1. Introduction
After the first cancer therapeutic drug nitrogen mustard was approved, cancer-related deaths have decreased at a steady rate. However, cancer remains one of the major causes of death in the world, with 1,958,310 new cancer cases and 609,820 cancer deaths in the United States alone [1]. According to the US Centers for Disease Control and Prevention (CDC), cancer overall represents 18.0% of all deaths that occur in the US. In current oncology studies, tumorigenesis is a hot topic that concerns scientists worldwide. In general, tumorigenesis is linked with self-stimulation of pro-tumour signal pathways and inhibition of apoptosis regulators. The tumour microenvironment (TME) is a crucial concept that describes the growing environment of tumours, encompassing a complex ecosystem between various biological factors. According to its nature and developmental stage, its composition varies, which provides valuable cancer hallmarks; researchers have shown that chronic inflammation can lead to myeloid-derived suppressor cells (MDSC) immunosuppression, whereas acute inflammation activates anti-tumour dendritic cells [2]. Immune cells in the TME have shown either pro-tumour or anti-tumour effects, contributing to tumour cell proliferation or cytotoxicity. For example, the regulatory T cells (Tregs) can secrete interleukins and growth factors to inhibit T cells [3]. On the other hand, natural killer (NK) cells contain activating and inhibitory receptors, directly recognise tumour cells and release cytotoxic granules [4]. Moreover, tumour-associated antigens (TAAs) in TME are usually overexpressed, and some other antigens are significantly reduced [5].
Furthermore, tumour cell modifications can lead to immune escape, where the human immune system can not perform an immunogenic response towards the cancerous cells. By focusing on the progression of TME, hallmarks can be identified and cancer could be more efficiently diagnosed and treated. For instance, when an immune-evasive TME overexpresses PD-L1 proteins, immune checkpoint inhibitors (ICIs) are expected to have more efficacy. However, despite the numerous benefits of investigating, TMEs are highly complex, and there is a lack of full understanding of the cellular interactions in TME. To illustrate, the role of B cells in TME is highly under-investigated, despite its high heterogeneity in tumorigenesis. Biologists are now working on its functionality [6]. Also, according to Dr Chiara Falcomatà, the types and mechanisms of inflammatory signals that recruit immunosuppressive immune cells are still unclear [7]. On the other hand, cancer hallmark identifications and ICIs have gained advancements since their invention. For the last two decades, the FDA has approved several monoclonal antibodies (mAbs), including ipilimumab, pembrolizumab and more [8]. Their applications are diverse, and they can tackle common cancer types such as lung cancer and renal cell carcinoma. By integrating the high efficacy of ICIs and the diverse cancer hallmarks within TME, this review aims to provide the medical field insight into identifying cancer types through TMEs, and the extent of ICIs in tackling TME abnormalities. This review uses independent research and cutting-edge reports as references to explore the different factors that contribute to TME, suggest the detectable hallmarks in TME development, and investigate the potency of ICI therapeutics.
2. Characteristics of TMEs
2.1. Inflammation
One key characteristic of TME is that it causes inflammation. Inflammation is an immune response that kills foreign cells, including tumour cells. When a proto-oncogene or tumour suppressor gene mutation causes tumourigenesis, the subsequent tumourigenesis releases several chemical signals. These include cytokines, growth factors, and more. For instance, tumour cells can secrete interleukins such as IL-6 and IL-8 [9].
IL-6 can cause either acute or chronic inflammations. This consequently allows IL-6 to activate the innate and adaptive immune system, exhibiting pro-tumour and anti-tumour effects. Primarily, IL-6 binds to IL-6 receptors (IL-6Rs) in T cells, initiating a dimerisation of glycoprotein 130 [9]. This activates the JAK-STAT pathway, causing a phosphorylation and dimerisation reaction of STAT proteins. Activated STAT proteins are transcription factors (TFs), and soon translocate to the T cell’s nucleus and increase the expressions of genes concerning clonal selection and expansion [9]. Also, IL-6 is a B-cell differentiating factor, and it stimulates B-cell proliferation, antibody secretion and cytokine production. IL-6 can also activate dendritic cells (DC), although the specific mechanism is unknown. When activated, T cells often express anti-tumour effects by binding their T cell receptors (TCRs) to abnormal self-antigens on the tumour cells' major histocompatibility complex I (MHCI) molecules. This interaction causes a release of perforins and granzymes, inducing the apoptosis of tumour cells. However, persistent T cell activation can lead to an overexpression of cytokines that creates chronic inflammation. This TME will then aid tumour-associated immune cells’ activities.
On the other hand, IL-8 is a pro-inflammatory cytokine that attracts neutrophils. It follows the same JAK-STAT pathway as IL-6, altering the transcription of neutrophils [10]. IL-8 also induces positive chemotaxis, recruiting tumour-associated neutrophils (TANs) into the TME. Neutrophils will then secrete more cytokines, including IL-1, IL-6 and tumour necrosis factor-alpha (TNF-α). This will recruit more immune cells to the TME, enhancing the inflammation [10]. As the TANs carry out phagocytosis, they often release reactive oxidation species (ROS) and neutrophil extracellular traps (NETs) that can damage tissue layers, contributing to inflammation.
