriers of enzymatic degradation and membrane permeability and possess greater specificity [40]. Wang et al. indicated that racemate-4 loaded with chitosan nanoparticles was better absorbed than free racemate-4 in the kidneys of Madin-Darby dogs and the intestines of rats [41].
Depending on the material used for production, nanoparticles can be categorized into four main types: lipid nanoparticles, polymer nanoparticles, mesoporous silica nanoparticles, and metal-organic framework nanoparticles [36]. In particular, lipid nanoparticles have a hydrophobic outer shell and a hydrophilic interior in which the drug is encapsulated. And the outer shell usually interacts with a coating, such as phenylboronic acid (PBA) conjugated hyaluronic acid (HA-PBA) or chondroitin sulfate-g-glycocholic acid (CSG), in order to release the drug when it reaches a specific area [36]. However, the encapsulation of lipid nanoparticles is less efficient, more costly and less stable [36]. The encapsulation of application programming interfaces in lipid nanoparticles involves the use of various lipids, including PEG lipids, which are hydrophobic, cholesterol, and 1,2-distearoyl-sn-glycero-3-phosphocholine, which can boost efficiency, and ionizable lipids, which are hydrophilic, as shown in Figure 5 [42].
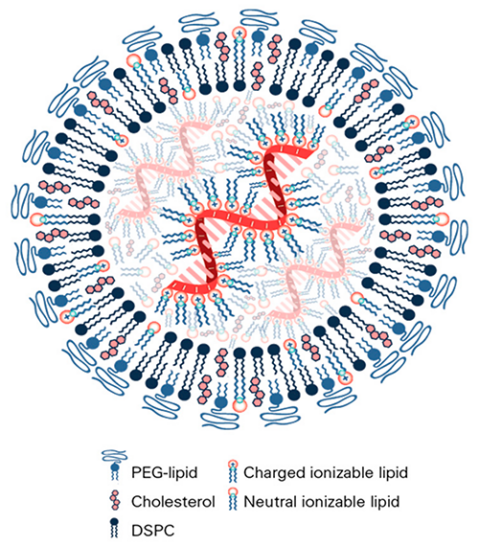
Figure 5: Structural Examples of Lipid Nanoparticles [42]
According to Figure 6, polymeric nanoparticles are classified as nanocapsules and nanospheres, and the main difference between the two is the way in which the drug is encapsulated: in nanocapsules the drug is in the center, while in nanospheres the drug is located on the surface [43]. Chitosan (CS) is one of the commonly used materials that modulates biodegradability and charge density and opens tight junctions between intestinal epithelial cells to increase drug permeability [36,44]. Synthetic polymers such as pHPMA exhibit high permeability and Shan et al. showed that PHPMA nanoparticles released drug in mucus with a 20-fold higher uptake rate than free insulin and were effective in lowering blood glucose in diabetic rats [45]. Despite the high encapsulation efficiency of nanopolymers, about 70%, these polymers tend to degrade after administration, which may lead to the release of the drug at an unintended site [36].
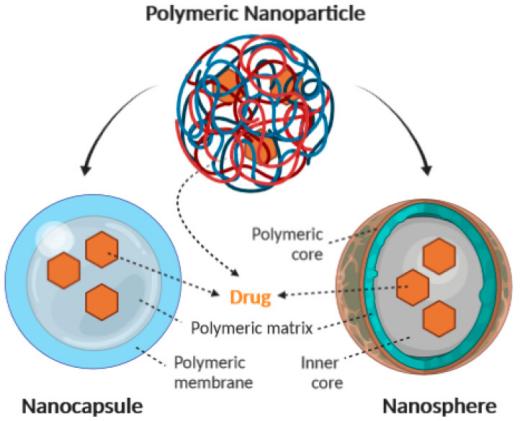
Figure 6: Types and Structures of Polymer Nanoparticles [43]
Mesoporous silica nanoparticles (MSN) are inorganic nanoparticles with high stability and biocompatibility, as shown in Figure 7. Drugs are loaded in the pores of MSNs, which usually need to be modified with organic materials to overcome gastrointestinal barriers [36,46]. It was found that chitosan and polylactic acid-co-polyethylene glycol modified MSNs are pH sensitive, have stronger interactions with intestinal cells, and are more permeable [47]. In addition, MSNs have high encapsulation efficiency, but require organic modification and are not biodegradable [36].
