1. Introduction
When we dream of flying across the sky and suddenly realize that we are dreaming, we are experiencing lucid dreaming. Lucid dreaming is a phenomenon in which one becomes aware of dreaming during the dream, and the dreamer can partly control the dream's content. A Dutch psychiatrist first proposed the concept of lucid dreaming when he kept records of his dreams [1]. This phenomenon was not scientifically verified until 1975 when electrooculography (EOG) was applied to record the prearranged special eye movement patterns of subjects during dreaming [2]. After that, a series of techniques to induce lucid dreaming were developed [3][4]. At the same time, scientists also studied the activity, connectivity, and composition of several cortical areas related to lucid dreaming [5][6][7]. Recently, some interesting applications of lucid dreaming, such as the control of virtual avatars from dreams [8], have been explored.
Lucid dreaming is prevalent among humans, as a meta-analysis from the period 1966-2016 shows that about 55% of people have experienced one or more lucid dreams in their whole life, and about 23% of people experience lucid dreaming at least once a month [9]. Such prevalence of lucid dreaming forms a solid foundation to carry out studies about lucid dreaming, especially large sample studies. Lucid dreaming is a research domain full of potential for cognitive neuroscience and dream research as it is an accessible case that allows us to carry out experimental manipulations to study the neural mechanisms of cognition, dream experience, and, most importantly, consciousness. What’s more, lucid dreaming also has many potential applications in both clinical and daily scenarios [10], which may provide new approaches to psychotherapy, creativity enhancement, motor skill improvement, human-computer interaction, education, and even entertainment. Thus, lucid dreaming is a topic with great potential in both basic research and daily scenarios, calling for more and deeper explorations.
This article reviews the studies about lucid dreaming from the perspective of neuroscience, aiming at summarizing the neurophysiological findings of lucid dreaming, discussing the relation between lucid dreaming and consciousness, and exploring the known and potential application of lucid dreaming.
2. Neurophysiological Features of Lucid Dreaming
Lucid dreaming was first thought to be a special mixed mental state. Scientists carried out an electrophysiological experiment, during which electroencephalogram (EEG), Electromyography (EMG), and EOG were applied to record the activities of the brain, eyes, and muscles [11]. The subjects were asked to signal lucid dreaming using predetermined patterns of horizontal eye movement, such as L-R-L.
They found that the power of lucid dreaming and non-lucid REM sleep is similar in low low-frequency bands, whereas the power of lucid dreaming in high high-frequency bands, especially around 40 Hz, is significantly higher than non-lucid REM sleep (see Figure 1). Such results indicate that lucid dreaming seems to be a mixed state of consciousness, with features of both waking and non-lucid REM sleep. Note that further research has verified that lucid dreaming is actually an activated REM sleep rather than a mixed state between waking and REM [12], which complements this pioneering study. It was also found that the CSD (current source densities) coherence of lucid dreaming is significantly higher than non-lucid REM sleep and waking at the frontal region and frontolateral front lateral region (see Figure 2), which may partly account for the self-awareness and internal attention of lucid dreaming, since the neural synchronization of these regions is highly related to such cognitive functions [4][13][14].
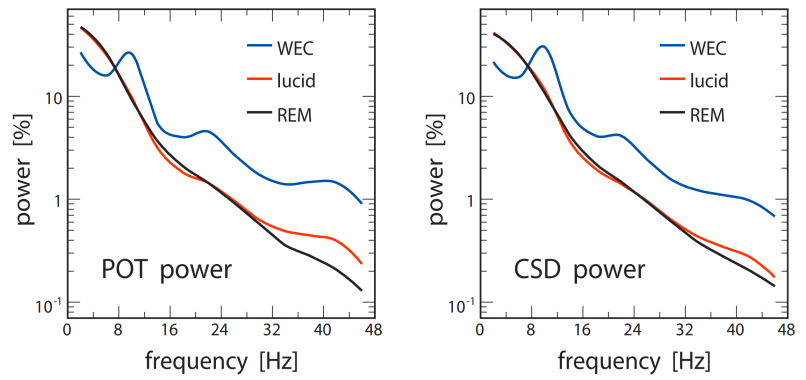
Figure 1: Power spectrum comparison of lucid dreaming, non-lucid REM sleep, and waking with eyes closed (WEC). The graph illustrates the relative power distribution across different frequency bands, highlighting the distinct increase in low γ band activity (around 40 Hz) during lucid dreaming compared to non-lucid REM sleep. POT = scalp potentials, CSD = current source densities [11].
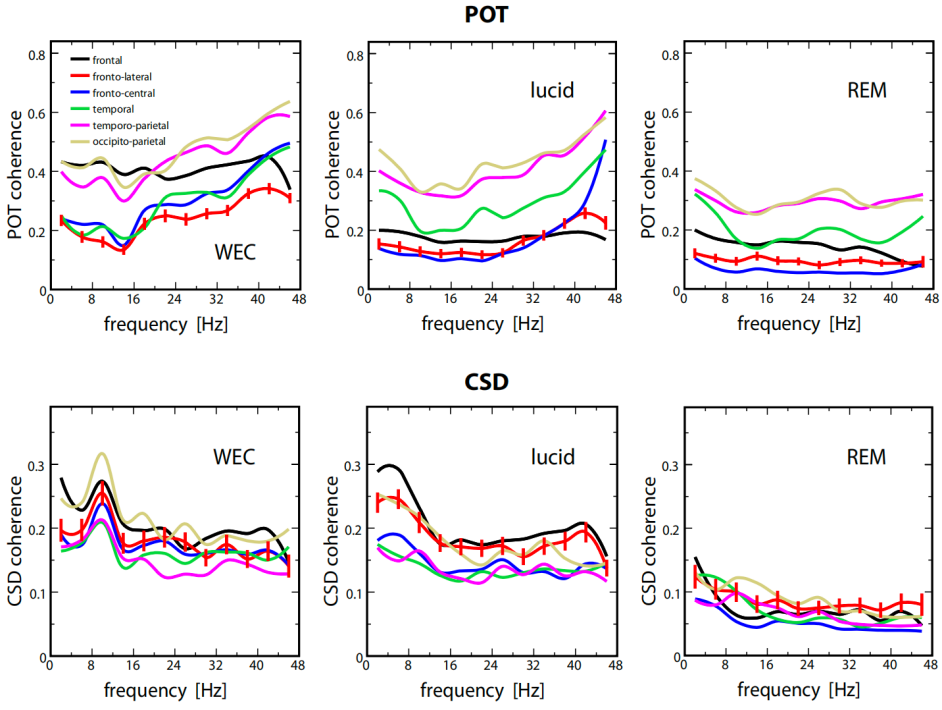
Figure 2: The average coherence values across selected regions of interest (ROIs) were derived from POT (scalp potentials) and CSD (current source densities) measurements. It highlights the increased coherence in the frontal and frontolateral regions during lucid dreaming compared to both WEC (wake with eyes closed) and non-lucid REM sleep, suggesting enhanced neural synchronization associated with self-awareness and internal attention in lucid dreaming [11].
The extrapolation of lucid dreaming above was further explored in 2012 through neuroimaging research [15]. Scientists found that several cortical regions, which are typically deactivated during non-lucid REM sleep, are reactivated during lucid dreaming with fMRI, which can account for the restoration of some cognitive functions, such as reflection, during lucid dreaming.
Blood Oxygen Level-Dependent (BOLD) signals during lucid dreaming showed increased activation mainly in the right dorsolateral prefrontal cortex, bilateral frontopolar areas, precuneus, bilateral cuneus cortices, and occipitotemporal cortices (see Figure 3). Such areas are thought to be associated with metacognitive evaluation, especially towards self [16], processing of internally generated information [17][18], operations of first-person perspective experience [19], and perceptual awareness [20], especially visual awareness, which is highly related to the awareness of dreaming state during lucid dreaming. Such results give us a comprehensive insight into the brain activities and corresponding phenomenological features of lucid dreaming.