Inflammation in TME overall benefits tumour growth. As tumour-associated neutrophils and macrophages release cytokines and ROS, it increases the blood flow near the tumour site which increases the nutrient supply of neoplastic tissues. In the later stages of TME, chronic inflammation stimulates blood vessel formation around the tumour, facilitating angiogenesis in tumour cells and worsening cancer. Moreover, the secreted ROS can cause DNA mutations, encouraging more cancerous cell formations.
2.2. Immune system
Tregs play an important role in TME by promoting tumour growth. In non-TME, Tregs exert immunosuppressive effects that inhibit the activity of effector T cells. This important function prevents excessive inflammation and subsequently protects body tissues from immune cells. Tregs regulate the overall response of the immune system, and they control the level of cytokine concentrations in the infected area.
However, Tregs are pro-tumour immune cells. In a typical TME, when Tregs encounter foreign antigens on the tumour cells’ membranes, IL-10 is produced. IL-10 primarily activates T cell’s JAK/STAT and JAK/TYK2 pathways; the phosphorylated TYK2 protein can dephosphorylate the CD28 receptor [3]. CD28 is essential for T cell activation, and when CD28 is dephosphorylated, it can’t be activated. This decreases the immune response of T cells overall.
In an inflammatory environment of high ROS and proteases, the GARP/LRRC33 protein on the Treg’s plasma membrane and the transforming growth factor-beta (TGF-β) binding proteins will dissociate from the TGF-β [11]. Then, the activated TGF-β will bind to serine/threonine kinases and induce the SMAD pathway. SMAD proteins will get phosphorylated and translocated to the nucleoplasm, modifying epigenetic tags or increasing gene expression that inhibits the proliferation of immune cells, such as T cells and NK cells [11]. Overall, Tregs exhibit an anti-inflammatory and pro-tumour effect in the TME.
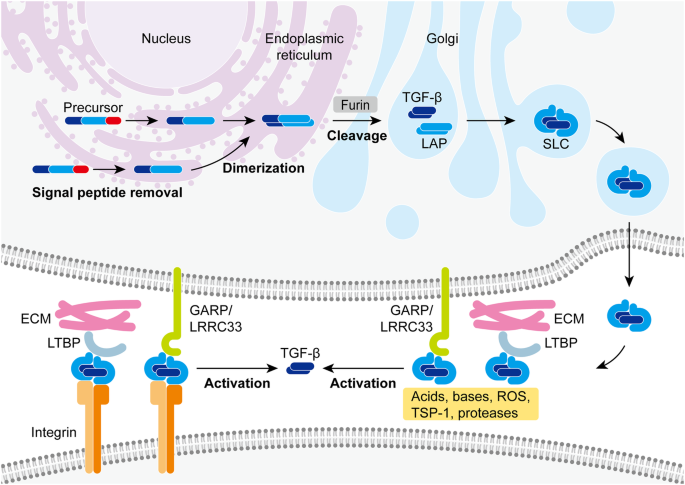
Figure 1: Activation of TGF-β proteins by Tregs [11].
At the tissue level, the immunosuppression caused by Tregs ultimately leads to less cytokine production, increased tumour cell survival, and the recruitment of other immunosuppressive cells (Figure 1).
2.3. Immune escape
Tumour cells have developed certain strategies to escape from immunosurveillance. This allows the tumour cells to grow further and develop secondary tumours.
Analogous to natural selection, tumour cells with more TAAs and TSAs on the plasma membrane are more likely to be detected by anti-tumour cells. However, tumour cells that downregulate TAAs and TSAs are less likely to be immunogenic. After several generations, most tumour cells will reduce their expressions of foreign antigens. Antigen loss, however, is not restricted to TAAs and TSAs. Tumour cells can also downregulate non-foreign antigens that were once overexpressed and immunogenic. For instance, during acute lymphoblastic leukaemia relapse, there is significant downregulation of CD19 in tumour cells [5], making conventional treatments inefficient (Figure 2).
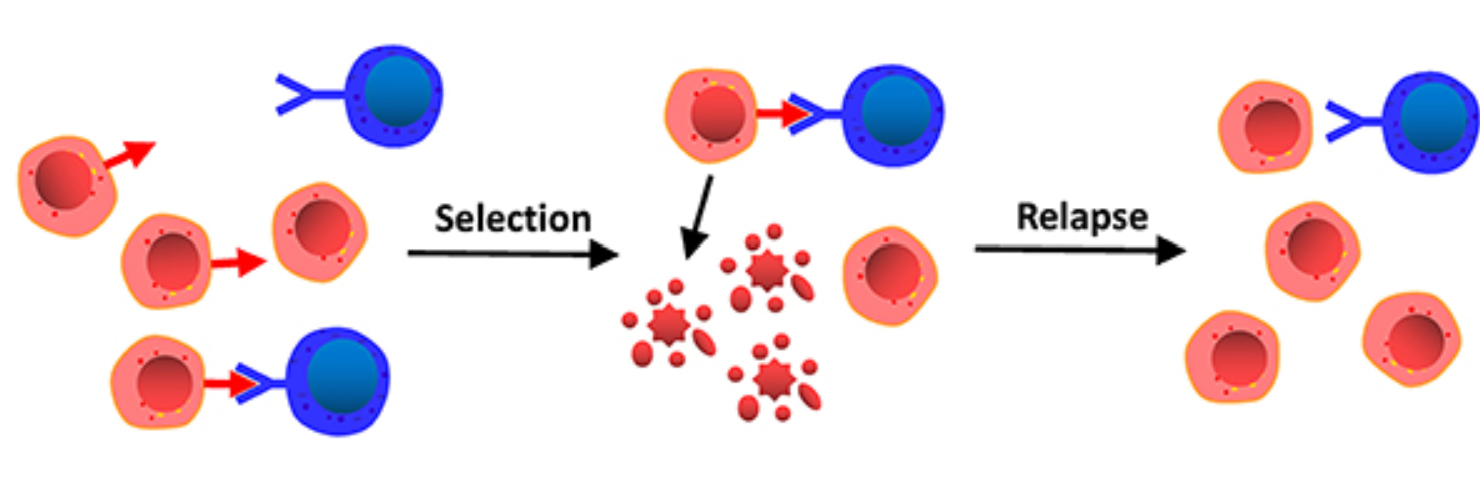
Figure 2: The red arrow integral protein represents CD19. During the relapse stage, the CD19 proteins are downregulated [5].
