1. Introduction
With the evolution and breakthrough of contemporary medical science, cancer, which was once a disease that people heard, has gradually become a fortress that we can conquer. Certainly, traditional therapies including radiotherapy, chemotherapy, and surgery can successfully prevent cancer from returning and increase a patient's chances of survival, but the return of tumors or resistance to drugs often makes the disease go downhill. In addition, the non-specific nature of chemotherapy and radiotherapy may cause significant side effects, and in some cases, even life-threatening. Therefore, we still need innovative cancer treatment strategies. The advent of CRISPR/Cas9 technology has triggered a potential revolution in cancer treatment. In the past, somatic gene therapy has mostly been used to introduce target genes into non-germline cells to deal with inherited diseases. Since the 1980s, the exploration of gene therapy has begun, but the existence of gene silencing, immune response in the host and off-target phenomenon makes its efficacy limited. Although many problems have not been completely solved, however, a series of studies have revealed that the future of somatic gene therapy is still bright, and its safety is better than that of traditional therapies. In 2014, the first clinical trial using zinc finger nuclease (ZFN) to replace CRISPR/Cas9 was conducted [1]. In patients with chronic HIV infection, CD4+T cells modified by ZFN are injected into the body to administer highly effective antiviral therapy [2].
The CRISPR/Cas9 system was first discovered in the immune mechanism of bacteria against foreign viruses and plasmids. CRISPR/Cas9 is mainly composed of Cas9 protein and guide RNA (sgRNA), in which Cas9 protein has two nuclease domains, which can respectively cut the double strand of DNA under the mediation of sgRNA, forming a double strand break (DSB), and then trigger the intracellular repair mechanism [3]. The technology is designed to provide an efficient and precise way to edit genes, and at the same time, CRISPR/Cas9 is more economical than earlier gene editing techniques.
The aim of cancer immunotherapy in clinical practice is to activate the host immune system so as to offer passive or active immunity against malignant tumors. CRISPR has expanded our understanding of cancer genetics by providing a formidable platform for the growth of mice with tumors and the screening of possible objectives for immuno-oncology. In translational medicine, the flexible CRISPR/Cas9 system holds great promise for overcoming the current limitations of cancer immunotherapy and boosting the feasibility of adoptive cell therapy (ACT) for the treatment of solid tumors [4].T-cell receptor (TCR) gene-modified T cells, chimeric antigen receptor (CAR) T-cell therapy, and therapy with tumor-infiltrating lymphocytes (TILs) are some of the methods used in adoptive cell therapy (ACT) to increase the activity of T lymphocytes and other effector cells that coordinate the antitumor immune response [5]. To increase the effectiveness of immunotherapy and lessen its negative effects, researchers can more accurately alter T cell receptors using CRISPR. They have also created novel immunotherapies that are more successful in combating cancer.
2. CAR-T structure and working principle
2.1. The structure of CAR-T
The most important structure of CAR-T is the part that recognizes tumor cell antigens, which is derived from the single-chain variable fragment (scFv) structure of the antibody and consists of the variable regions of the light and heavy chains of the antibody. After being screened for antigen-binding affinity in vitro, scFvs have the potential to identify antigens on all cell surfaces, in contrast to the T-cell receptor (TCR) on T cells, which primarily targets intracellular peptides given by major histocompatibility complex (MHC) molecules on target cells. In normal T cells, antigen recognition is mostly restricted to MHC-presented peptides, while scFvs are not limited by this requirement.
2.2. The working principle of CAR-T
CAR-T recognizes surface antigens of target cells through scFv, such as receptors, ligands and
other proteins on the cell surface, which increases the breadth of target recognition, and this
recognition is not limited by MHC [6]. The formation of immune synapses depends on the gap between T cells and target cells.The size of extracellular antigens is typically not defined, while the size of antigenic peptide-MHC is, the gap in immune synapses changes accordingly and can be regulated by scFv.