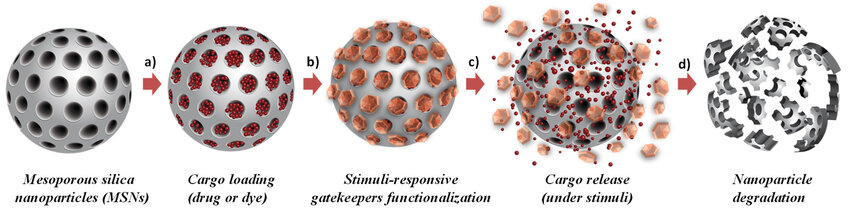
Figure 7: structure and mechanism of MSN [46]
Metal-organic frameworks (MOFs) are porous materials with homogeneous crystal structures that regulate pore size and functionality by altering the organic ligands and metal units [36]. Zhou et al. showed that iron-based MOF nanoparticles modified with sodium dodecyl sulfate (SDS) showed increased permeability and stability in acidic environments, and compared to the injection of free insulin, the plasma levels of insulin in diabetic rats were were higher [48]. MOFs, as an emerging technology, need to be further investigated [36].
4.2. Microneedle Devices
The basic principle of microneedle devices is the release of drugs at the target site by the puncture of a microneedle, and rationally designed peptides are effective against the gastrointestinal environment [12]. Abramson et al. developed an intraluminal unfolding microneedle injector, which is a swallowable capsule. The drug is released when the pH is ≥5.5 (usually in the intestinal environment). The microneedle was able to penetrate the intestinal cell membrane and release the drug directly into the bloodstream. Experimental results showed that in a porcine model, the method resulted in more than 10% insulin uptake over 4 hours as compared to conventional subcutaneous injections. In addition, microneedle materials are biodegradable and are usually manufactured using polymers such as polyvinyl alcohol (PVA) [49,50].
4.3. Self-Emulsifying Drug Delivery Systems
Self-emulsifying drug delivery systems (SEDDS) consist of drug, oil, surfactant and co-surfactant that form tiny emulsions in the gastrointestinal tract [51]. These emulsions are absorbed via the lymphatic route and are effective in avoiding first-pass effects and degradation caused by proteases [51]. In SEDDS, drugs are usually dissolved in oil, but for peptides, special design is required due to their high hydrophilicity and protein properties [52]. One approach is ion pairing, for example, insulin can be complexed with amphiphilic surfactants such as dialkyldimethylammonium bromide or soy phosphatidylcholine to enhance its lipid solubility [53]. Another strategy is double emulsification (SDEDDS), where Qi et al. developed an oil-in-water-based self-double emulsification drug delivery system capable of sequestering the hydrophilic peptide drug pidomod in the internal aqueous phase [54]. Experimental results showed a 2.56-fold increase in drug uptake in SDEDDS, and it was stable for 6 months at 25°C [54]. To protect the drug from lipase, enteric coating can be designed to prevent the drug from being degraded by lipase in the stomach. In the intestine, some excipients (e.g., Tween 80, sodium dodecyl sulfate, and dodecyltrimethylammonium bromide) are able to inhibit lipase activity [52]. In addition, excipients such as Cremophor RH 40 and glyceryl triacetate improve drug permeability [55].
4.4. Peptide Conjugation
Peptide conjugation is the process of linking peptides with other compounds to boost their intrinsic properties, including stability, acidity and basicity. Peptides are usually conjugated in two ways, pegylated and lipidated [12]. Tregopil was a developing ultra-rapid oral insulin that composed of the an insulin analogue which conjugate with alkylated polyethylene glycol [12]. This conjugation increase the water solubility and the resistance to enzymes [12]. Anh et al also conducted a research related to another type of conjugation, which is exendin-4 conjugated with low molecular weight chitosan, this conjugation can be cleaved red in vivo, in rats test, this conjugate peptide shows 6.4% bioavailability and effects of lower plasma glucose [56].
4.5. Permeation Enhancers
Compared with other technologies, two of the approved oral peptide drugs employ osmotic enhancers, Rybelsus® and Mycapssa® for the treatment of type 2 diabetes mellitus and acromegaly. Rybelsus utilizes sodium salt hexanedioate (SNAC) as an osmotic enhancer, whereas Mycapssa may use sodium caprylate (C8) [57].
Rybelsus is a white tablet designed to release the drug in the stomach. Clinical trials have demonstrated that when Rybelsus tablets are dissolved in the stomach, SNAC not only raises the local pH and reduces pepsin activity, thereby protecting the API semaglutide from enzymatic degradation, but also prevents its oligomerization [24,58,59]. When SNAC interacts with the lipid membranes of gastric epithelial cells, it fluidizes these membranes and facilitates the transcellular transport of the API into the bloodstream [24]. Mycapssa is a liquid capsule designed to release drugs in the gut with an enteric coating [57,60]. However, the half-life of the API octreotide in the intestinal fluid is only 6.8 minutes, thus requiring protective measures [61]. Since the enzyme is inactive in the oil phase, the drug was designed as a capsule encapsulated in an oily suspension [60]. While C8 may play a major role in enhancing permeability, the only known information is that TPE extends the tight junctions (TJs) of the small intestine, thereby facilitating the absorption of the API [57,62].