On the other hand, tumour cells can also overexpress antigens. Tumour cells in TME can present excessive MHC class I chain-related protein A (MICA) on their plasma membrane. They bind to natural-killer group 2, member D (NKG2D) receptors expressed on CD 8+ T cells and NK cells. NKG2D receptors’ main function is to recognise MICA, cause signal transduction, and increase cytokine production. However, tumour cells can shed MICA, releasing it to the TME. The soluble MICAs (sMICAs) will then act as decoys, as they bind to NKG2D receptors and prevent immune cells from recognising the tumour cells [5].
Moreover, tumour cells can express immune checkpoint molecules. These ligands on the surface of tumour cells bind to inhibitory receptors, leading to the immunosuppression of immune cells. One of the most common examples is programmed death ligand 1 proteins (PD-L1). When PD-L1 binds to PD-1 receptors on T cells, B cells, NK cells and DC cells, it induces a pathway that phosphorylates SHP-2 proteins [12]. These proteins disrupt the TCR pathway, dephosphorylating important secondary messengers such as ZAP-70 and LAT. On the cellular level, it deactivates the immune cells.
2.4. Different types of TME
2.4.1. Inflammatory TME
Inflammatory TME is one of the most common TMEs in all types of cancer. This includes colorectal, breast, liver, lung cancer and others. Some key characteristics of inflammatory TME are persisting chronic inflammation and well-developed angiogenesis [13]. The uncontrollable high concentration of cytokines and chemokines in TME constantly activates the immune system, causing more signal production and recruitment of immune cells. This positive chemotaxis often recruits pro-inflammatory and pro-tumour immune cells such as TANs and MDSCs, sustaining the inflammatory environment. Also, the increased blood supply for tumour cells increases their nutritional uptake, which fuels tumour cell proliferation and growth.
2.4.2. Desmoplastic TME
Desmoplastic TME is unique for its dense connective tissue that forms around the TME. This is mostly present in pancreatic cancer, but it is also common in invasive breast and lung cancer. The extracellular matrix outside of tumour cells is made up of collagen and fibronectin proteins, and the strong intermolecular interactions between these structural proteins form a fibrous network that prevents some molecules from entering the tumour cells [14]. This includes chemotherapeutic drugs, so cancers with desmoplastic TME are relatively harder to treat by chemotherapy means. Also, desmoplastic TME contains special pro-tumour cells called cancer-associated fibroblasts (CAFs) [14]. CAF’s function in TME is to remodel the ECM structure; this makes the ECM stiffen and stronger, preventing immune cell infiltration. CAF also secrete cytokines, recruiting more immune cells to the TME. The heterogeneity of CAF adds complexity to desmoplastic TME, making classification and treatments much harder (Figure 3).
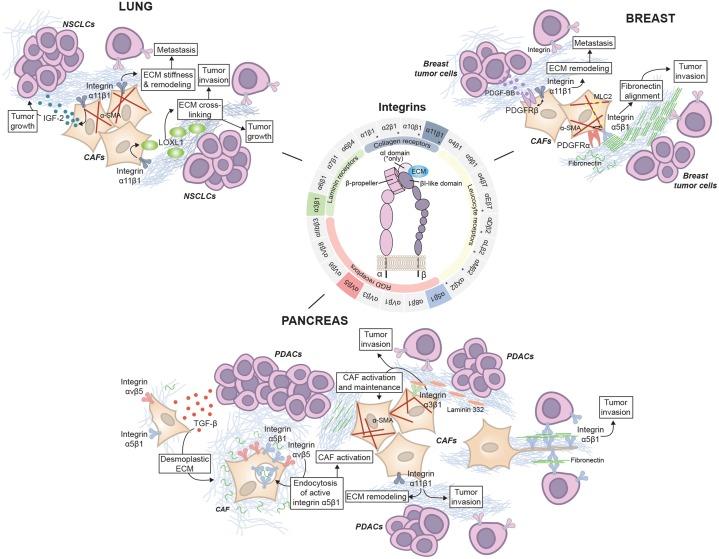
Figure 3: ECM remodelling by CAFs in lung, breast and pancreas cancer [14].
