1. Introduction
Biomedical engineering, an interdisciplinary fusion of biology, medicine, and engineering, is revolutionizing healthcare through technological innovations. Within the dynamic field, optical biosensors have emerged as groundbreaking tools, blending optical science with biological recognition to deliver unparalleled sensitivity and specificity in biomolecular detection. The devices utilize phenomena such as light absorption, scattering, and fluorescence to decode molecular information, enabling real-time, non-invasive, and cost-effective analysis, with applications ranging from early disease diagnosis to environmental monitoring.
This paper addresses limitations and explores strategies to enhance sensitivity and portability. It examines the principles and advancements of optical biosensors, focusing on technologies like Surface Plasmon Resonance (SPR), Evanescent Wave Fluorescence (EWF), and emerging solutions. Present studies are selected to bridge the gaps by examining the design and functionality of cutting-edge optical biosensor technologies. Delving into advancements such as photonic crystal cavities (PCC), wearable devices, and Lab-on-a-Chip (LOC) biosensors, this research outlines strategies to overcome existing barriers. The objective is to develop highly-sensitive, portable, and versatile biosensors that cater to medical and environmental demands. They stand poised to redefine precision diagnostics, facilitate sustainable environmental monitoring, and democratize healthcare. By tackling persistent technical challenges and expanding applications of optical biosensors, this study enriches their scientific understandings and illuminates their pivotal roles in fostering improved global health and environmental stewardship.
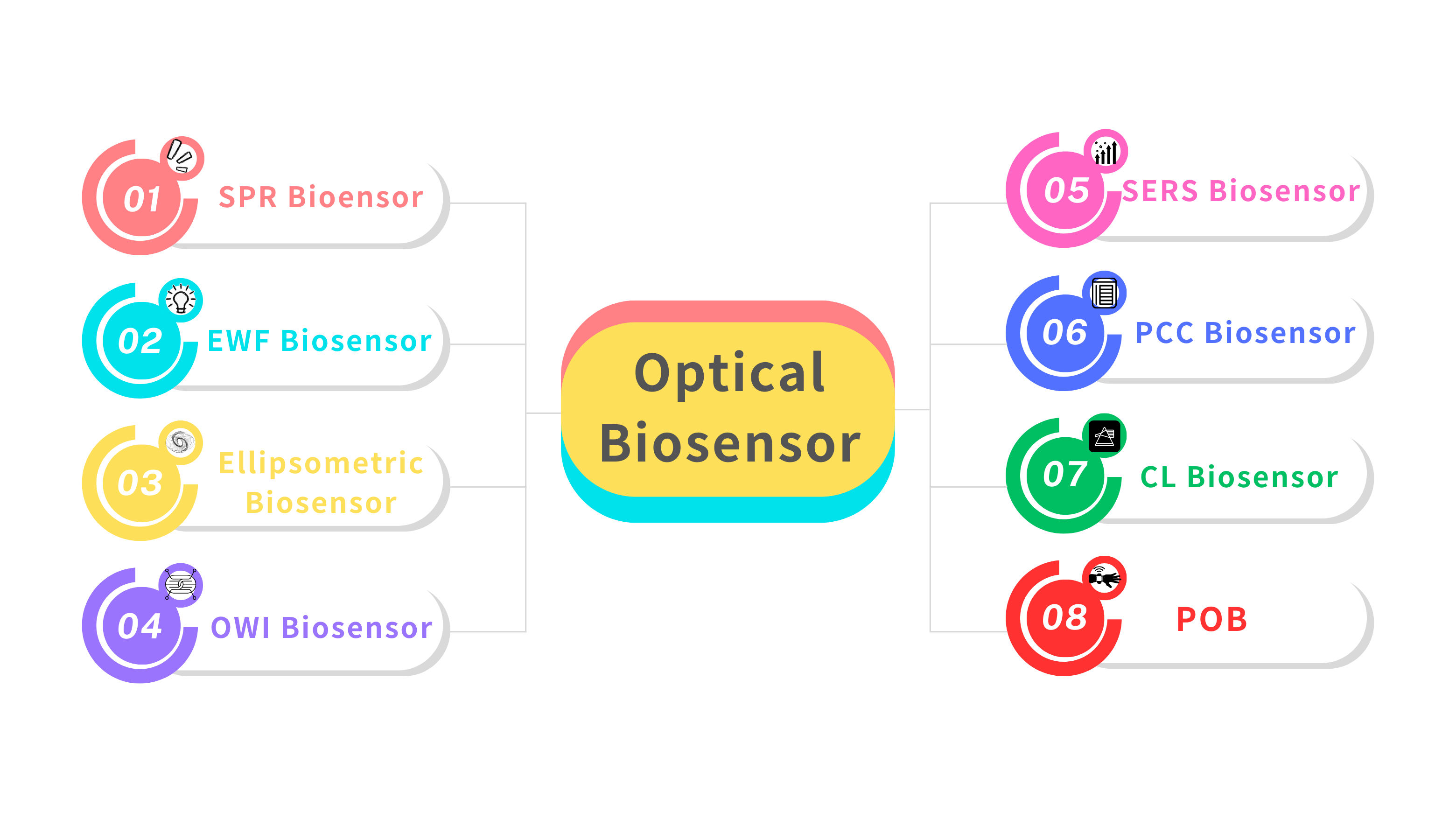
Figure 1: Classification of optical biosensors
2. Technological Advances and Trends in Optical Biosensors
Optical biosensors rely on principles including light absorption, scattering, fluorescence, and resonance phenomena. Methodologies employed are SPR and L-SPR (Utilizing fluorophore excitation, emission phenomena to provide fast response, enhanced detection, fluorescence signal concentration, and color compatibility dyed for various probe domains), Waveguides and Interferometry (Amplifying evanescent molecular interactions), SERS (Exploiting plasmonic nanostructures to enhance Raman signals), Nanomaterials Integration (Employing quantum dots, gold nanoparticles, and photonic crystals to refine sensitivity, specificity), Portable Platforms (Combining microfluidic and AI technologies for real-time, point-of-care diagnostics)[1][2].