4.6. Combinations
In the technology of oral delivery of peptides, it is common to combine different technologies in the same drug. For example, chitosan can be used as a material for polymeric nanoparticles and as a permeation enhancer. It has been shown that chitosan and its derivatives are able to reduce the transepithelial electrical resistance (TEER) values and that free chitosan is superior to nanoparticles in promoting insulin permeation [63]. Chitosan's biocompatibility and enhanced penetration are its advantages in polymeric nanoparticle applications [64]. Qinna et al. showed that low molecular weight chitosan (LMWC) of different molecular weights and degrees of deacetylation were effective in lowering blood glucose levels in rats [65]. Rybelsus® is another example of binding technology. By adding a C18 lipoic acid chain to the peptide, the affinity with human serum albumin (HSA) is enhanced, thereby extending the drug half-life [66]. HSA is a plasma protein secreted by the human liver in the highest amount with an average half-life of 19 days. HSA have outstanding ability of binding with drugs and metal ions like Cu2+, Ni2+, Ca2+, Zn2+ and bilirubin, and also can increase the solubility of long chain fatty acid [67]. In Rybelsus® totally, apart from attaching C18 fatty di-acid to increase the half-life, permeation enhancer SNAC is also formulated.
5. Conclusion
Though oral delivery of peptide has many potential advantages like easy to take and administer, easy to manufacture, large-scale oral delivery of peptide drugs is not practical due to biological barriers, such as complex environments and the susceptible nature of peptides, as well as cost. Moreover, some technologies like nanoparticles, microneedle, SEDDS, peptide conjugation and permeation enhancer are developing to overcome the biological barriers of oral delivery peptide, and there are successful cases like Rybelsus®. However, due to the low bioavailability of orally administered peptides, manufacturers may not be inclined to promote, expand and invest in the research and production of orally administered peptides unless the cost of production of the API can be covered by the lower cost of the formulation.
References
[1]. Sapra, A. and Bhandari, P. (2023) Diabetes.In: StatPearls [Internet]. Treasure Island (FL): StatPearls.
[2]. Karamanou, M., Protogerou, A., Tsoucalas, G., Androutsos, G. and Poulakou-Rebelakou, E. (2016) Milestones in the history of diabetes mellitus: The main contributors. World J. Diabetes, 7: 1-7.
[3]. World Health Organization. (n.d.) Diabetes. https://www.who.int/health-topics/diabetes.
[4]. Ong, K.L. et al. (2023) Global, regional, and national burden of diabetes from 1990 to 2021, with projections of prevalence to 2050: a systematic analysis for the Global Burden of Disease Study 2021. The Lancet 402, 203-234.
[5]. Word Health Organization. (n.d.) Diabetes. https://www.who.int/news-room/fact-sheets/detail/diabetes.
[6]. Wilcox, G.(2005) Insulin and Insulin Resistance. Clin. Biochem. Rev. 26, 19-39.
[7]. Rashid, A. and Xie, V. (2024) Top 15 Diabetes Drugs in 2023 by 2022 Sales Statistics. https://xtalks.com/top-15-diabetes-drugs-in-2023-by-2022-sales-statistics-3715/.
[8]. Ahrén, B. (2011) GLP-1 for type 2 diabetes. Exp. Cell Res. 317, 1239-1245 (2011).
[9]. Sheahan, K.H., Wahlberg, E.A. and Gilbert, M.P. (2010) An overview of GLP-1 agonists and recent cardiovascular outcomes trials. Postgrad. Med. J. 96, 156-161.
[10]. Collins, L. and Costello, R.A. (2024) Glucagon-Like Peptide-1 Receptor Agonists. In: StatPearls [Internet], Treasure Island (FL).
[11]. Tyagi, P., Pechenov, S. and Anand Subramony, J. (2018) Oral peptide delivery: Translational challenges due to physiological effects. J. Controlled Release 287, 167-176.
[12]. Zizzari, A.T., et al. (2021) New perspectives in oral peptide delivery. Drug Discov. Today 26, 1097-1105.
[13]. Saffran, M., et al. (2011) A model for the study of the oral administration of peptide hormones. Can. J. Biochem.
[14]. Alqahtani, M.S., et al. (2021) Advances in Oral Drug Delivery. Front. Pharmacol. 12, 618411.
[15]. Richter, B. and Neises, G. (2005) Human’ insulin versus animal insulin in people with diabetes mellitus. Cochrane Database Syst. Rev., CD003816.
[16]. Kramer, C.K., Retnakaran, R. and Zinman, B. (2021) Insulin and insulin analogs as antidiabetic therapy: A perspective from clinical trials. Cell Metab. 33, 740-747.
[17]. Baeshen, N.A., et al. (2014) Cell factories for insulin production. Microb. Cell Factories 13, 141.
[18]. Heller, S., et al. (2013) Meta-analysis of insulin aspart versus regular human insulin used in a basal–bolus regimen for the treatment of diabetes mellitus. J. Diabetes 5, 482-491.