2.4.3. Hypoxic TME
Hypoxic TME is formed when there is a lack of oxygen molecules, especially at the core of a tumour site. This can occur when rapid tumour growth is much faster than angiogenesis, as insufficient blood vessels cannot supply adequate oxygen to the tumour cells. Hypoxic TME is common in solid tumours such as lung cancer, prostate cancer, and melanoma. This is because solid tumours have restricted mobility, where they are more likely to be stuck in a position and have less access to oxygen. What is special about hypoxic TME is that the hypoxia condition activates hypoxia-inducible factors (HIFs). HIFs are TFs that alter tumour cells’ gene expressions, which include vascular endothelial growth factor (VEGF) [15]. VEGF increases the vascular permeability of endothelial walls, encouraging blood vessel formation and angiogenesis. Moreover, tumour cells in low oxygen conditions carry out anaerobic respiration, and the hypoxic TME will subsequently contain high lactate concentrations. Lactate can inhibit the activity of effector T cells (Figure 4).
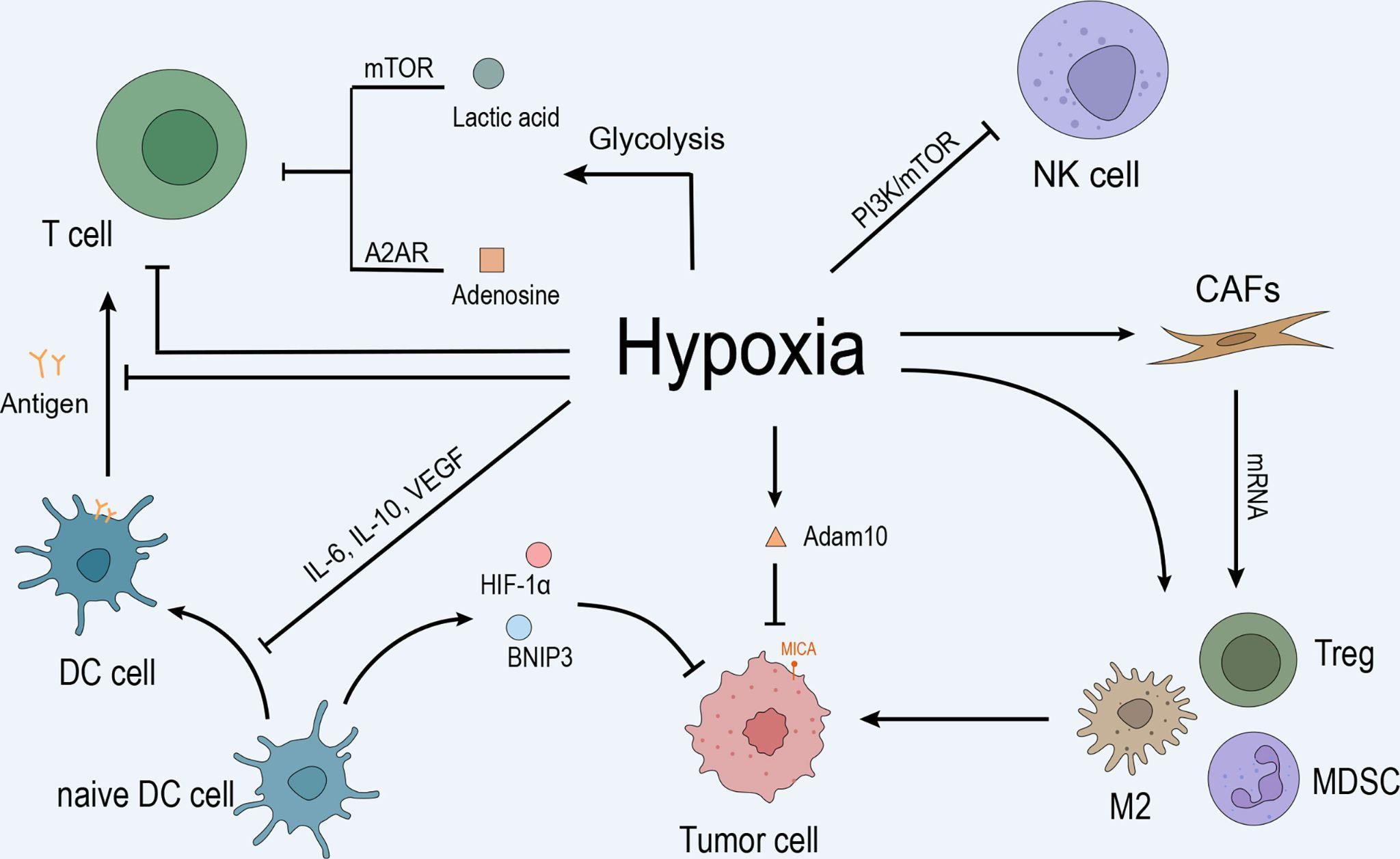
Figure 4: Hypoxia inhibits T cell and dendritic cell activity through VEGF, encouraging tumour growth [15].
3. Development of cancer hallmark identification
Identifying cancer hallmarks in TME is about recognising specific characteristics in tumour cells that are not found in normal cells. There are many traditional cancer hallmarks, including uncontrolled cell proliferation, apoptosis inhibition, angiogenesis induction, ECM remodelling, activating metastasis and more. Almost all of these hallmarks are associated with an overexpression of protein or a tumour-specific protein. Thus, one of the most promising cancer hallmark identification technologies, flow cytometry, is related to the identification of intracellular proteins.