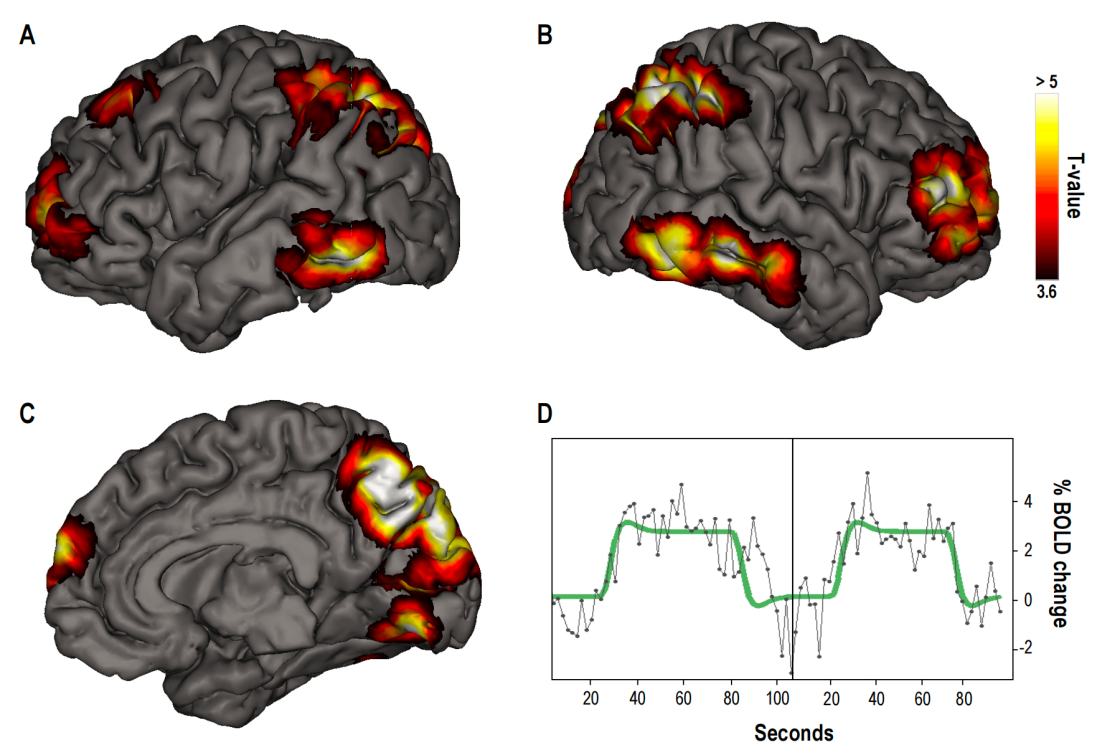
Figure 3: Neural activations associated with lucid dreaming. Color-coded clusters represent areas of increased activity, indicating higher neural engagement in these regions during lucid dreaming. The panels (A) and (B) show the left and right hemispheres, and panel (C) provides a midline view. Panel (D) illustrates the Blood Oxygen Level-Dependent (BOLD) signal change over time in the right precuneus [15].
As a profound feature in lucid dreaming and a higher-order cognitive capability, metacognitive function in lucid dreaming was further researched. It was revealed that the thought monitoring during lucid dreaming may share the same neural basis with metacognitive function, especially the frontopolar cortex [7]. During the experiment, the subjects participated in two kinds of thought-monitoring tasks. In the non-monitor trials, subjects need to move the red cursor so that it matches the target circle. In the monitor trials, subjects needed to move the cursor depending on whether their mind was more focused on internal thinking (INT) or external circumstances (EXT).
A greater volume of gray matter was found for those high-lucidity dreamers compared to the low-lucidity dreamers using fMRI, especially in the frontopolar cortex (BA9/10). Furthermore, for both the high-lucidity and low-lucidity groups, a significant difference in BOLD occurred between non-monitor trials and monitor trials in BA9/10 (see Figure 4). Together, these results showed that BA9/10 directly participates in the metacognitive functions, especially thought monitoring and such functions are highly related to lucid dreaming, which may indicate that the activation of metacognition during sleep probably makes lucid dreaming possible.
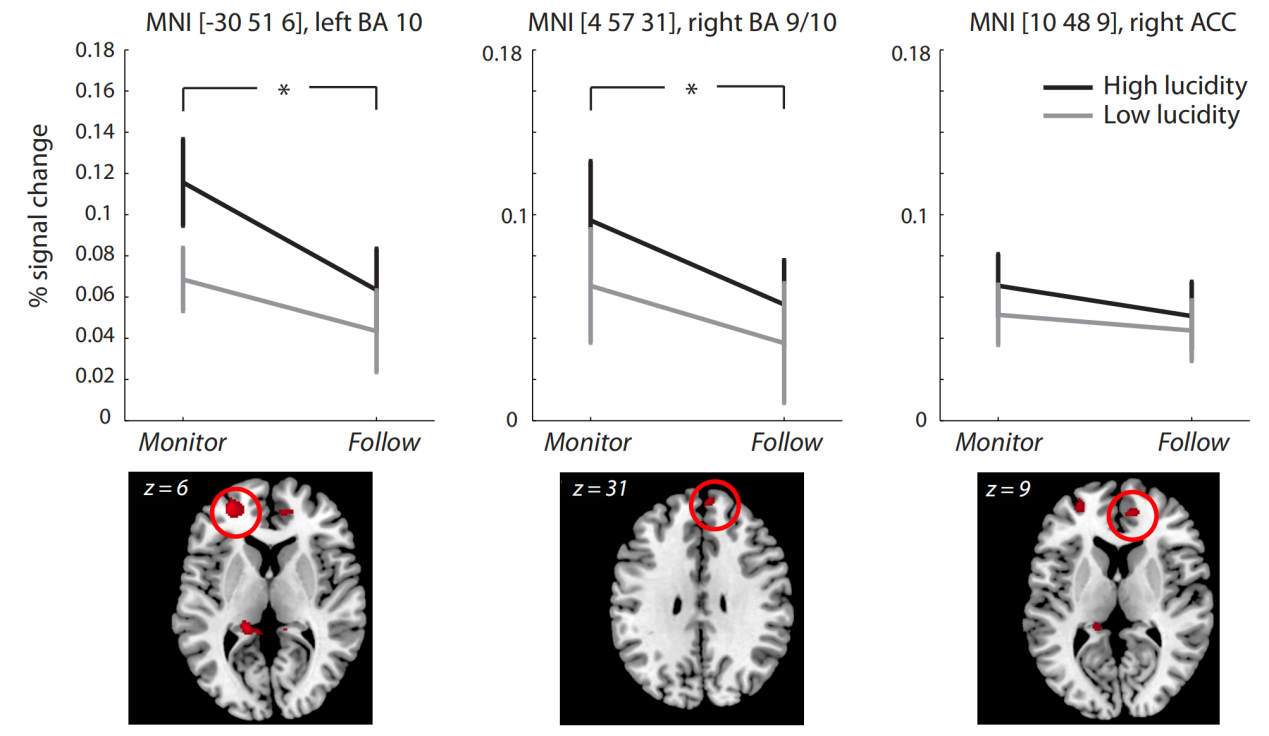
Figure 4: The signal changes of BOLD during the thought-monitoring task within ROIs (Regions of Interest). The signal changes for high and low lucidity groups within the left BA 10 (MNI coordinate [-30 51 6]), right BA 9/10 (MNI coordinate [4 57 31]), and right ACC (MNI coordinate [10 48 9]) [7].