3. Progress in the application of CRISPR-Cas9 to CAR-T and TRC-T therapy
3.1. CAR-T
CRISPR-Cas9 technology is a revolutionary gene editing tool that is currently being extensively studied and applied to CAR T cell therapies to improve the efficacy and safety of the therapy. CAR T cell therapy is a type of immunotherapy that uses genetic engineering to modify a patient's T cells so that they can specifically recognize and attack cancer cells. The application of CRISPR-Cas9 technology in CAR-T therapy mainly focuses on optimizing CAR structure, strengthening the persistence and anti-tumor activity of T cells, reducing immune rejection, and overcoming the inhibition of tumor microenvironment (TME).
CRISPR-Cas9 technology can precisely edit the genome of CAR T cells, such as by knocking out TCR and β2 microglobulin (β2M) genes, to reduce immune rejection after allotransplantation, and then create so-called "universal" CAR T cells.Furthermore, CRISPR-Cas9 can be employed to eliminate T cell activity inhibition and improve the function of CAR T cells by knocking down immune checkpoint molecules.
CAR's discovery in 1987 has led to its effectiveness in cancer therapy. The efficiency of CAR-T cell treatment is improved by the collection, modification in-vitro, and re-infusion of patient's T lymphocytes [7].
Before the native T cell can deliver its knockout punch, it must bond with the target cell, building a complex interface of connections known as immune synapses. The same sophisticated construction applies to CAR-T cells, although the synapses they form when they make contact with their target have a slightly different architecture, resulting in different intensity and timing of the signal transmission. Like T cells, CAR T cells use immunological synapses to join forces with tumor cells and kill them in three different ways. First, perforin and grease are secreted by CAR-T cells; perforin can puncture in tumor cells' surfaces, allowing grease to enter the cells and either physically kill the tumor cells or cause them to undergo apoptosis. Secondly, TNF ligands are prominently displayed on CAR-T cells' surface, which may also cause tumor cell apoptosis.
Along with the two processes mentioned above, CAR-T cells also release certain cytokines, which allows them to alter the TME, increase the anti-tumor effectiveness of CAR-T, and promote CAR-T activity.
Cell therapy, represented by CAR-T, works by reengineering a patient's own immune cells to act as a form of cancer immunotherapy. These new therapies have been very successful in treating blood cancers, but so far, most cancer patients do not respond well to cell therapy, and solid tumors, which account for the majority of cancer types, have not been tackled by cell therapy. After receiving cell therapy, while some cancer patients develop a lasting response, most do not, and each patient's unique TME can suppress the activity of immune cells and prevent the cell therapy from working.
Through lentiviral electrical delivery of CRISPR/Cas9 mRNA and gRNA, CAR T cells eliminate three TRAC, beta2-B2M, and PD-1 genes, producing more effective "self" CAR T cells that have been shown to lower GVHD and increase mouse survival [8].
CAR-T therapy's most common adverse effect is cytokine release syndrome (CRS). Numerous interleukins, including as IL-1, IL-2, IL-4, IL-6, IL-8, IL-10, TNF-α, and others, are produced, which causes it. To put it another way, IL-12 and IL-15 also increase anti-cancer activity, and IL-18 has been demonstrated to increase IFN-γ, which further encourages CAR-T proliferation [9]. When CAR-T cells are formed, CRISPR/Cas9 is employed to help knock out all the genes linked to toxicity and to help knock in the necessary cytokine genes while encouraging cytokine production.
3.2. TCR-T
Although CAR-T therapy has made a great breakthrough in blood tumors, it has not achieved a greater breakthrough in the treatment of solid tumors. So the researchers turned their hopes for a cure for solid tumors to another approach of ACT called TCR-T.TCR-T is engineered T cells that can specifically recognize antigens by modifying T cells in vitro, and the final expression of T cells can target and eliminate tumor cells to achieve the purpose of cancer treatment (Figure 1). TCRs, in contrast to CARs, recognize epitopes made from proteins that are found in any subcellular compartment, such as the membrane, cytoplasm, or nucleus. TCRs can identify a variety of targets with this, including viral oncoproteins, cancer germline antigens, and neoantigens [10]. Tumor cells and immunosuppressive cells have immune checkpoint receptors that attach to T cell negative regulatory ligands to inhibit T cells.In this TME lacking essential amino acids and low oxygen, T cells will be senescent and exhausted. Therefore, there is no cure in order to treat solid tumors, and its development can only be controlled through various ways.