Firstly, the extracted tumour cells are immersed in a formaldehyde solution to fix the intracellular proteins in position. Then, fluorescent antibodies are introduced to the tumour cells, allowing them to bind to specific proteins [16]. Different antibody combinations can be chosen according to the oncologists’ interests. For example, Ki67 staining is often selected to investigate tumour cell proliferation. Ki67 is a protein that acts as a marker for proliferating cells. In the G0 phase, there will be no Ki67 proteins present in the cell. However, during G1, S, G2 and M phases, Ki67 proteins can be found in the cytosol. Specifically, the Ki67 proteins are on the surface of chromosomes during mitosis [17]. Likewise, to investigate apoptosis inhibition, Bcl-2 family proteins can be selected for antigen binding. It is because bcl-2 proteins bind directly to pro-apoptosis proteins such as bcl-2-associated x protein (Bax) and bcl-2 antagonist/killer (Bak). It stops the formation of oligomer pores and prevents the subsequent release of cytochrome c from the intermembrane space, inhibiting apoptosis. High concentrations of bcl-2 will indicate tumour activities [18].
However, antibodies cannot enter the cells due to the plasma membrane. Chemicals such as methanol can be introduced to create pores in the tumour cells, increasing the permeability of antibodies. The sample is passed through a fluid-filled chamber after all targeted proteins are bound to antigens. A laser beam is projected onto the tumour cell one at a time, exciting the fluorescent marks on the antibodies. The light sensors then capture the scattered light, displaying a graph showing targeted protein concentrations [16].
What is important about the flow cytometry technology is that antibodies are not restricted to intracellular protein concentration detections. Antibodies can bind to histone markers or methylated DNA bases, informing the epigenetics of the tumour cells. Also, the TAAs and TSAs can be marked by antibodies, identifying the cell surface markers [16].
However, as the oncologists shift focus to the heterogeneity of TMEs and the molecular-level cancer hallmarks, flow cytometry alone may not identify all cancer hallmarks. Flow cytometry technology measures the cell’s activity at an instantaneous moment, in which the interactions between different cells are not captured. Chemicals in the vicinity of cells can’t be detected, so the chemical modification of ECMs and the extracellular cytokine concentrations cannot be identified. Moreover, antibodies are not specific enough to bind to mutated oncogenes or tumour suppressor genes. Therefore, the flow cytometry can’t detect genetic cancer hallmarks.
Different identification methods are used widely to overcome these disadvantages. Next-generation sequencing (NGS) extracts the DNA sample from a potential tumour cell. Then, the sample DNA is fragmented and adaptors are added to the ends of DNA fragments. These fragments are attached to a flow cell for bridge amplification, and they are replicated by DNA polymerases using fluorescently labelled nucleotides. The colour produced by the cell plate can be analysed using a computer, in which the tumour cell’s DNA sequence is generated. Microscopic cancer hallmarks can be identified successfully using a more molecular-based technology. For an in vivo analysis of TME, molecular imaging such as PET shows promising results. Recent studies have shown that CAFs that modulate ECM can be detected using a combination of fibroblast activation protein inhibitors and PET imaging.
4. ICIs
Immune checkpoint inhibitors (ICIs) are one of the most promising immunotherapies that effectively utilise the cancer hallmarks in TME. Their function is to block the ligand-receptor immune checkpoint interaction between the tumour cell and the T cell. Two prominent ICIs used widely in clinics include Pembrolizumab and Ipilimumab, which target the PD-1 and CTLA-4 checkpoints.
ICIs are essentially monoclonal antibodies (mAbs). Each mAb contains two light chains and two heavy chains linked by disulfide bridges, and its variable region can bind to a specific integral protein within the immune checkpoint. For example, once Pembrolizumab is introduced in the TME, it can bind to the PD-1 receptor and compete with the PD-L1 ligand on the tumour cell. The reversible binding decreases the successful immune checkpoint binding rate. Without the subsequent signal transduction stimulated by PD-1, T cells will not be inhibited and will attack the tumour cells through the TCR binding, initiating apoptosis [19]. Overall, the tumourigenesis can be controlled and patients can enter the remission stage.