Together, these results showed that BA9/10 directly participates in the metacognitive functions, especially thought monitoring and such functions are highly related to lucid dreaming, which may indicate that the activation of metacognition during sleep probably makes lucid dreaming possible. Together, the studies above showed that lucid dreaming was thought to be a mixed state of consciousness (but an activated REM sleep), with features of both waking and non-lucid REM sleep, in which several brain regions related to self and consciousness were abnormally activated, and possibly being a specific manifestation of metacognition during dreaming. With these insights from the neuroscientific perspective, scientists began to wonder if lucid dreaming could be used to study one of the most mysterious and difficult problems of science the consciousness.
3. The Study of Consciousness About Lucid Dreaming
Scientists studied higher-order consciousness through lucid dreaming and claimed that higher-order consciousness is distinctively related to the synchronous oscillations around 25-40 Hz of brain activity [4]. During the experiment, EEG, EOG, and EMG were applied to record related activities. Once the subject entered REM sleep, they would perform frontotemporal transcranial alternating current stimulation (tACS) in a certain frequency band, varying from 0 to 100Hz. After that, they woke up the subject to report the content of their dream, as well as complete the LuCid scale, a validated scale to rate the lucidity or consciousness of the dream [21].
The ratio of the instantaneous power during stimulation versus the average power before stimulation is used to show the result (see Figure 5). Under the tACS of 25 Hz and 40 Hz, the power ratio of lucid dreaming notably increased. Together with the self-reports and LuCid scales, they found that such tACS stimulation can induce self-awareness, which results in lucid dreaming. Though the study is doubted by later researchers [22], the result still provides causal evidence indicating that specific oscillation frequencies of brain activity in frontal and temporal parts may be related to self-consciousness. Besides, the study also provides a possible electrophysiological method to induce lucid dreaming.
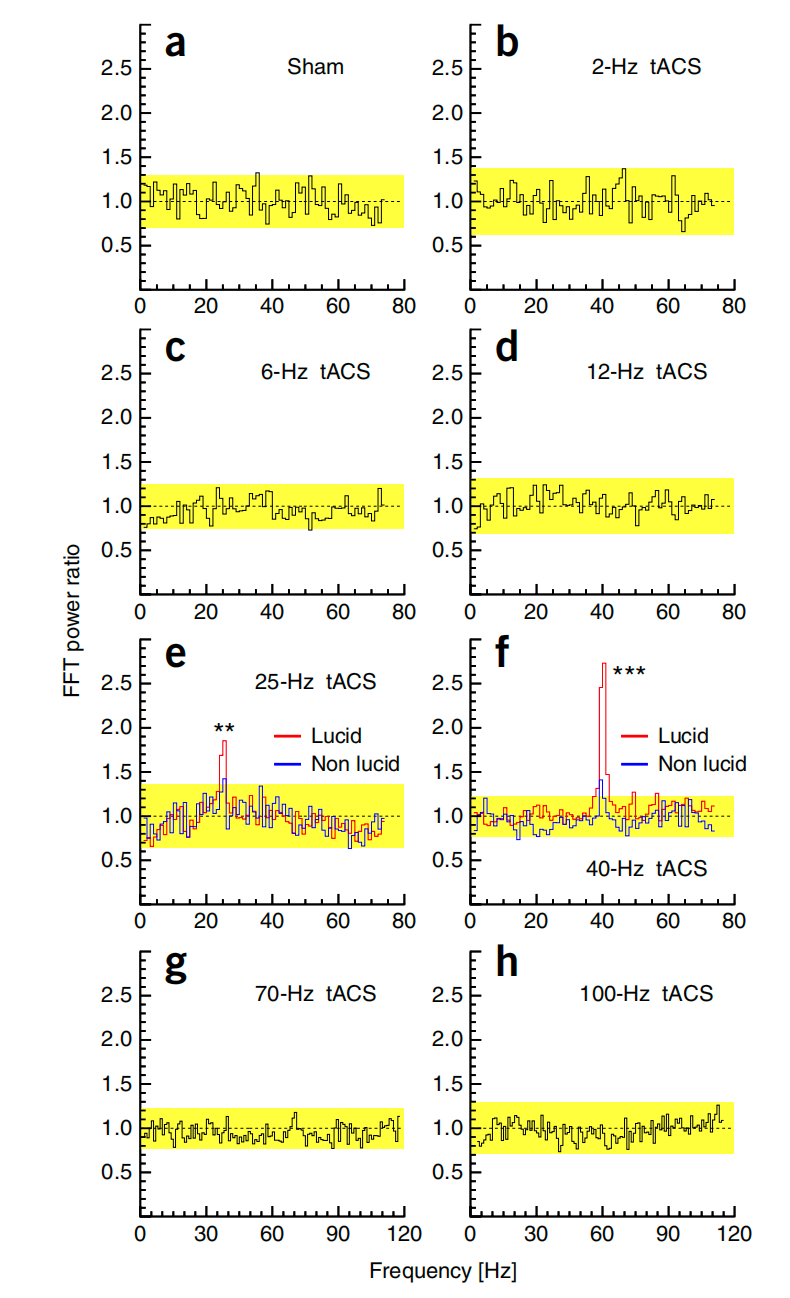
Figure 5: FFT power ratios of activity during tACS stimulation versus activity before stimulation for different stimulation conditions showed significant increases in low γ band activity associated with lucid dreaming during 25 Hz and 40 Hz tACS [4].
Except for directly exploring the consciousness in lucid dreaming, lucid dreaming also allows us to look into perceptions in a different state of consciousness, such as visual perception. Scientists discovered that the eye movement pattern in lucid dreaming is smooth pursuit eye movements (SPEMs), which is highly similar to the waking state, whereas saccadic eye movements prevail during visuomotor imagination [23]. More interestingly, it was further verified that SPEMs can occur without retinal stimulation and can be driven by the specific voluntary change in the direction of gaze in dreams.
During the experiment, the subjects performed eye-tracking tasks in 3 different conditions, including lucid dreaming, waking, and imaging. A series of methods were used to make sure the subjects entered REM and marked the beginning of lucid dreaming (see Figure 6). The EMG signals showed muscle paralysis, specifically during REM, which is known as atonia. Skin potential response (SPR) was used to mark the beginning of lucid dreaming. It was found that SPEMs occur most of the time during waking perception and lucid dreaming when tracking a target, whereas significantly more saccades were observed during visuomotor imagination (see Figure 7). This research is of particular significance since it gives tentative answers to several tricky questions [10], one of which is “Whether dreams are generated in a ‘bottom-up’ or a ‘top-down’ manner?” [24]. The study answers the question by revealing that dream is more like perception (‘bottom-up’) than imagination (‘top-down’), at least in the context of visual perception, which opens up a new possibility to explore a “simplified version” of perceptual consciousness and its integration in dreams, since a lot of activities are deactivated during dreaming, which remains a popular and crucial question in the study of consciousness.