[19]. Mapelli, C., et al. (2009) Eleven amino acid glucagon-like peptide-1 receptor agonists with antidiabetic activity. J. Med. Chem. 52, 7788-7799.
[20]. Sjöholm Å. (2010) Liraglutide Therapy for Type 2 Diabetes: Overcoming Unmet Needs. Pharmaceuticals (Basel). 3(3): 764-781.
[21]. Damholt, A.B., Kofod, H. and Buchan, A.M.J. (1999) Immunocytochemical evidence for a paracrine interaction between GIP and GLP-1-producing cells in canine small intestine. Cell Tissue Res. 298, 287-293.
[22]. Gupta, V. (2013) Glucagon-like peptide-1 analogues: An overview. Indian J. Endocrinol. Metab. 17, 413.
[23]. Kalra, S. and Sahay, R. (2020) A Review on Semaglutide: An Oral Glucagon-Like Peptide 1 Receptor Agonist in Management of Type 2 Diabetes Mellitus. Diabetes Ther., 11(9): 1965-1982.
[24]. Aroda, V.R., Blonde, L. and Pratley, R.E. (2022) A new era for oral peptides: SNAC and the development of oral semaglutide for the treatment of type 2 diabetes. Rev. Endocr. Metab. Disord. 23, 979-994.
[25]. Scitable. (2010) Enzyme Catalysis: The Serine Proteases www.nature.com/scitable/topicpage/enzyme-catalysis-the-serine-proteases-14398894.
[26]. Ahmad, A. and Khan, J.M. (2022) pH-sensitive endosomolytic peptides in gene and drug delivery: Endosomal escape and current challenges. J. Drug Deliv. Sci. Technol. 76, 103786.
[27]. Britannica, T. (2024) pH. Encyclopedia Britannica. https://www.britannica.com/science/pH
[28]. Lee, Y.H. et al. (1999) Impact of Regional Intestinal pH Modulation on Absorption of Peptide Drugs: Oral Absorption Studies of Salmon Calcitonin in Beagle Dogs. Pharm. Res. 16, 1233-1239.
[29]. Hadji, H. and Bouchemal, K. (2022) Advances in the treatment of inflammatory bowel disease: Focus on polysaccharide nanoparticulate drug delivery systems. Adv. Drug Deliv. Rev. 181, 114101.
[30]. Russell, T.L., et al. (1993) Upper Gastrointestinal pH in Seventy-Nine Healthy, Elderly, North American Men and Women. Pharm. Res. 10, 187-196.
[31]. Queckenberg, C. and Fuhr U. (2009) Influence of posture on pharmacokinetics. Eur. J. Clin. Pharmacol. 65.
[32]. Akbarian, M. and Chen, S.H. (2022) Instability Challenges and Stabilization Strategies of Pharmaceutical Proteins. Pharmaceutics 14, 2533.
[33]. Wang, X.Q. and Zhang, Q. (2012) pH-sensitive polymeric nanoparticles to improve oral bioavailability of peptide/protein drugs and poorly water-soluble drugs. Eur. J. Pharm. Biopharm. 82, 219-229.
[34]. Mitragotri, S., Burke, P.A. and Langer, R. (2014) Overcoming the challenges in administering biopharmaceuticals: formulation and delivery strategies. Nat. Rev. Drug Discov. 13, 655-672.
[35]. Lewis, A.L., McEntee, N., Holland, J. et al. (2022) Development and approval of rybelsus (oral semaglutide): ushering in a new era in peptide delivery. Drug Deliv. and Transl. Res. 12, 1-6.
[36]. Li, Y., et al. (2022) Advances in oral peptide drug nanoparticles for diabetes mellitus treatment. Bioact. Mater. 15, 392-408.
[37]. Turner, P.V., et al. (2011) Administration of Substances to Laboratory Animals: Routes of Administration and Factors to Consider. J. Am. Assoc. Lab. Anim. Sci. JAALAS 50, 600-613.
[38]. Joudeh, N. and Linke, D. (2022) Nanoparticle classification, physicochemical properties, characterization, and applications: a comprehensive review for biologists. J. Nanobiotechnology 20, 262.
[39]. Gelperina, S., Kisich, K., Iseman, M.D. and Heifets, L. (2005) The Potential Advantages of Nanoparticle Drug Delivery Systems in Chemotherapy of Tuberculosis. Am. J. Respir. Crit. Care Med. 172, 1487-1490.
[40]. Cao, S., et al. (2019) Nanoparticles: Oral Delivery for Protein and Peptide Drugs. AAPS PharmSciTech 20, 190.
[41]. Wang, M., et al. (2014) Permeability of Exendin-4-Loaded Chitosan Nanoparticles across MDCK Cell Monolayers and Rat Small Intestine. Biol. Pharm. Bull. 37, 740-747.