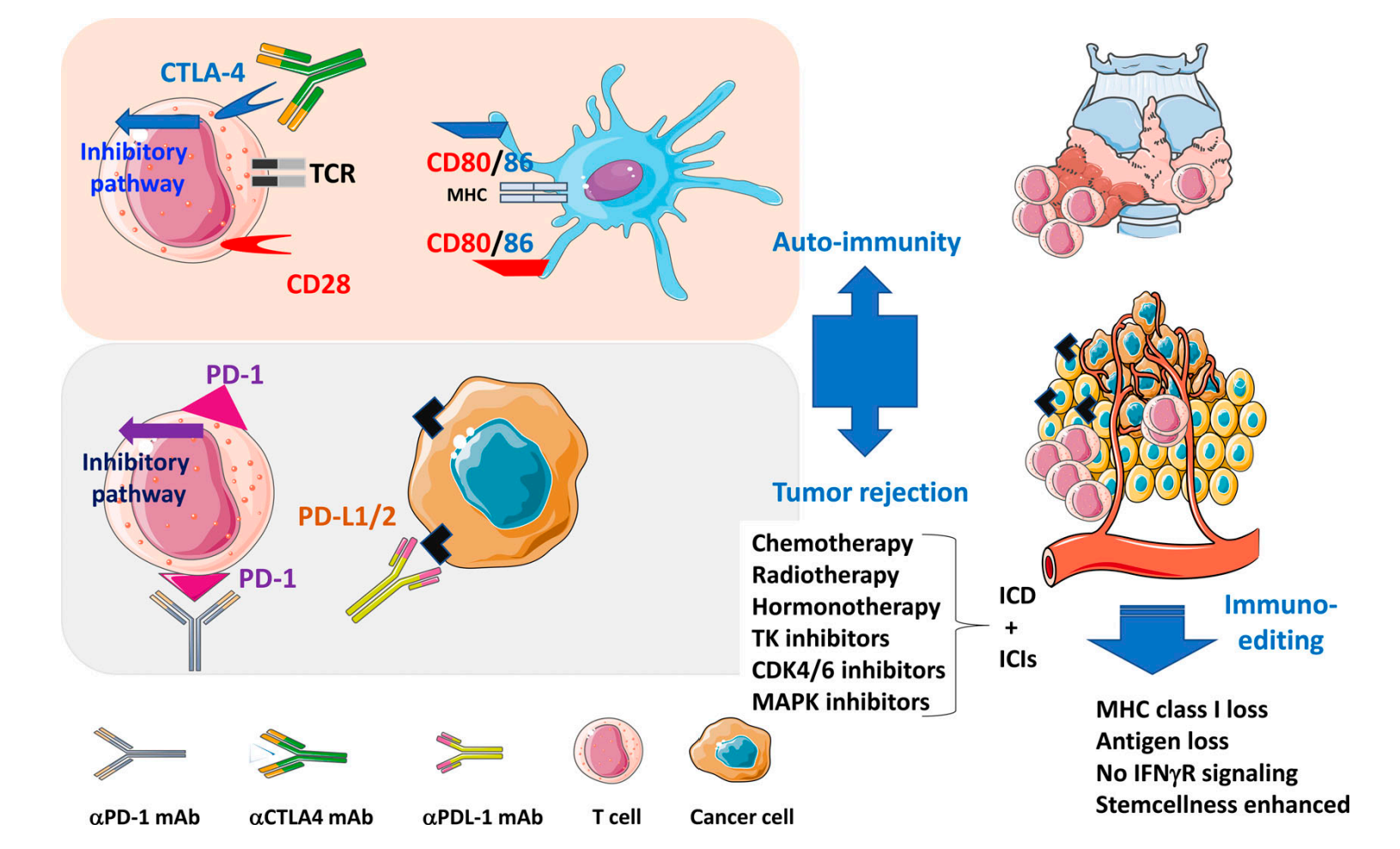
Figure 5: Pembrolizumab is an αPD-1 mAb. It binds to the PD-1 receptor and prevents an inhibitory pathway [19].
ICIs have a wide range of applications. ICIs are commonly used to treat melanoma, Hodgkin lymphoma, lung, bladder, colorectal, cervical cancer and more. ICIs often have a higher efficacy on solid cancers than liquid cancers [19]; due to the extensive immunosuppressive TME within solid tumours, the reactivation of the immune system by the ICIs yields a more significant result. Moreover, solid tumours tend to have more mutations than liquid tumours, which leads to a higher chance for solid tumour cells to develop immune checkpoint mechanisms (Figure 5).
However, ICIs can lead to some side effects, which are often referred to as immune-related adverse effects (irAEs). Some common irAEs include fatigue, fever and diarrhoea. Yet, researchers have shown that ICIs can cause rare but fatal side effects, including cytokine release syndrome (CRS) and macrophage-activation syndrome (MAS). These symptoms often appear 9 days after the ICI introduction, and this effect is mostly shown in IL-6 antibody treatment [19]. These syndromes extend to devastating effects: CRS gives rise to an ample release of cytokines, speeding up white blood cell proliferation. The overstimulated immune cells may attack self-cells, which causes more inflammation. On the other hand, MAS is characterised by the overactivation of T cells and macrophages. It can also cause severe inflammation, leading to swollen lymph nodes and persistent fever. Therefore, even though ICI is an FDA-approved and widely recognised immunotherapy, its side effects should not be neglected, and the oncology field should focus more on side effects prevention to mitigate the uncertainties.
5. Conclusion
Overall, this review report highlights the importance of TME when analysing tumour growth. The common characteristics and induced pathways are shown, and the heterogeneity of TME is considered by exploring different niches of TME. Working from the immense data that TME provides, cancer hallmarks can be identified using various cutting-edge technologies. Moreover, ICI immunotherapy effectively utilises the immune checkpoint hallmarks to treat liquid cancers, which has unique advantages and costs. Reviewing this topic reminds the oncology field that categorising different TMEs is crucial for therapy decisions. Despite ICIs being very specific, the complexity of TME inevitably will cause some side effects during the treatment process. Also, this report highlights the power of cancer hallmark identification techniques, where one discovery can potentially create a new divergence of therapy. However, the report failed to capture all different variants of TMEs due to their diversity, and there are other important chemicals in all types of TMEs that are not explored; for instance, the stromal cells in TME play an important role in pro-tumour and pro-inflammatory effects. Lastly, oncologists can continue to work on the research gaps in TMEs, including the role of B cells and mast cells. Also, ICIs may have a wider application than inhibiting immune checkpoints, as they can also be used in ligand inhibition. The great potential of ICIs should be investigated in the near future.