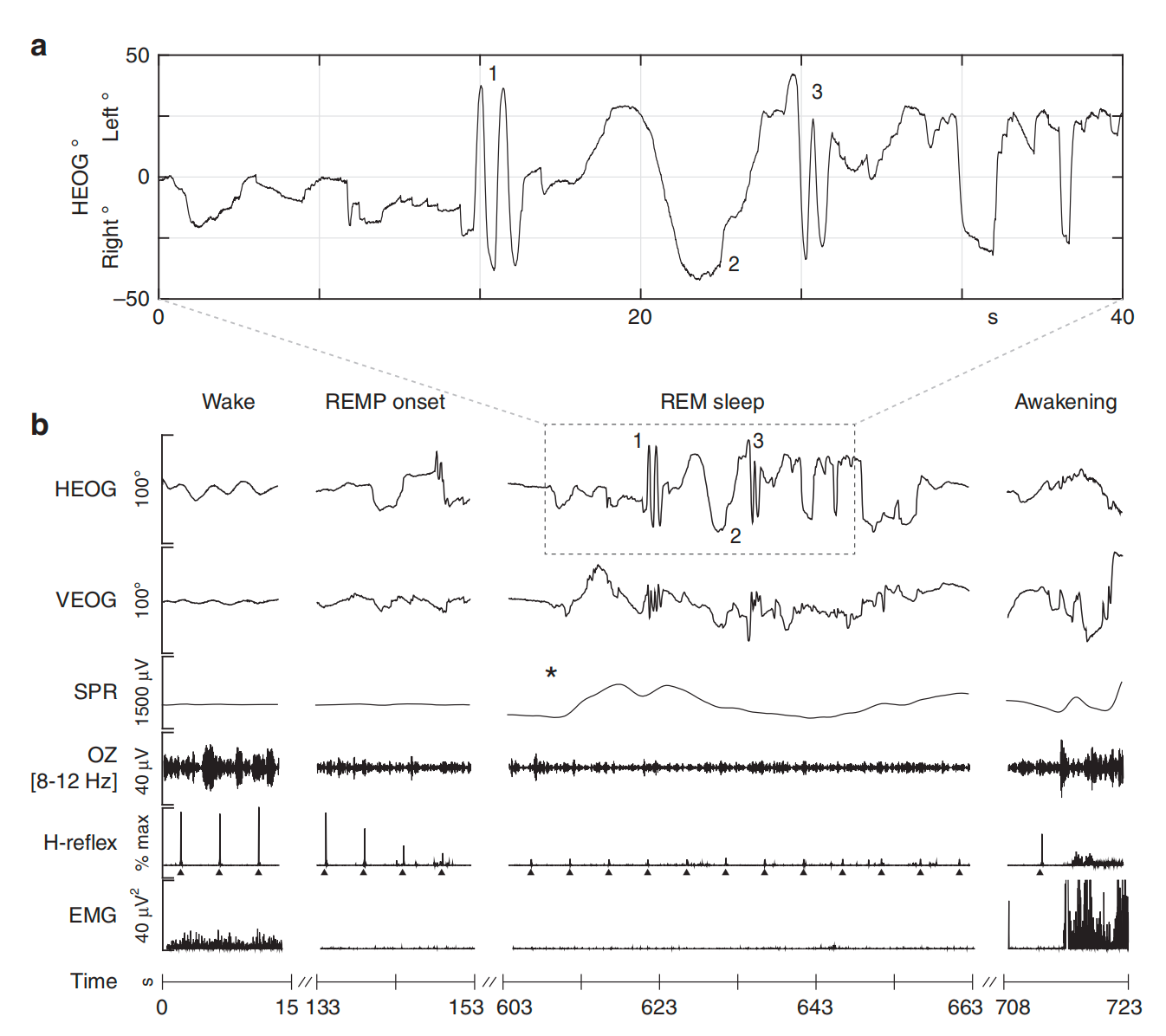
Figure 6: Data was tracked during a lucid REM sleep dream in a participant. 1 and 3 are L-R-L-R eye movements, and 2 is the actual eye tracking task, in which the EOG pattern is similar to the trajectory during waking. Asterisk depicts the beginning of lucid dreaming [23].
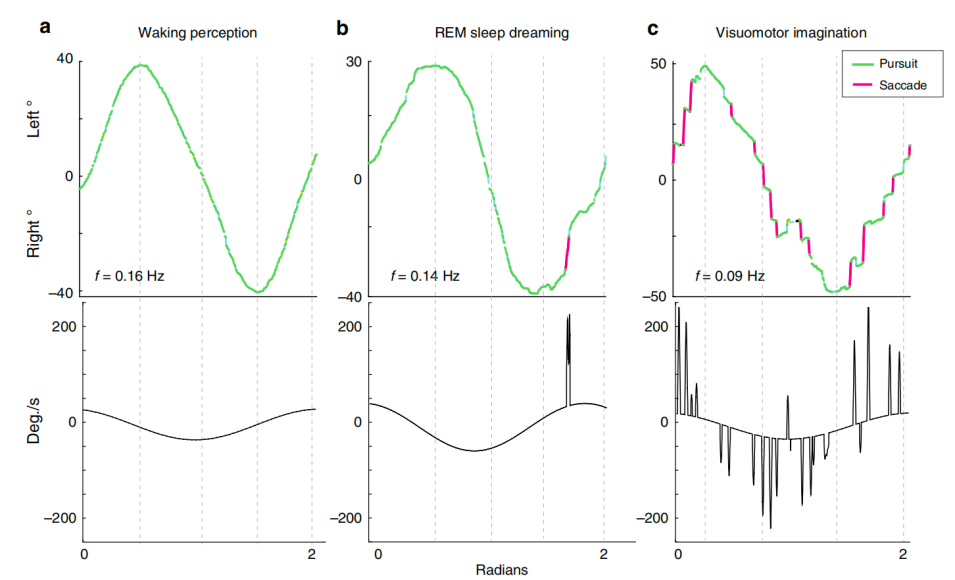
Figure 7: Eye tracking during 1D horizontal meridian line tracking tasks in waking perception, lucid dreaming, and visuomotor imagination illustrate the contrast between smooth pursuit eye movements and saccadic intrusions [23].
Based on empirical findings of lucid dreaming, a theoretical framework was proposed to integrate the phenomenological features and neural mechanisms of lucid dreaming, in which many aspects of consciousness are involved [25]. The most fundamental component of the framework is predictive coding (PC). PC regards our brain as a hierarchical system. Different levels of the brain would predict the external environment and internal state based on former experience and sensory stimulation (prior). Such predictions would be evaluated by the following actual input (posterior), and the difference between prior and posterior is prediction error. A key claim of the framework is that our brains tend to minimize prediction error, which is quite reasonable since such a tendency helps us adapt to the environment.
Prediction error minimization is mainly done by higher-order consciousness, especially during dreaming, which can partly explain the disorganized dream since the higher hierarchy can change prediction coding freely without too many external constraints. Furthermore, higher consciousness attenuates prediction error by producing a more comprehensive internal model, which requires multisensory integration. Multisensory integration is thought to play a vital role in the maintenance of lucid dreaming since it enhances the consistency of dream content and sensory input. For example, when we dream of diving under the water in lucid dreaming, corresponding respiratory behavior like central apnoea may occur [26]. Such a phenomenon reminds us that the consistency of experience is crucial for consciousness, which may partly explain the role of multisensory integration in consciousness.
Apart from multisensory integration, dynamic attention also attenuates prediction error by giving different precision weights to different priors. When we pay attention to certain sensory stimulation, the precision weight of the stimulation increases during the PC. Such a mechanism allows a flexible shift of PC and promotes the balance of information processing between various hierarchies. According to the theory, we identify the dream state during lucid dreaming when we pay attention to certain abnormal aspects in dreams compared to reality, which is the precondition of controlling the dream. We control the dream content during lucid dreaming by flexibly shifting our dynamic attention. For example, when we intend to fly in a dream, we can pay less attention to vestibular sensation and more attention to our flying movement.