[42]. ABP Biosciences. (n.d.) Lipid Nanoparticles (LNPs). www.abpbio.com/product/lipid-nanoparticles-lnps/.
[43]. Zielińska, A., et al. (2020) Polymeric Nanoparticles: Production, Characterization, Toxicology and Ecotoxicology. Molecules 25, 3731.
[44]. Lin, P., et al. (2018) Oral Nonviral Gene Delivery for Chronic Protein Replacement Therapy. Advanced Science, 5.
[45]. Shan, W. et al. (2015) Overcoming the Diffusion Barrier of Mucus and Absorption Barrier of Epithelium by Self-Assembled Nanoparticles for Oral Delivery of Insulin. ACS nano, 9(3): 2345-2356.
[46]. Poscher, V. and Salinas Y. (2020) Trends in Degradable Mesoporous Organosilica-Based Nanomaterials for Controlling Drug Delivery: A Mini Review. Materials (Basel).
[47]. Araújo, F., et al. (2015) Microfluidic Assembly of a Multifunctional Tailorable Composite System Designed for Site Specific Combined Oral Delivery of Peptide Drugs. ACS Nano 9: 8291-8302.
[48]. Zhou, Y., et al. (2020) A Nanocomposite Vehicle Based on Metal-Organic Framework Nanoparticle Incorporated Biodegradable Microspheres for Enhanced Oral Insulin Delivery. ACS Appl. Mater. Interfaces 12, 22581-22592.
[49]. Abramson, A., et al. (2019) A luminal unfolding microneedle injector for oral delivery of macromolecules. Nat. Med. 25: 1512-1518.
[50]. Yan, Q., et al. (2021) Finite Element Analysis for Biodegradable Dissolving Microneedle Materials on Skin Puncture and Mechanical Performance Evaluation. Polymers 13, 3043.
[51]. Zhu, Y., Ye, J. and Zhang, Q. (2020) Self-emulsifying Drug Delivery System Improve Oral Bioavailability: Role of Excipients and Physico-chemical Characterization. Pharm. Nanotechnol. 8, 290-301.
[52]. Leonaviciute, G. and Bernkop-Schnürch, A. (2015) Self-emulsifying drug delivery systems in oral (poly)peptide drug delivery. Expert Opin. Drug Deliv.
[53]. Li, P., et al. (2013) Preparation and characterization of insulin-surfactant complexes for loading into lipid-based drug delivery systems. J. Pharm. Sci. 102: 268-2698.
[54]. Qi, X., et al. (2011) Self-double-emulsifying drug delivery system (SDEDDS): a new way for oral delivery of drugs with high solubility and low permeability. Int. J. Pharm. 409: 245-251.
[55]. Friedl, H. et al. (2013) Development and evaluation of a novel mucus diffusion test system approved by self-nanoemulsifying drug delivery systems. J. Pharm. Sci. 102: 4406-441.
[56]. Ahn, S. et al. (2013) Oral delivery of an anti-diabetic peptide drug via conjugation and complexation with low molecular weight chitosan. J. Controlled Release 170: 226-232.
[57]. Kim, J.C., Park, E.J. and Na, D.H. (2022) Gastrointestinal Permeation Enhancers for the Development of Oral Peptide Pharmaceuticals. Pharmaceuticals, 15: 1585.
[58]. Bækdal, T.A., et al. (2021) Relationship Between Oral Semaglutide Tablet Erosion and Pharmacokinetics: A Pharmacoscintigraphic Study. Clin. Pharmacol. Drug Dev. 10: 453-462.
[59]. European Medicines Agency. (n.d) Rybelsus. www.ema.europa.eu/en/medicines/human/EPAR/rybelsus.
[60]. Brayden, D.J. and Maher, S. (2021) Transient Permeation Enhancer® (TPE®) technology for oral delivery of octreotide: a technological evaluation. Expert Opin. Drug Deliv. 18: 1501-1512.
[61]. Wang, J., Yadav, V., Smart, A.L., Tajiri, S. and Basit, A.W. (2015) Toward oral delivery of biopharmaceuticals: an assessment of the gastrointestinal stability of 17 peptide drugs. Mol. Pharm. 12: 966-973.
[62]. MyCapssa. (n.d) What is MyCapssa. https://mycapssa.com/patients/what-is-mycapssa.
[63]. Sadeghi, A.M.M. et al. (2008) Permeation enhancer effect of chitosan and chitosan derivatives: Comparison of formulations as soluble polymers and nanoparticulate systems on insulin absorption in Caco-2 cells. Eur. J. Pharm. Biopharm. 70: 270-278.
[64]. Wong, C.Y., Al-Salami, H. and Dass, C.R. (2018) The role of chitosan on oral delivery of peptide-loaded nanoparticle formulation. J. Drug Target.
[65]. Qinna, N.A. et al. (2015) Influence of Molecular Weight and Degree of Deacetylation of Low Molecular Weight Chitosan on the Bioactivity of Oral Insulin Preparations. Mar. Drugs 13: 1710-1725.