References
[1]. Siegel, R. L., Miller, K. D., Wagle, N. S., & Jemal, A. (2023). Cancer statistics, 2023. CA: A Cancer Journal for Clinicians, 73(1), 17–48.
[2]. Zhao, H., Wu, L., Yan, G., et al. (2021). Inflammation and tumor progression: Signaling pathways and targeted intervention. Signal Transduction and Targeted Therapy, 6, 263.
[3]. Li, C., Jiang, P., Wei, S., Xu, X., & Wang, J. (2020). Regulatory T cells in tumor microenvironment: New mechanisms, potential therapeutic strategies and future prospects. Molecular Cancer, 19(1), Article 1234.
[4]. Blunt, M. D., & Khakoo, S. I. (2023). Harnessing natural killer cell effector function against cancer. OUP Academic.
[5]. Xu, X., Sun, Q., Liang, X., Chen, Z., Zhang, X., Zhou, X., Li, M., Tu, H., Liu, Y., Tu, S., & Li, Y. (2019). Mechanisms of relapse after CD19 CAR T-cell therapy for acute lymphoblastic leukemia and its prevention and treatment strategies. Frontiers in Immunology, 10.
[6]. Yang, H., Zhang, Z., Li, J., Wang, K., Zhu, W., & Zeng, Y. (2024). The dual role of B cells in the tumor microenvironment: Implications for cancer immunology and therapy. Molecules, 25(21), Article 11825.
[7]. Falcomatà, C., Bärthel, S., Schneider, G., Rad, R., Schmidt-Supprian, M., & Saur, D. (2023). Context-specific determinants of the immunosuppressive tumor microenvironment in pancreatic cancer. Cancer Discovery, 13(2), 278–297.
[8]. EG;, B. S. R. (2022). Immune checkpoint inhibitors for the treatment of cancer: Clinical impact and mechanisms of response and resistance. Annual Review of Pathology.
[9]. Rašková, M., Lacina, L., Kejík, Z., Venhauerová, A., Skaličková, M., Kolář, M., Jakubek, M., Rosel, D., Smetana, K., & Brábek, J. (2022). The role of IL-6 in cancer cell invasiveness and metastasis—Overview and therapeutic opportunities. Cells, 11(22), Article 3698.
[10]. Matsushima, K., Yang, D., & Oppenheim, J. J. (2022). Interleukin-8: An evolving chemokine. Cytokine, 153, Article 155828.
[11]. Deng, Z., Fan, T., Xiao, C., Tian, H., Zheng, Y., Li, C., & He, J. (2024). TGF-β signaling in health, disease and therapeutics. Nature News.
[12]. Yi, M., Zheng, X., Niu, M., Zhu, S., Ge, H., & Wu, K. (2022). Combination strategies with PD-1/PD-L1 blockade: Current advances and future directions. Molecular Cancer, 21(1), Article 28.
[13]. Tan, Z., Xue, H., Sun, Y., Zhang, C., Song, Y., & Qi, Y. (2021). The role of tumor inflammatory microenvironment in lung cancer. Frontiers in Pharmacology, 12.
[14]. Zeltz C., Primac I., Erusappan P., Alam J., Noel A., & Gullberg D. (2020). Cancer-associated fibroblasts in desmoplastic tumors: Emerging role of integrins. In Seminars in Cancer Biology (Vol. 62 pp. 166-181).
[15]. Chen G., Wu K., Li H., Xia D., & He T., (2022). Role of hypoxia in the tumor microenvironment and targeted therapy. Frontiers in Oncology, 12.
[16]. Bonilla D.L., Reinin G., & Chua E., (2021). Full spectrum flow cytometry as a powerful technology for cancer immunotherapy research. Frontiers in Molecular Biosciences, 7.
[17]. Simonetti S., Natalini A., Peruzzi G., Nicosia A., Folgori A., Capone S. & Di Rosa F., (2021). A DNA/Ki67-based flow cytometry assay for cell cycle analysis of antigen-specific CD8 T cells in vaccinated mice.Journal of Visualized Experiments, 167.
[18]. Warren C.F., Wong-Brown M.W., & Bowden N.A., (2019). BCL-2 family isoforms in apoptosis and cancer. Cell Death & Disease, 10(3), Article 177.
[19]. Liu L.L., Skribek M., Harmenberg U., & Gerling M., (2023). Systemic inflammatory syndromes as life-threatening side effects of immune checkpoint inhibitors: Case report and systematic review of the literature.Journal for ImmunoTherapy of Cancer, 11(3), Article e005841.
Cite this article
Jin,C. (2025). Research Developments in Cancer Hallmarks and Immune Checkpoints Based on Mechanisms of Tumour Microenvironment. Theoretical and Natural Science,90,37-46.