In this section, two experiments exploring consciousness during lucid dreaming are reviewed, which show that self-consciousness may be related to certain synchronous oscillations of the brain activity, and perceptions in lucid dreaming are more similar to waking compared to imagination, at least for visual perception. In the future, we can delve deeper into the synchronization of biological neural networks through lucid dreaming [27], as well as explore a wide range of perceptions in lucid dreaming to enrich our knowledge of perceptual consciousness. Apart from experimental research, a theoretical framework of lucid dreaming is also reviewed, which mainly organizes multisensory integration and dynamic attention based on predictive coding. Such a theoretical framework is also indispensable to understanding such a complex phenomenon as consciousness. In addition to all these basic neuroscientific studies, the potential application of lucid dreaming has also aroused the interest of scientists.
4. Applications of Lucid Dreaming
Since we can control our dream content in lucid dreaming, scientists were thinking about using lucid dreaming as a therapy for nightmares, especially for patients with post-traumatic stress disorder (PTSD), as 80% of them suffer from nightmares [28]. The American Academy of Sleep Medicine regards lucid dreaming therapy (LDT) as one of the cognitive behavioral therapy (CBT) approaches to deal with nightmares [29], and LDT is the only CBT to deal with nightmares during dreaming rather than waking so far. One of the reasons for LDT to be effective might be that the induction exercises help patients to perceive their dreams more critically [30]. To further explore its effectiveness, Scientists carried out research and found that LDT can significantly lower the anxiety and depression levels of PTSD patients, even though it was not observed to directly lower the severity or frequency of nightmares [31]. Such result shows that LDT can be seen as a complementary treatment for other pharmacological treatments and CBT methods in the process of treating nightmares. However, more convincing evidence and consistent results of LDT are needed in the future.
Apart from clinical applications, lucid dreaming may also encourage us to outperform some motor skills in our daily lives. It was discovered that lucid dream practice (LDP) could improve our performance during waking with an effect close to mental practice and a bit smaller than physical practice [32]. The study result showed that LDP, Mental Practice (MP), and Physical Practice (PP) all improved their performance significantly compared with the control group. And the effect size of LDP is similar to MP, a widely used training method in sports [33]. This study provides a possibility to effectively train our motor skills in lucid dreaming, which can be quite helpful for athletes. Furthermore, this study also partly expands the simulation theory, which states that the same neural basis is shared between motor performance and motor simulation since similar regions of our brain are activated during observation, imagination, and execution of certain movements [34]. Future studies could focus on the neural mechanism of motor performance improvement during lucid dreaming, which is likely to provide new insights into bridging the gap between cognitive science and neuroscience.
In addition to applying lucid dreaming to individuals as either therapy or training, a more imaginative application is gradually becoming a reality: human-computer interaction in lucid dreaming. It was tentatively found that a two-way control of the avatar can be accomplished during lucid dreaming, indicating dreamers in lucid dreaming can control virtual objects through muscle contractions, as well as receive feedback to adjust the control [8]. During the experiment, the subjects were first trained to control a virtual automobile (avatar) with muscle contractions of legs and arms through EMG signals. Then, subjects spent 1-4 nights in the laboratory and controlled the avatar during lucid dreaming, in which EEG, EOG, and EMG were applied to record the data. When the subjects woke up the next morning, they were asked to report their dream experiences.
The most prominent result came from the fourth attempt of the first subject (see Figure 8). Such a result shows that signals were consciously sent by the subject to control the avatar, and the feedback information can also reach the subject, allowing him to adjust his control. This study provides a possibility to develop a new human-computer interaction interface based on lucid dreaming. For example, we may be able to control our smart home devices during lucid dreaming. What’s more, this study also expands our understanding of the potential high-level functions of our brain during lucid dreaming, as well as provides a new approach to exploring our brain activities during lucid dreaming, especially in terms of cognitive control and consciousness.
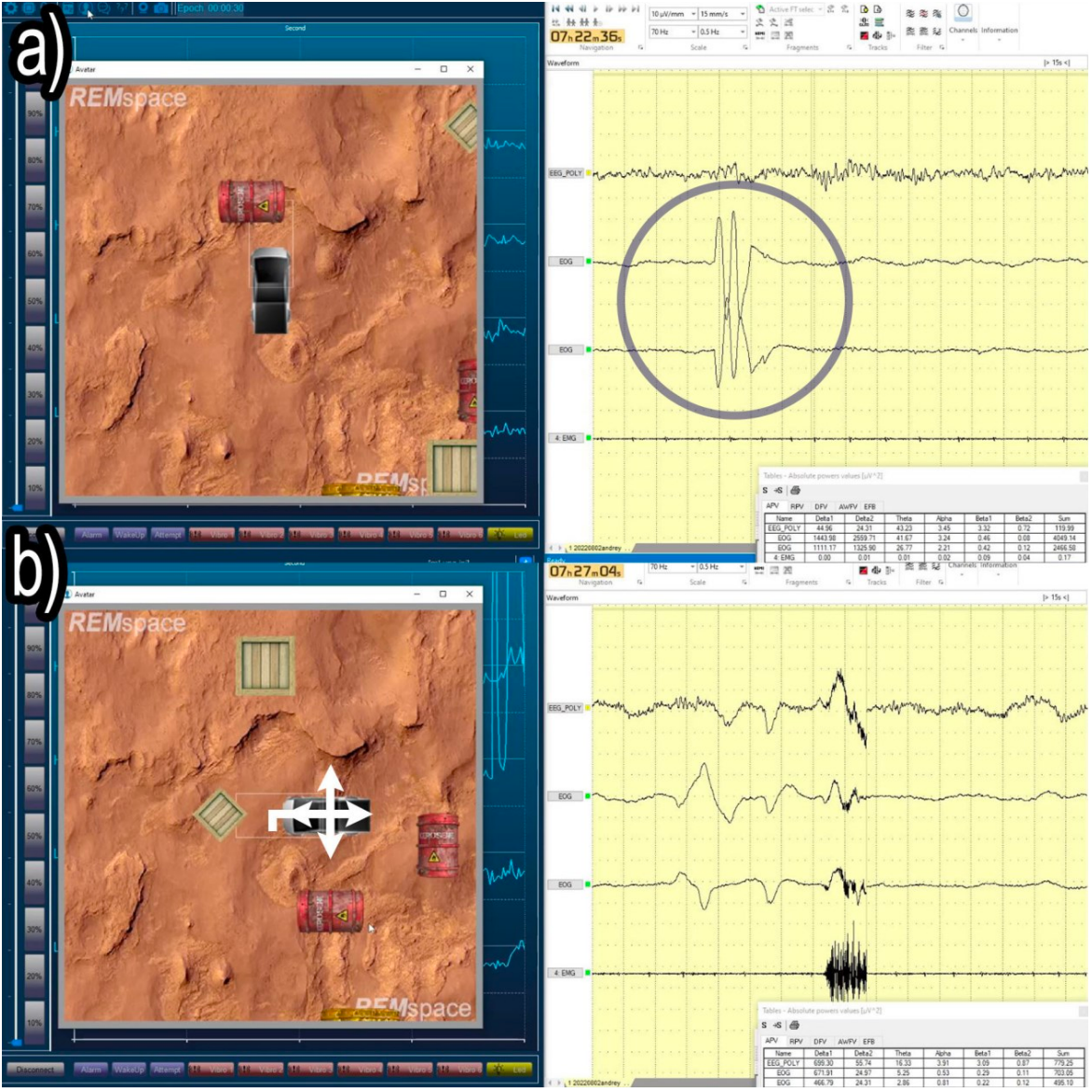
Figure 8: Real-time monitoring screenshots depicting the first subject successfully controlling the avatar during the fourth attempt. The PSG observation with predetermined eye signals on EOG channels (encircled), the corresponding EMG impulses, and their decoded outcomes are shown, interpreting the two-way control during a lucid dream. The arrows show the moving direction of the avatar [8].