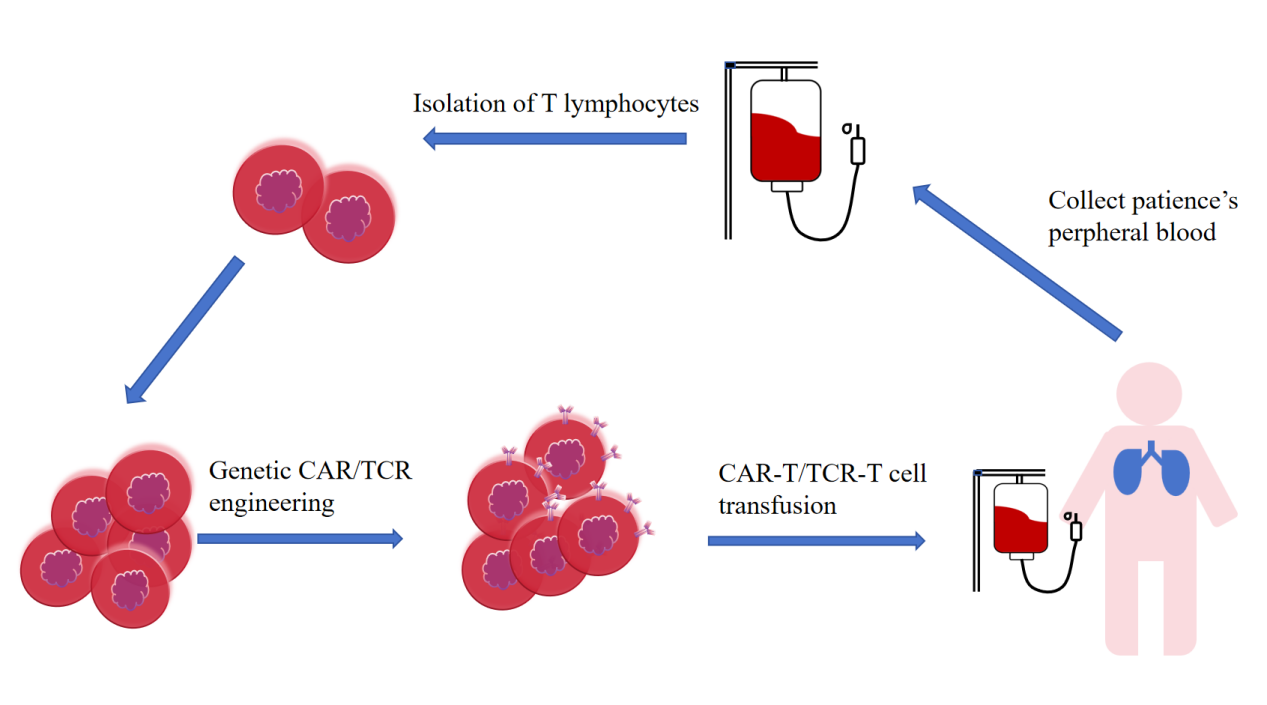
Figure 1: Clinical operation of T cell-based adoptive therapy.
Using CRISPR-Cas9, a revolutionary genetic scissors, researchers were able to accurately eliminate endogenous TCR (T cell receptor) genes in T cells, effectively avoiding the possible cross-interference between endogenous and transgenic TCR that is common in TCR-T therapy. Through such genetic engineering, T cells are able to specialize in the expression of a uniquely specified TCR, greatly increasing the therapy's effectiveness and targeting. Moreover, CRISPR-Cas9 can also play a key role by removing the immune checkpoint molecule PD-1 on T cells, freeing the shackles of T cell activity and enhancing its natural anti-tumor potential. This procedure also reduces the autoimmune risk and potential adverse effects associated with treatment. At present, TCR-T cells modified with CRISPR-Cas9 have entered the stage of clinical trials to verify their safety and efficacy. One cutting-edge study focused on a range of patients with intractable solid tumors, infusing them with CRISPR-Cas9-engineered TCR-T cells that carry TCRS specifically designed to recognize patient-specific tumor markers. Initial data show a positive safety profile, and in some cases have witnessed treatment results, bringing new hope for the fight against cancer. In short, the application of CRISPR-Cas9 technology in TCR-T cell therapy not only overcomes technical barriers, improves specificity and functional performance, but also opens up an innovation path in the field of anti-cancer, demonstrating its great potential as a future medical means.