[66]. Knudsen, L.B. and Lau, J. (2019) The Discovery and Development of Liraglutide and Semaglutide. Front. Endocrinol. 10.
[67]. Kratz, F. (2008) Albumin as a drug carrier: Design of prodrugs, drug conjugates and nanoparticles. J. Contro Release, 132: 171-183.
Cite this article
Feng,Y. (2025). Applications and Challenges of Oral Peptide Drug Delivery in Diabetes Treatment. Theoretical and Natural Science,68,234-244.
Data availability
The datasets used and/or analyzed during the current study will be available from the authors upon reasonable request.
Disclaimer/Publisher's Note
The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of EWA Publishing and/or the editor(s). EWA Publishing and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
About volume
Volume title: Proceedings of the 3rd International Conference on Modern Medicine and Global Health
© 2024 by the author(s). Licensee EWA Publishing, Oxford, UK. This article is an open access article distributed under the terms and
conditions of the Creative Commons Attribution (CC BY) license. Authors who
publish this series agree to the following terms:
1. Authors retain copyright and grant the series right of first publication with the work simultaneously licensed under a Creative Commons
Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this
series.
2. Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the series's published
version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial
publication in this series.
3. Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and
during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See
Open access policy for details).
References
[1]. Sapra, A. and Bhandari, P. (2023) Diabetes.In: StatPearls [Internet]. Treasure Island (FL): StatPearls.
[2]. Karamanou, M., Protogerou, A., Tsoucalas, G., Androutsos, G. and Poulakou-Rebelakou, E. (2016) Milestones in the history of diabetes mellitus: The main contributors. World J. Diabetes, 7: 1-7.
[3]. World Health Organization. (n.d.) Diabetes. https://www.who.int/health-topics/diabetes.
[4]. Ong, K.L. et al. (2023) Global, regional, and national burden of diabetes from 1990 to 2021, with projections of prevalence to 2050: a systematic analysis for the Global Burden of Disease Study 2021. The Lancet 402, 203-234.
[5]. Word Health Organization. (n.d.) Diabetes. https://www.who.int/news-room/fact-sheets/detail/diabetes.
[6]. Wilcox, G.(2005) Insulin and Insulin Resistance. Clin. Biochem. Rev. 26, 19-39.
[7]. Rashid, A. and Xie, V. (2024) Top 15 Diabetes Drugs in 2023 by 2022 Sales Statistics. https://xtalks.com/top-15-diabetes-drugs-in-2023-by-2022-sales-statistics-3715/.
[8]. Ahrén, B. (2011) GLP-1 for type 2 diabetes. Exp. Cell Res. 317, 1239-1245 (2011).
[9]. Sheahan, K.H., Wahlberg, E.A. and Gilbert, M.P. (2010) An overview of GLP-1 agonists and recent cardiovascular outcomes trials. Postgrad. Med. J. 96, 156-161.
[10]. Collins, L. and Costello, R.A. (2024) Glucagon-Like Peptide-1 Receptor Agonists. In: StatPearls [Internet], Treasure Island (FL).
[11]. Tyagi, P., Pechenov, S. and Anand Subramony, J. (2018) Oral peptide delivery: Translational challenges due to physiological effects. J. Controlled Release 287, 167-176.
[12]. Zizzari, A.T., et al. (2021) New perspectives in oral peptide delivery. Drug Discov. Today 26, 1097-1105.
[13]. Saffran, M., et al. (2011) A model for the study of the oral administration of peptide hormones. Can. J. Biochem.
[14]. Alqahtani, M.S., et al. (2021) Advances in Oral Drug Delivery. Front. Pharmacol. 12, 618411.
[15]. Richter, B. and Neises, G. (2005) Human’ insulin versus animal insulin in people with diabetes mellitus. Cochrane Database Syst. Rev., CD003816.
[16]. Kramer, C.K., Retnakaran, R. and Zinman, B. (2021) Insulin and insulin analogs as antidiabetic therapy: A perspective from clinical trials. Cell Metab. 33, 740-747.
[17]. Baeshen, N.A., et al. (2014) Cell factories for insulin production. Microb. Cell Factories 13, 141.
[18]. Heller, S., et al. (2013) Meta-analysis of insulin aspart versus regular human insulin used in a basal–bolus regimen for the treatment of diabetes mellitus. J. Diabetes 5, 482-491.
[19]. Mapelli, C., et al. (2009) Eleven amino acid glucagon-like peptide-1 receptor agonists with antidiabetic activity. J. Med. Chem. 52, 7788-7799.
[20]. Sjöholm Å. (2010) Liraglutide Therapy for Type 2 Diabetes: Overcoming Unmet Needs. Pharmaceuticals (Basel). 3(3): 764-781.