In this section, applications of lucid dreaming, including clinical application as a therapy for nightmares, individual application as a training for motor skills, and daily application as an approach to human-computer interaction, are reviewed and discussed. Furthermore, these applications go beyond pure applications to provide new ideas and new methods for future neuroscience research, as well as give us a deeper understanding of the complicated emotional, cognitive, and behavioral functions of our brain in the context of lucid dreaming.
5. Conclusion
To sum up, this article gives a brief review of lucid dreaming, mainly from the perspective of neuroscience. First, several general neurobiological features detected by bioelectric signal measurements and neuroimaging technologies are discussed, which lay the foundation for us to delve into consciousness through lucid dreaming. Then, both experimental studies and the theoretical framework of lucid dreaming concentrating on various aspects of consciousness are reviewed. These researches not only give answers to some tricky and basic questions about consciousness but also open up more possibilities to explore consciousness further through lucid dreaming. Such opportunities are researching the synchronization of our neural network, investigating various perceptual consciousnesses during lucid dreaming, finding the neural mechanism of predictive coding, and so on. Finally, a few applications of lucid dreaming are shown, such as nightmare treatment, motor skill training, and human-computer interaction, illustrating the great application potential of lucid dreaming. Besides, these applications are also helpful for future basic research since they provide new approaches and even guide new directions, which is mainly due to their focus on practical problems in reality, the source of almost all scientific questions.
Lucid dreaming remains a mysterious phenomenon with great potential to be further researched, especially when it comes to all the aspects of consciousness in lucid dreaming. Fortunately, some inherent characteristics of lucid dreaming, especially the awareness and control of the dreamers, make the study of lucid dreaming accessible as long as we can find more reliable and effective methods to induce stable lucid dreaming. Apart from the accessibility, lucid dreaming, as a form of dreaming, naturally simplifies our brain activities a lot by deactivating a bunch of brain regions compared to waking, making it easier for us to study our extremely complex and exquisite brain. Above all, it is the captivating nature of lucid dreaming, together with our insatiable curiosity, that drives us to explore this phenomenon consistently and passionately.
References
[1]. Van Eeden, F. (1913). A Study of Dreams. Proceedings of the Society for Psychical Research, 26(47), 431–461. https://www.dreamscience.ca/en/documents/New%20content/lucid%20dreaming%20pdfs/vanEeden_PSPR_26_1-12_1913.pdf
[2]. Hearne, K. M. T. (1978). Lucid dreams: an electro-physiological and psychological study. PhD dissertation, University of Liverpool. https://www.keithhearne.com/wp-content/uploads/2014/12/Lucid-Dreams-LQ.pdf
[3]. LaBerge, S. P. (1980). Lucid dreaming as a learnable skill: A case study. Perceptual and Motor Skills, 51(3, Pt 2), 1039–1042. https://doi.org/10.2466/pms.1980.51.3f.1039
[4]. Voss, U., Holzmann, R., Hobson, A., Paulus, W., Koppehele-Gossel, J., Klimke, A., & Nitsche, M. A. (2014). Induction of self-awareness in dreams through frontal low current stimulation of gamma activity. Nature Neuroscience, 17(6), 810–812. https://doi.org/10.1038/nn.3719
[5]. Dresler, M., Koch, S. P., Wehrle, R., Spoormaker, V. I., Holsboer, F., Steiger, A., Sämann, P. G., Obrig, H., & Czisch, M. (2011). Dreamed movement elicits activation in the sensorimotor cortex. Current Biology, 21(21), 1833–1837. https://doi.org/10.1016/j.cub.2011.09.029
[6]. Baird, B., Castelnovo, A., Gosseries, O., & Tononi, G. (2018). Frequent lucid dreaming associated with increased functional connectivity between frontopolar cortex and temporoparietal association areas. Scientific Reports, 8(1). https://doi.org/10.1038/s41598-018-36190-w
[7]. Filevich, E., Dresler, M., Brick, T. R., & Kühn, S. (2015). Metacognitive mechanisms underlying lucid dreaming. Journal of Neuroscience, 35(3), 1082–1088. https://doi.org/10.1523/jneurosci.3342-14.2015
[8]. Raduga, M., Shashkov, A., & Vanin, A. (2024). Two-way control of a virtual avatar from lucid dreams. International Journal of Dream Research, 17(1), 38-54. https://doi.org/10.11588/ijodr.2024.1.100322
[9]. Saunders, D. T., Roe, C. A., Smith, G., & Clegg, H. (2016). Lucid dreaming incidence: A quality effects meta-analysis of 50 years of research. Consciousness and Cognition, 43, 197–215. https://doi.org/10.1016/j.concog.2016.06.002
[10]. Baird, B., Mota-Rolim, S. A., & Dresler, M. (2019). The cognitive neuroscience of lucid dreaming. Neuroscience & biobehavioral reviews, 100, 305–323. https://doi.org/10.1016/j.neubiorev.2019.03.008
[11]. Voss, U., Holzmann, R., Tuin, I., & Hobson, A. J. (2009). Lucid Dreaming: a State of Consciousness with Features of Both Waking and Non-Lucid Dreaming. SLEEP, 32(9), 1191–1200. https://doi.org/10.1093/sleep/32.9.1191
[12]. Baird, B., Tononi, G., & LaBerge, S. (2022). Lucid dreaming occurs in activated rapid eye movement sleep, not a mixture of sleep and wakefulness. SLEEP, 45(4). https://doi.org/10.1093/sleep/zsab294
[13]. Lückmann, H. C., Jacobs, H. I., & Sack, A. T. (2014). The cross-functional role of frontoparietal regions in cognition: internal attention as the overarching mechanism. Progress in Neurobiology, 116, 66–86. https://doi.org/10.1016/j.pneurobio.2014.02.002
[14]. Lou, H., Changeux, J., & Rosenstand, A. (2016). Towards a cognitive neuroscience of self-awareness. Neuroscience & Biobehavioral Reviews, 83, 765–773. https://doi.org/10.1016/j.neubiorev.2016.04.004
[15]. Dresler, M., Wehrle, R., Spoormaker, V. I., Koch, S. P., Holsboer, F., Steiger, A., Obrig, H., Sämann, P. G., & Czisch, M. (2012). Neural Correlates of Dream Lucidity Obtained from Contrasting Lucid versus Non-Lucid REM Sleep: A Combined EEG/fMRI Case Study. SLEEP, 35(7), 1017–1020. https://doi.org/10.5665/sleep.1974
[16]. Schmitz, T. W., Kawahara-Baccus, T. N., & Johnson, S. C. (2004). Metacognitive evaluation, self-relevance, and the right prefrontal cortex. NeuroImage, 22(2), 941–947. https://doi.org/10.1016/j.neuroimage.2004.02.018
[17]. Christoff, K., & Gabrieli, J. D. E. (2000). The frontopolar cortex and human cognition: Evidence for a rostrocaudal hierarchical organization within the human prefrontal cortex. Psychobiology, 28(2), 168–186. https://doi.org/10.3758/bf03331976