Recently, TAEST16001, the first TCR-T therapy in China, was the subject of a Phase I clinical investigation. The results showed that the cell therapy is well tolerated and may have anticancer effect for expressing certain advanced soft tissue sarcomas. The core technology of TCR-T is to modify the binding of TCR to tumor antigens, and the natural TCR in the human body has a relatively low affinity for these antigens, so it is unable to recognize and kill tumor cells. When tumor cells are recognized, the synthetically created high-affinity TCR improves specific recognition and affinity. However, another drawback of TCR-T is that it is MHC cell-restricted and depends on the presentation of MHC molecules to identify and activate T cells.Patients receiving TCR-T must express not only the targeted antigen, but also the corresponding HLA allele antigen. Therefore, TCR-T therapy usually uses TCRS that are limited to relatively common HLA alleles. TAEST16001 is a product developed by Xiangxue Life Sciences and is currently undergoing Phase II clinical trials. TCR-T still faces many challenges in tumor therapy, such as the need to identify more specific antigens of tumor cells and recognize TCR, and how to improve its specific recognition and affinity with antigens by modifying TCR still need to be further studied. In any case, adoptive cell therapy represented by TCR-T has shown its great advantages in tumor treatment and is a key technology to solve cancer [10].
4. Limitations and future development trend
As an important part of cancer immunotherapy, CAR-T and TCR-T have broad development prospects in the future, but they still have certain limitations at present. For CAR T cells, at present, they are mainly targeted at some specific antigens of hematoma, and more antigens may be found for solid tumors in the future, thus expanding their application range. For TCR-T cells, the design and screening of TCRS should be further optimized so that they can more accurately recognize the antigens in tumor cells and improve the therapeutic effect.
Off-target effects are a potential risk of CAR-T and TCR-T therapies, which can lead to damage to normal tissue. In the future, the probability of off-target effects can be minimized by choosing antigens carefully, improving the specificity of TCR and improving gene editing technology.
CAR-T and TCR-T cells may induce immune reactions such as CRS, which may endanger patients' lives in severe cases [11,12]. In the future, it is necessary to further study the mechanism of immune response and develop more effective monitoring and control methods, such as predicting the occurrence of CRS in advance and adjusting the treatment plan in time, so as to reduce the risk of immune reaction.
5. Conclusion
CRISPR technology has brought an unprecedented boost to adoptive cell immunotherapy (CAR-T and TCR-T) in cancer research. In CAR-T and TCR-T therapies, CRISPR technology, with its ability to precisely edit the genome, reduces immune rejection after allotransplantation through gene editing, increases the availability of "universal" immune cells, and can also overcome the inhibition of the TME and enhance the activity of immune cells. These results show that CRISPR technology has great potential to enhance the safety and effectiveness of adoptive cell immunotherapy, which is expected to bring more innovative strategies and better treatment results for cancer treatment, and is an important help for cancer research to develop more effective and more accurate treatment. However, despite these advances, CRISPR technology still faces challenges in practical applications such as off-target effects and CRS immune responses, and more in-depth research is needed to further optimize the use of this technology in adoptive cell immunotherapy.
References
[1]. Wang, S. W., Gao, C., Zheng, Y. M., Yi, L., Lu, J. C., Huang, X. Y., Cai, J. B., Zhang, P. F., Cui, Y. H., & Ke, A. W. (2022). Current applications and future perspective of CRISPR/Cas9 gene editing in cancer. Molecular Cancer, 21(1), 57.
[2]. Liu, Z., Chen, S., Jin, X., Wang, Q., Yang, K., Li, C., Xiao, Q., Hou, P., Liu, S., Wu, S., Hou, W., Xiong, Y., Kong, C., Zhao, X., Wu, L., Li, C., Sun, G., & Guo, D. (2017). Genome editing of the HIV co-receptors CCR5 and CXCR4 by CRISPR-Cas9 protects CD4+ T cells from HIV-1 infection. Cell Bioscience, 7, 47.
[3]. Yang, H., Ren, S., Yu, S., Pan, H., Li, T., Ge, S., Zhang, J., & Xia, N. (2020). Methods favoring homology-directed repair choice in response to CRISPR/Cas9 induced-double strand breaks. International Journal of Molecular Sciences, 21(18), 6461.