[21]. Damholt, A.B., Kofod, H. and Buchan, A.M.J. (1999) Immunocytochemical evidence for a paracrine interaction between GIP and GLP-1-producing cells in canine small intestine. Cell Tissue Res. 298, 287-293.
[22]. Gupta, V. (2013) Glucagon-like peptide-1 analogues: An overview. Indian J. Endocrinol. Metab. 17, 413.
[23]. Kalra, S. and Sahay, R. (2020) A Review on Semaglutide: An Oral Glucagon-Like Peptide 1 Receptor Agonist in Management of Type 2 Diabetes Mellitus. Diabetes Ther., 11(9): 1965-1982.
[24]. Aroda, V.R., Blonde, L. and Pratley, R.E. (2022) A new era for oral peptides: SNAC and the development of oral semaglutide for the treatment of type 2 diabetes. Rev. Endocr. Metab. Disord. 23, 979-994.
[25]. Scitable. (2010) Enzyme Catalysis: The Serine Proteases www.nature.com/scitable/topicpage/enzyme-catalysis-the-serine-proteases-14398894.
[26]. Ahmad, A. and Khan, J.M. (2022) pH-sensitive endosomolytic peptides in gene and drug delivery: Endosomal escape and current challenges. J. Drug Deliv. Sci. Technol. 76, 103786.
[27]. Britannica, T. (2024) pH. Encyclopedia Britannica. https://www.britannica.com/science/pH
[28]. Lee, Y.H. et al. (1999) Impact of Regional Intestinal pH Modulation on Absorption of Peptide Drugs: Oral Absorption Studies of Salmon Calcitonin in Beagle Dogs. Pharm. Res. 16, 1233-1239.
[29]. Hadji, H. and Bouchemal, K. (2022) Advances in the treatment of inflammatory bowel disease: Focus on polysaccharide nanoparticulate drug delivery systems. Adv. Drug Deliv. Rev. 181, 114101.
[30]. Russell, T.L., et al. (1993) Upper Gastrointestinal pH in Seventy-Nine Healthy, Elderly, North American Men and Women. Pharm. Res. 10, 187-196.
[31]. Queckenberg, C. and Fuhr U. (2009) Influence of posture on pharmacokinetics. Eur. J. Clin. Pharmacol. 65.
[32]. Akbarian, M. and Chen, S.H. (2022) Instability Challenges and Stabilization Strategies of Pharmaceutical Proteins. Pharmaceutics 14, 2533.
[33]. Wang, X.Q. and Zhang, Q. (2012) pH-sensitive polymeric nanoparticles to improve oral bioavailability of peptide/protein drugs and poorly water-soluble drugs. Eur. J. Pharm. Biopharm. 82, 219-229.
[34]. Mitragotri, S., Burke, P.A. and Langer, R. (2014) Overcoming the challenges in administering biopharmaceuticals: formulation and delivery strategies. Nat. Rev. Drug Discov. 13, 655-672.
[35]. Lewis, A.L., McEntee, N., Holland, J. et al. (2022) Development and approval of rybelsus (oral semaglutide): ushering in a new era in peptide delivery. Drug Deliv. and Transl. Res. 12, 1-6.
[36]. Li, Y., et al. (2022) Advances in oral peptide drug nanoparticles for diabetes mellitus treatment. Bioact. Mater. 15, 392-408.
[37]. Turner, P.V., et al. (2011) Administration of Substances to Laboratory Animals: Routes of Administration and Factors to Consider. J. Am. Assoc. Lab. Anim. Sci. JAALAS 50, 600-613.
[38]. Joudeh, N. and Linke, D. (2022) Nanoparticle classification, physicochemical properties, characterization, and applications: a comprehensive review for biologists. J. Nanobiotechnology 20, 262.
[39]. Gelperina, S., Kisich, K., Iseman, M.D. and Heifets, L. (2005) The Potential Advantages of Nanoparticle Drug Delivery Systems in Chemotherapy of Tuberculosis. Am. J. Respir. Crit. Care Med. 172, 1487-1490.
[40]. Cao, S., et al. (2019) Nanoparticles: Oral Delivery for Protein and Peptide Drugs. AAPS PharmSciTech 20, 190.
[41]. Wang, M., et al. (2014) Permeability of Exendin-4-Loaded Chitosan Nanoparticles across MDCK Cell Monolayers and Rat Small Intestine. Biol. Pharm. Bull. 37, 740-747.
[42]. ABP Biosciences. (n.d.) Lipid Nanoparticles (LNPs). www.abpbio.com/product/lipid-nanoparticles-lnps/.
[43]. Zielińska, A., et al. (2020) Polymeric Nanoparticles: Production, Characterization, Toxicology and Ecotoxicology. Molecules 25, 3731.
[44]. Lin, P., et al. (2018) Oral Nonviral Gene Delivery for Chronic Protein Replacement Therapy. Advanced Science, 5.