[18]. Christoff, K., Ream, J. M., Geddes, L. P. T., & Gabrieli, J. D. E. (2003). Evaluating Self-Generated Information: anterior prefrontal contributions to human cognition. Behavioral Neuroscience, 117(6), 1161–1168. https://doi.org/10.1037/0735-7044.117.6.1161
[19]. Vogeley, K., Bussfeld, P., Newen, A., Herrmann, S., Happé, F., Falkai, P., Maier, W., Shah, N., Fink, G., & Zilles, K. (2001). Mind Reading: Neural Mechanisms of Theory of Mind and Self-Perspective. NeuroImage, 14(1), 170–181. https://doi.org/10.1006/nimg.2001.0789
[20]. Grill-Spector, K., Kushnir, T., Hendler, T., & Malach, R. (2000). The dynamics of object-selective activation correlate with recognition performance in humans. Nature Neuroscience, 3(8), 837–843. https://doi.org/10.1038/77754
[21]. Voss, U., Schermelleh-Engel, K., Windt, J., Frenzel, C., & Hobson, A. (2013). Measuring consciousness in dreams: The lucidity and consciousness in dreams scale. Consciousness and Cognition, 22(1), 8–21. https://doi.org/10.1016/j.concog.2012.11.001
[22]. Blanchette-Carrière, C., Julien, S., Picard-Deland, C., Bouchard, M., Carrier, J., Paquette, T., & Nielsen, T. (2020). Attempted induction of signaled lucid dreaming by transcranial alternating current stimulation. Consciousness and Cognition, 83, 102957. https://doi.org/10.1016/j.concog.2020.102957
[23]. LaBerge, S., Baird, B., & Zimbardo, P. G. (2018). Smooth tracking of visual targets distinguishes lucid REM sleep dreaming and waking perception from imagination. Nature Communications, 9(1). https://doi.org/10.1038/s41467-018-05547-0
[24]. Nir, Y., & Tononi, G. (2010). Dreaming and the brain: from phenomenology to neurophysiology. Trends in Cognitive Sciences, 14(2), 88–100. https://doi.org/10.1016/j.tics.2009.12.001
[25]. Simor, P., Bogdány, T., & Peigneux, P. (2022). Predictive coding, multisensory integration, and attentional control: A multicomponent framework for lucid dreaming. Proceedings of the National Academy of Sciences, 119(44). https://doi.org/10.1073/pnas.2123418119
[26]. Oudiette, D., Dodet, P., Ledard, N., Artru, E., Rachidi, I., Similowski, T., & Arnulf, I. (2018). REM sleep respiratory behaviours match mental content in narcoleptic lucid dreamers. Scientific Reports, 8(1). https://doi.org/10.1038/s41598-018-21067-9
[27]. Arenas, A., Díaz-Guilera, A., Kurths, J., Moreno, Y., & Zhou, C. (2008). Synchronization in complex networks. Physics Reports, 469(3), 93–153. https://doi.org/10.1016/j.physrep.2008.09.002
[28]. Morgenthaler, T. I., Auerbach, S., Casey, K. R., Kristo, D., Maganti, R., Ramar, K., Zak, R., & Kartje, R. (2018). Position Paper for the Treatment of Nightmare Disorder in Adults: An American Academy of Sleep Medicine Position Paper. Journal of Clinical Sleep Medicine, 14(06), 1041–1055. https://doi.org/10.5664/jcsm.7178
[29]. Aurora, R. N., Zak, R. S., Auerbach, S. H., Casey, K. R., Chowdhuri, S., Karippot, A., Maganti, R. K., Ramar, K., Kristo, D. A., Bista, S. R., Lamm, C. I., & Morgenthaler, T. I. (2010). Best Practice Guide for the Treatment of Nightmare Disorder in Adults. Journal of Clinical Sleep Medicine, 06(04), 389–401. https://doi.org/10.5664/jcsm.27883
[30]. De Macêdo, T. C. F., Ferreira, G. H., De Almondes, K. M., Kirov, R., & Mota-Rolim, S. A. (2019). My Dream, my rules: Can lucid dreaming treat nightmares? Frontiers in Psychology, 10. https://doi.org/10.3389/fpsyg.2019.02618
[31]. Holzinger, B., Saletu, B., & Klösch, G. (2020). Cognitions in sleep: Lucid dreaming as an intervention for nightmares in patients with posttraumatic stress disorder. Frontiers in Psychology, 11. https://doi.org/10.3389/fpsyg.2020.01826
[32]. Stumbrys, T., Erlacher, D., & Schredl, M. (2015). Effectiveness of motor practice in lucid dreams: a comparison with physical and mental practice. Journal of Sports Sciences, 34(1), 27–34. https://doi.org/10.1080/02640414.2015.1030342
[33]. Morris, T., Spittle, M., & Watt, A. P. (2005). Imagery in sport. Human Kinetics.
[34]. Jeannerod, M. (2001). Neural Simulation of action: a unifying mechanism for motor cognition. NeuroImage, 14(1), S103–S109. https://doi.org/10.1006/nimg.2001.0832
Cite this article
Pan,B. (2025). Light Sleeper, Heavy Dreamer: A Neuroscientific Perspective of Lucid Dreaming. Theoretical and Natural Science,82,152-161.
Data availability
The datasets used and/or analyzed during the current study will be available from the authors upon reasonable request.
Disclaimer/Publisher's Note
The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of EWA Publishing and/or the editor(s). EWA Publishing and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
About volume
Volume title: Proceedings of the 3rd International Conference on Modern Medicine and Global Health
© 2024 by the author(s). Licensee EWA Publishing, Oxford, UK. This article is an open access article distributed under the terms and
conditions of the Creative Commons Attribution (CC BY) license. Authors who
publish this series agree to the following terms:
1. Authors retain copyright and grant the series right of first publication with the work simultaneously licensed under a Creative Commons
Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this
series.
2. Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the series's published
version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial
publication in this series.
3. Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and
during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See
Open access policy for details).
References
[1]. Van Eeden, F. (1913). A Study of Dreams. Proceedings of the Society for Psychical Research, 26(47), 431–461. https://www.dreamscience.ca/en/documents/New%20content/lucid%20dreaming%20pdfs/vanEeden_PSPR_26_1-12_1913.pdf
[2]. Hearne, K. M. T. (1978). Lucid dreams: an electro-physiological and psychological study. PhD dissertation, University of Liverpool. https://www.keithhearne.com/wp-content/uploads/2014/12/Lucid-Dreams-LQ.pdf
[3]. LaBerge, S. P. (1980). Lucid dreaming as a learnable skill: A case study. Perceptual and Motor Skills, 51(3, Pt 2), 1039–1042. https://doi.org/10.2466/pms.1980.51.3f.1039
[4]. Voss, U., Holzmann, R., Hobson, A., Paulus, W., Koppehele-Gossel, J., Klimke, A., & Nitsche, M. A. (2014). Induction of self-awareness in dreams through frontal low current stimulation of gamma activity. Nature Neuroscience, 17(6), 810–812. https://doi.org/10.1038/nn.3719
[5]. Dresler, M., Koch, S. P., Wehrle, R., Spoormaker, V. I., Holsboer, F., Steiger, A., Sämann, P. G., Obrig, H., & Czisch, M. (2011). Dreamed movement elicits activation in the sensorimotor cortex. Current Biology, 21(21), 1833–1837. https://doi.org/10.1016/j.cub.2011.09.029
[6]. Baird, B., Castelnovo, A., Gosseries, O., & Tononi, G. (2018). Frequent lucid dreaming associated with increased functional connectivity between frontopolar cortex and temporoparietal association areas. Scientific Reports, 8(1). https://doi.org/10.1038/s41598-018-36190-w