[4]. Chen, C., Wang, Z., & Qin, Y. (2023). CRISPR/Cas9 system: recent applications in immuno-oncology and cancer immunotherapy. Experimental Hematology & Oncology, 12(1), 95.
[5]. Albarrán, V., San Román, M., Pozas, J., Chamorro, J., Rosero, D. I., Guerrero, P., Calvo, J. C., González, C., García de Quevedo, C., Pérez de Aguado, P., Moreno, J., Cortés, A., & Soria, A. (2024). Adoptive T cell therapy for solid tumors: current landscape and future challenges. Frontiers in Immunology, 15, 1352805.
[6]. Benmebarek, M. R., Karches, C. H., Cadilha, B. L., Lesch, S., Endres, S., & Kobold, S. (2019). Killing mechanisms of chimeric antigen receptor (CAR) T cells. International Journal of Molecular Sciences, 20(6), 1283.
[7]. Mehrabadi, A. Z., Ranjbar, R., Farzanehpour, M., Shahriary, A., Dorostkar, R., Hamidinejad, M. A., & Ghaleh, H. E. G. (2022). Therapeutic potential of CAR T cell in malignancies: A scoping review. Biomedicine & Pharmacotherapy, 146, 112512.
[8]. Ren, J., Liu, X., Fang, C., Jiang, S., June, C. H., & Zhao, Y. (2017). Multiplex genome editing to generate universal CAR T cells resistant to PD1 inhibition. Clinical Cancer Research, 23(9), 2255–2266.
[9]. Hurton, L. V., Singh, H., Najjar, A. M., Switzer, K. C., Mi, T., Maiti, S., Olivares, S., Rabinovich, B., Huls, H., Forget, M. A., Datar, V., Kebriaei, P., Lee, D. A., Champlin, R. E., & Cooper, L. J. (2016). Tethered IL-15 augments antitumor activity and promotes a stem-cell memory subset in tumor-specific T cells. Proceedings of the National Academy of Sciences, 113(48), E7788–E7797.
[10]. Chandran, S. S., & Klebanoff, C. A. (2019). T cell receptor-based cancer immunotherapy: Emerging efficacy and pathways of resistance. Immunological Reviews, 290(1), 127–147.
[11]. Pan, Q., Weng, D., Liu, J., Han, Z., Ou, Y., Xu, B., Peng, R., Que, Y., Wen, X., Yang, J., Zhong, S., Zeng, L., Chen, A., Gong, H., Lin, Y., Chen, J., Ma, K., Lau, J. Y. N., Li, Y., Fan, Z., & Zhang, X. (2023). Phase 1 clinical trial to assess safety and efficacy of NY-ESO-1-specific TCR T cells in HLA-A*02:01 patients with advanced soft tissue sarcoma. Cell Reports Medicine, 4(8), 101133.
[12]. Jia, Z., Ragoonanan, D., Mahadeo, K. M., Gill, J., Gorlick, R., Shpal, E., & Li, S. (2022). IL12 immune therapy clinical trial review: Novel strategies for avoiding CRS-associated cytokines. Frontiers in Immunology, 13, 952231.
Cite this article
Li,Y. (2025). CRISPR Technology Advances Immunotherapy in Cancer Research: Research Progress. Theoretical and Natural Science,82,70-75.
Data availability
The datasets used and/or analyzed during the current study will be available from the authors upon reasonable request.
Disclaimer/Publisher's Note
The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of EWA Publishing and/or the editor(s). EWA Publishing and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
About volume
Volume title: Proceedings of the 3rd International Conference on Modern Medicine and Global Health
© 2024 by the author(s). Licensee EWA Publishing, Oxford, UK. This article is an open access article distributed under the terms and
conditions of the Creative Commons Attribution (CC BY) license. Authors who
publish this series agree to the following terms:
1. Authors retain copyright and grant the series right of first publication with the work simultaneously licensed under a Creative Commons
Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this
series.
2. Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the series's published
version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial
publication in this series.
3. Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and
during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See
Open access policy for details).