[45]. Shan, W. et al. (2015) Overcoming the Diffusion Barrier of Mucus and Absorption Barrier of Epithelium by Self-Assembled Nanoparticles for Oral Delivery of Insulin. ACS nano, 9(3): 2345-2356.
[46]. Poscher, V. and Salinas Y. (2020) Trends in Degradable Mesoporous Organosilica-Based Nanomaterials for Controlling Drug Delivery: A Mini Review. Materials (Basel).
[47]. Araújo, F., et al. (2015) Microfluidic Assembly of a Multifunctional Tailorable Composite System Designed for Site Specific Combined Oral Delivery of Peptide Drugs. ACS Nano 9: 8291-8302.
[48]. Zhou, Y., et al. (2020) A Nanocomposite Vehicle Based on Metal-Organic Framework Nanoparticle Incorporated Biodegradable Microspheres for Enhanced Oral Insulin Delivery. ACS Appl. Mater. Interfaces 12, 22581-22592.
[49]. Abramson, A., et al. (2019) A luminal unfolding microneedle injector for oral delivery of macromolecules. Nat. Med. 25: 1512-1518.
[50]. Yan, Q., et al. (2021) Finite Element Analysis for Biodegradable Dissolving Microneedle Materials on Skin Puncture and Mechanical Performance Evaluation. Polymers 13, 3043.
[51]. Zhu, Y., Ye, J. and Zhang, Q. (2020) Self-emulsifying Drug Delivery System Improve Oral Bioavailability: Role of Excipients and Physico-chemical Characterization. Pharm. Nanotechnol. 8, 290-301.
[52]. Leonaviciute, G. and Bernkop-Schnürch, A. (2015) Self-emulsifying drug delivery systems in oral (poly)peptide drug delivery. Expert Opin. Drug Deliv.
[53]. Li, P., et al. (2013) Preparation and characterization of insulin-surfactant complexes for loading into lipid-based drug delivery systems. J. Pharm. Sci. 102: 268-2698.
[54]. Qi, X., et al. (2011) Self-double-emulsifying drug delivery system (SDEDDS): a new way for oral delivery of drugs with high solubility and low permeability. Int. J. Pharm. 409: 245-251.
[55]. Friedl, H. et al. (2013) Development and evaluation of a novel mucus diffusion test system approved by self-nanoemulsifying drug delivery systems. J. Pharm. Sci. 102: 4406-441.
[56]. Ahn, S. et al. (2013) Oral delivery of an anti-diabetic peptide drug via conjugation and complexation with low molecular weight chitosan. J. Controlled Release 170: 226-232.
[57]. Kim, J.C., Park, E.J. and Na, D.H. (2022) Gastrointestinal Permeation Enhancers for the Development of Oral Peptide Pharmaceuticals. Pharmaceuticals, 15: 1585.
[58]. Bækdal, T.A., et al. (2021) Relationship Between Oral Semaglutide Tablet Erosion and Pharmacokinetics: A Pharmacoscintigraphic Study. Clin. Pharmacol. Drug Dev. 10: 453-462.
[59]. European Medicines Agency. (n.d) Rybelsus. www.ema.europa.eu/en/medicines/human/EPAR/rybelsus.
[60]. Brayden, D.J. and Maher, S. (2021) Transient Permeation Enhancer® (TPE®) technology for oral delivery of octreotide: a technological evaluation. Expert Opin. Drug Deliv. 18: 1501-1512.
[61]. Wang, J., Yadav, V., Smart, A.L., Tajiri, S. and Basit, A.W. (2015) Toward oral delivery of biopharmaceuticals: an assessment of the gastrointestinal stability of 17 peptide drugs. Mol. Pharm. 12: 966-973.
[62]. MyCapssa. (n.d) What is MyCapssa. https://mycapssa.com/patients/what-is-mycapssa.
[63]. Sadeghi, A.M.M. et al. (2008) Permeation enhancer effect of chitosan and chitosan derivatives: Comparison of formulations as soluble polymers and nanoparticulate systems on insulin absorption in Caco-2 cells. Eur. J. Pharm. Biopharm. 70: 270-278.
[64]. Wong, C.Y., Al-Salami, H. and Dass, C.R. (2018) The role of chitosan on oral delivery of peptide-loaded nanoparticle formulation. J. Drug Target.
[65]. Qinna, N.A. et al. (2015) Influence of Molecular Weight and Degree of Deacetylation of Low Molecular Weight Chitosan on the Bioactivity of Oral Insulin Preparations. Mar. Drugs 13: 1710-1725.
[66]. Knudsen, L.B. and Lau, J. (2019) The Discovery and Development of Liraglutide and Semaglutide. Front. Endocrinol. 10.
[67]. Kratz, F. (2008) Albumin as a drug carrier: Design of prodrugs, drug conjugates and nanoparticles. J. Contro Release, 132: 171-183.