[7]. Filevich, E., Dresler, M., Brick, T. R., & Kühn, S. (2015). Metacognitive mechanisms underlying lucid dreaming. Journal of Neuroscience, 35(3), 1082–1088. https://doi.org/10.1523/jneurosci.3342-14.2015
[8]. Raduga, M., Shashkov, A., & Vanin, A. (2024). Two-way control of a virtual avatar from lucid dreams. International Journal of Dream Research, 17(1), 38-54. https://doi.org/10.11588/ijodr.2024.1.100322
[9]. Saunders, D. T., Roe, C. A., Smith, G., & Clegg, H. (2016). Lucid dreaming incidence: A quality effects meta-analysis of 50 years of research. Consciousness and Cognition, 43, 197–215. https://doi.org/10.1016/j.concog.2016.06.002
[10]. Baird, B., Mota-Rolim, S. A., & Dresler, M. (2019). The cognitive neuroscience of lucid dreaming. Neuroscience & biobehavioral reviews, 100, 305–323. https://doi.org/10.1016/j.neubiorev.2019.03.008
[11]. Voss, U., Holzmann, R., Tuin, I., & Hobson, A. J. (2009). Lucid Dreaming: a State of Consciousness with Features of Both Waking and Non-Lucid Dreaming. SLEEP, 32(9), 1191–1200. https://doi.org/10.1093/sleep/32.9.1191
[12]. Baird, B., Tononi, G., & LaBerge, S. (2022). Lucid dreaming occurs in activated rapid eye movement sleep, not a mixture of sleep and wakefulness. SLEEP, 45(4). https://doi.org/10.1093/sleep/zsab294
[13]. Lückmann, H. C., Jacobs, H. I., & Sack, A. T. (2014). The cross-functional role of frontoparietal regions in cognition: internal attention as the overarching mechanism. Progress in Neurobiology, 116, 66–86. https://doi.org/10.1016/j.pneurobio.2014.02.002
[14]. Lou, H., Changeux, J., & Rosenstand, A. (2016). Towards a cognitive neuroscience of self-awareness. Neuroscience & Biobehavioral Reviews, 83, 765–773. https://doi.org/10.1016/j.neubiorev.2016.04.004
[15]. Dresler, M., Wehrle, R., Spoormaker, V. I., Koch, S. P., Holsboer, F., Steiger, A., Obrig, H., Sämann, P. G., & Czisch, M. (2012). Neural Correlates of Dream Lucidity Obtained from Contrasting Lucid versus Non-Lucid REM Sleep: A Combined EEG/fMRI Case Study. SLEEP, 35(7), 1017–1020. https://doi.org/10.5665/sleep.1974
[16]. Schmitz, T. W., Kawahara-Baccus, T. N., & Johnson, S. C. (2004). Metacognitive evaluation, self-relevance, and the right prefrontal cortex. NeuroImage, 22(2), 941–947. https://doi.org/10.1016/j.neuroimage.2004.02.018
[17]. Christoff, K., & Gabrieli, J. D. E. (2000). The frontopolar cortex and human cognition: Evidence for a rostrocaudal hierarchical organization within the human prefrontal cortex. Psychobiology, 28(2), 168–186. https://doi.org/10.3758/bf03331976
[18]. Christoff, K., Ream, J. M., Geddes, L. P. T., & Gabrieli, J. D. E. (2003). Evaluating Self-Generated Information: anterior prefrontal contributions to human cognition. Behavioral Neuroscience, 117(6), 1161–1168. https://doi.org/10.1037/0735-7044.117.6.1161
[19]. Vogeley, K., Bussfeld, P., Newen, A., Herrmann, S., Happé, F., Falkai, P., Maier, W., Shah, N., Fink, G., & Zilles, K. (2001). Mind Reading: Neural Mechanisms of Theory of Mind and Self-Perspective. NeuroImage, 14(1), 170–181. https://doi.org/10.1006/nimg.2001.0789
[20]. Grill-Spector, K., Kushnir, T., Hendler, T., & Malach, R. (2000). The dynamics of object-selective activation correlate with recognition performance in humans. Nature Neuroscience, 3(8), 837–843. https://doi.org/10.1038/77754
[21]. Voss, U., Schermelleh-Engel, K., Windt, J., Frenzel, C., & Hobson, A. (2013). Measuring consciousness in dreams: The lucidity and consciousness in dreams scale. Consciousness and Cognition, 22(1), 8–21. https://doi.org/10.1016/j.concog.2012.11.001
[22]. Blanchette-Carrière, C., Julien, S., Picard-Deland, C., Bouchard, M., Carrier, J., Paquette, T., & Nielsen, T. (2020). Attempted induction of signaled lucid dreaming by transcranial alternating current stimulation. Consciousness and Cognition, 83, 102957. https://doi.org/10.1016/j.concog.2020.102957
[23]. LaBerge, S., Baird, B., & Zimbardo, P. G. (2018). Smooth tracking of visual targets distinguishes lucid REM sleep dreaming and waking perception from imagination. Nature Communications, 9(1). https://doi.org/10.1038/s41467-018-05547-0
[24]. Nir, Y., & Tononi, G. (2010). Dreaming and the brain: from phenomenology to neurophysiology. Trends in Cognitive Sciences, 14(2), 88–100. https://doi.org/10.1016/j.tics.2009.12.001
[25]. Simor, P., Bogdány, T., & Peigneux, P. (2022). Predictive coding, multisensory integration, and attentional control: A multicomponent framework for lucid dreaming. Proceedings of the National Academy of Sciences, 119(44). https://doi.org/10.1073/pnas.2123418119
[26]. Oudiette, D., Dodet, P., Ledard, N., Artru, E., Rachidi, I., Similowski, T., & Arnulf, I. (2018). REM sleep respiratory behaviours match mental content in narcoleptic lucid dreamers. Scientific Reports, 8(1). https://doi.org/10.1038/s41598-018-21067-9
[27]. Arenas, A., Díaz-Guilera, A., Kurths, J., Moreno, Y., & Zhou, C. (2008). Synchronization in complex networks. Physics Reports, 469(3), 93–153. https://doi.org/10.1016/j.physrep.2008.09.002
[28]. Morgenthaler, T. I., Auerbach, S., Casey, K. R., Kristo, D., Maganti, R., Ramar, K., Zak, R., & Kartje, R. (2018). Position Paper for the Treatment of Nightmare Disorder in Adults: An American Academy of Sleep Medicine Position Paper. Journal of Clinical Sleep Medicine, 14(06), 1041–1055. https://doi.org/10.5664/jcsm.7178
[29]. Aurora, R. N., Zak, R. S., Auerbach, S. H., Casey, K. R., Chowdhuri, S., Karippot, A., Maganti, R. K., Ramar, K., Kristo, D. A., Bista, S. R., Lamm, C. I., & Morgenthaler, T. I. (2010). Best Practice Guide for the Treatment of Nightmare Disorder in Adults. Journal of Clinical Sleep Medicine, 06(04), 389–401. https://doi.org/10.5664/jcsm.27883
[30]. De Macêdo, T. C. F., Ferreira, G. H., De Almondes, K. M., Kirov, R., & Mota-Rolim, S. A. (2019). My Dream, my rules: Can lucid dreaming treat nightmares? Frontiers in Psychology, 10. https://doi.org/10.3389/fpsyg.2019.02618
[31]. Holzinger, B., Saletu, B., & Klösch, G. (2020). Cognitions in sleep: Lucid dreaming as an intervention for nightmares in patients with posttraumatic stress disorder. Frontiers in Psychology, 11. https://doi.org/10.3389/fpsyg.2020.01826
[32]. Stumbrys, T., Erlacher, D., & Schredl, M. (2015). Effectiveness of motor practice in lucid dreams: a comparison with physical and mental practice. Journal of Sports Sciences, 34(1), 27–34. https://doi.org/10.1080/02640414.2015.1030342
[33]. Morris, T., Spittle, M., & Watt, A. P. (2005). Imagery in sport. Human Kinetics.
[34]. Jeannerod, M. (2001). Neural Simulation of action: a unifying mechanism for motor cognition. NeuroImage, 14(1), S103–S109. https://doi.org/10.1006/nimg.2001.0832