Data availability
The datasets used and/or analyzed during the current study will be available from the authors upon reasonable request.
Disclaimer/Publisher's Note
The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of EWA Publishing and/or the editor(s). EWA Publishing and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
About volume
Volume title: Proceedings of ICMMGH 2025 Workshop: Computational Modelling in Biology and Medicine
© 2024 by the author(s). Licensee EWA Publishing, Oxford, UK. This article is an open access article distributed under the terms and
conditions of the Creative Commons Attribution (CC BY) license. Authors who
publish this series agree to the following terms:
1. Authors retain copyright and grant the series right of first publication with the work simultaneously licensed under a Creative Commons
Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this
series.
2. Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the series's published
version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial
publication in this series.
3. Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and
during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See
Open access policy for details).
References
[1]. Siegel, R. L., Miller, K. D., Wagle, N. S., & Jemal, A. (2023). Cancer statistics, 2023. CA: A Cancer Journal for Clinicians, 73(1), 17–48.
[2]. Zhao, H., Wu, L., Yan, G., et al. (2021). Inflammation and tumor progression: Signaling pathways and targeted intervention. Signal Transduction and Targeted Therapy, 6, 263.
[3]. Li, C., Jiang, P., Wei, S., Xu, X., & Wang, J. (2020). Regulatory T cells in tumor microenvironment: New mechanisms, potential therapeutic strategies and future prospects. Molecular Cancer, 19(1), Article 1234.
[4]. Blunt, M. D., & Khakoo, S. I. (2023). Harnessing natural killer cell effector function against cancer. OUP Academic.
[5]. Xu, X., Sun, Q., Liang, X., Chen, Z., Zhang, X., Zhou, X., Li, M., Tu, H., Liu, Y., Tu, S., & Li, Y. (2019). Mechanisms of relapse after CD19 CAR T-cell therapy for acute lymphoblastic leukemia and its prevention and treatment strategies. Frontiers in Immunology, 10.
[6]. Yang, H., Zhang, Z., Li, J., Wang, K., Zhu, W., & Zeng, Y. (2024). The dual role of B cells in the tumor microenvironment: Implications for cancer immunology and therapy. Molecules, 25(21), Article 11825.
[7]. Falcomatà, C., Bärthel, S., Schneider, G., Rad, R., Schmidt-Supprian, M., & Saur, D. (2023). Context-specific determinants of the immunosuppressive tumor microenvironment in pancreatic cancer. Cancer Discovery, 13(2), 278–297.
[8]. EG;, B. S. R. (2022). Immune checkpoint inhibitors for the treatment of cancer: Clinical impact and mechanisms of response and resistance. Annual Review of Pathology.
[9]. Rašková, M., Lacina, L., Kejík, Z., Venhauerová, A., Skaličková, M., Kolář, M., Jakubek, M., Rosel, D., Smetana, K., & Brábek, J. (2022). The role of IL-6 in cancer cell invasiveness and metastasis—Overview and therapeutic opportunities. Cells, 11(22), Article 3698.
[10]. Matsushima, K., Yang, D., & Oppenheim, J. J. (2022). Interleukin-8: An evolving chemokine. Cytokine, 153, Article 155828.
[11]. Deng, Z., Fan, T., Xiao, C., Tian, H., Zheng, Y., Li, C., & He, J. (2024). TGF-β signaling in health, disease and therapeutics. Nature News.
[12]. Yi, M., Zheng, X., Niu, M., Zhu, S., Ge, H., & Wu, K. (2022). Combination strategies with PD-1/PD-L1 blockade: Current advances and future directions. Molecular Cancer, 21(1), Article 28.
[13]. Tan, Z., Xue, H., Sun, Y., Zhang, C., Song, Y., & Qi, Y. (2021). The role of tumor inflammatory microenvironment in lung cancer. Frontiers in Pharmacology, 12.
[14]. Zeltz C., Primac I., Erusappan P., Alam J., Noel A., & Gullberg D. (2020). Cancer-associated fibroblasts in desmoplastic tumors: Emerging role of integrins. In Seminars in Cancer Biology (Vol. 62 pp. 166-181).
[15]. Chen G., Wu K., Li H., Xia D., & He T., (2022). Role of hypoxia in the tumor microenvironment and targeted therapy. Frontiers in Oncology, 12.
[16]. Bonilla D.L., Reinin G., & Chua E., (2021). Full spectrum flow cytometry as a powerful technology for cancer immunotherapy research. Frontiers in Molecular Biosciences, 7.
[17]. Simonetti S., Natalini A., Peruzzi G., Nicosia A., Folgori A., Capone S. & Di Rosa F., (2021). A DNA/Ki67-based flow cytometry assay for cell cycle analysis of antigen-specific CD8 T cells in vaccinated mice.Journal of Visualized Experiments, 167.
[18]. Warren C.F., Wong-Brown M.W., & Bowden N.A., (2019). BCL-2 family isoforms in apoptosis and cancer. Cell Death & Disease, 10(3), Article 177.
[19]. Liu L.L., Skribek M., Harmenberg U., & Gerling M., (2023). Systemic inflammatory syndromes as life-threatening side effects of immune checkpoint inhibitors: Case report and systematic review of the literature.Journal for ImmunoTherapy of Cancer, 11(3), Article e005841.