References
[1]. Wang, S. W., Gao, C., Zheng, Y. M., Yi, L., Lu, J. C., Huang, X. Y., Cai, J. B., Zhang, P. F., Cui, Y. H., & Ke, A. W. (2022). Current applications and future perspective of CRISPR/Cas9 gene editing in cancer. Molecular Cancer, 21(1), 57.
[2]. Liu, Z., Chen, S., Jin, X., Wang, Q., Yang, K., Li, C., Xiao, Q., Hou, P., Liu, S., Wu, S., Hou, W., Xiong, Y., Kong, C., Zhao, X., Wu, L., Li, C., Sun, G., & Guo, D. (2017). Genome editing of the HIV co-receptors CCR5 and CXCR4 by CRISPR-Cas9 protects CD4+ T cells from HIV-1 infection. Cell Bioscience, 7, 47.
[3]. Yang, H., Ren, S., Yu, S., Pan, H., Li, T., Ge, S., Zhang, J., & Xia, N. (2020). Methods favoring homology-directed repair choice in response to CRISPR/Cas9 induced-double strand breaks. International Journal of Molecular Sciences, 21(18), 6461.
[4]. Chen, C., Wang, Z., & Qin, Y. (2023). CRISPR/Cas9 system: recent applications in immuno-oncology and cancer immunotherapy. Experimental Hematology & Oncology, 12(1), 95.
[5]. Albarrán, V., San Román, M., Pozas, J., Chamorro, J., Rosero, D. I., Guerrero, P., Calvo, J. C., González, C., García de Quevedo, C., Pérez de Aguado, P., Moreno, J., Cortés, A., & Soria, A. (2024). Adoptive T cell therapy for solid tumors: current landscape and future challenges. Frontiers in Immunology, 15, 1352805.
[6]. Benmebarek, M. R., Karches, C. H., Cadilha, B. L., Lesch, S., Endres, S., & Kobold, S. (2019). Killing mechanisms of chimeric antigen receptor (CAR) T cells. International Journal of Molecular Sciences, 20(6), 1283.
[7]. Mehrabadi, A. Z., Ranjbar, R., Farzanehpour, M., Shahriary, A., Dorostkar, R., Hamidinejad, M. A., & Ghaleh, H. E. G. (2022). Therapeutic potential of CAR T cell in malignancies: A scoping review. Biomedicine & Pharmacotherapy, 146, 112512.
[8]. Ren, J., Liu, X., Fang, C., Jiang, S., June, C. H., & Zhao, Y. (2017). Multiplex genome editing to generate universal CAR T cells resistant to PD1 inhibition. Clinical Cancer Research, 23(9), 2255–2266.
[9]. Hurton, L. V., Singh, H., Najjar, A. M., Switzer, K. C., Mi, T., Maiti, S., Olivares, S., Rabinovich, B., Huls, H., Forget, M. A., Datar, V., Kebriaei, P., Lee, D. A., Champlin, R. E., & Cooper, L. J. (2016). Tethered IL-15 augments antitumor activity and promotes a stem-cell memory subset in tumor-specific T cells. Proceedings of the National Academy of Sciences, 113(48), E7788–E7797.
[10]. Chandran, S. S., & Klebanoff, C. A. (2019). T cell receptor-based cancer immunotherapy: Emerging efficacy and pathways of resistance. Immunological Reviews, 290(1), 127–147.
[11]. Pan, Q., Weng, D., Liu, J., Han, Z., Ou, Y., Xu, B., Peng, R., Que, Y., Wen, X., Yang, J., Zhong, S., Zeng, L., Chen, A., Gong, H., Lin, Y., Chen, J., Ma, K., Lau, J. Y. N., Li, Y., Fan, Z., & Zhang, X. (2023). Phase 1 clinical trial to assess safety and efficacy of NY-ESO-1-specific TCR T cells in HLA-A*02:01 patients with advanced soft tissue sarcoma. Cell Reports Medicine, 4(8), 101133.
[12]. Jia, Z., Ragoonanan, D., Mahadeo, K. M., Gill, J., Gorlick, R., Shpal, E., & Li, S. (2022). IL12 immune therapy clinical trial review: Novel strategies for avoiding CRS-associated cytokines. Frontiers in Immunology, 13, 952231.