According to the quantitative analysis, biomedical diagnostic application frequency is 60% while environmental monitoring is 25%, 15% of drug screening. About performance metrics, sensitivity (Detected molecules as low as 2.1 nM for EWF Biosensors), specificity (SPR capability to differentiate cancer biomarkers like circulating tumor), and detection limits (SERS achieving analyte detection at 5 pg/mL concentration) stand out [3]. Reflecting figures and data, application distribution by pie chart, detection principle contributions by bar chart, performance metrics by comparative table are presented, revealing biomedical prioritization trends [4]. Descriptive statistics suggest that SPR and SERS dominate in high-sensitivity applications and novel platforms like PCC, tattoo biosensors emerge as transformative niche solutions. Emphasis on reducing non-specific binding and enhancing portability indicates an explicit trajectory towards personalized and accessible healthcare diagnostics.
3. Performance and Applications of Optical Biosensor Technologies
3.1. Surface Plasmon Resonance (SPR) Biosensors
Regarding SPR Biosensors, Figure 2 exhibits a classic experimental system where analyte with ligand fastened to the gold epi-film. The method includes detecting and quantifying analyte-binding wavelength shifts using nucleic acid hybridization and immunoassay techniques (Figure 3). U-bent plasmonic fiber optic absorbance biosensors are composed of a sandwich compact probe-surface immunoassay, steeping an antibody-functionalized probe into test specimen and AuNP label suspension mixture. It's simple and low-cost to detect human immunoglobulin G on zeptomolar concentrations (Figure 4) [5]. In Figure 5, when there are no biomolecules, maximum SPR imaging is realized by exposing sensor chip to the laser beam at a particular angle. When biomolecules exist, a distinct resonance shift increases reflected light intensity at an angle. With biomolecules mass changing, a further shift in resonance angle occurs. To address photo-bleaching, Metal-Enhanced-Fluorescence Biosensors utilize fluorophore interaction with metal surfaces, thereby improving optical performance stability. In addition, in the SPR module configuration of extrinsic electromagnet to produce alterable magnetic field (Figure 6), Magneto-Optical SPR Biosensors assist in evaluating bioaffinity interactions and resolving refractive index changes, equipped to adjust reflectance curves. However, SPR biosensors face limitations in distinguishing surface-specific binding interactions. Hence, it is imperative to use control samples. Even though they can effectively detect sensitive high molecular weight (HMW) molecules, they cannot detect lower types. As for exposure to liquid brine samples, MO-SPR involve metal multi-layers or nanostructures must remain [6].
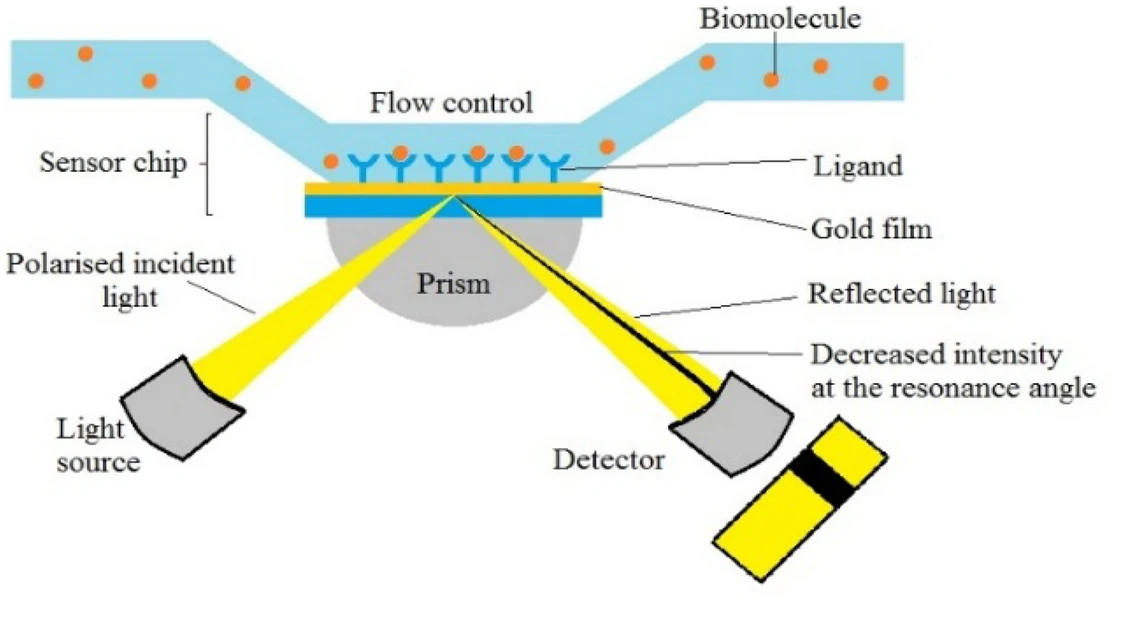
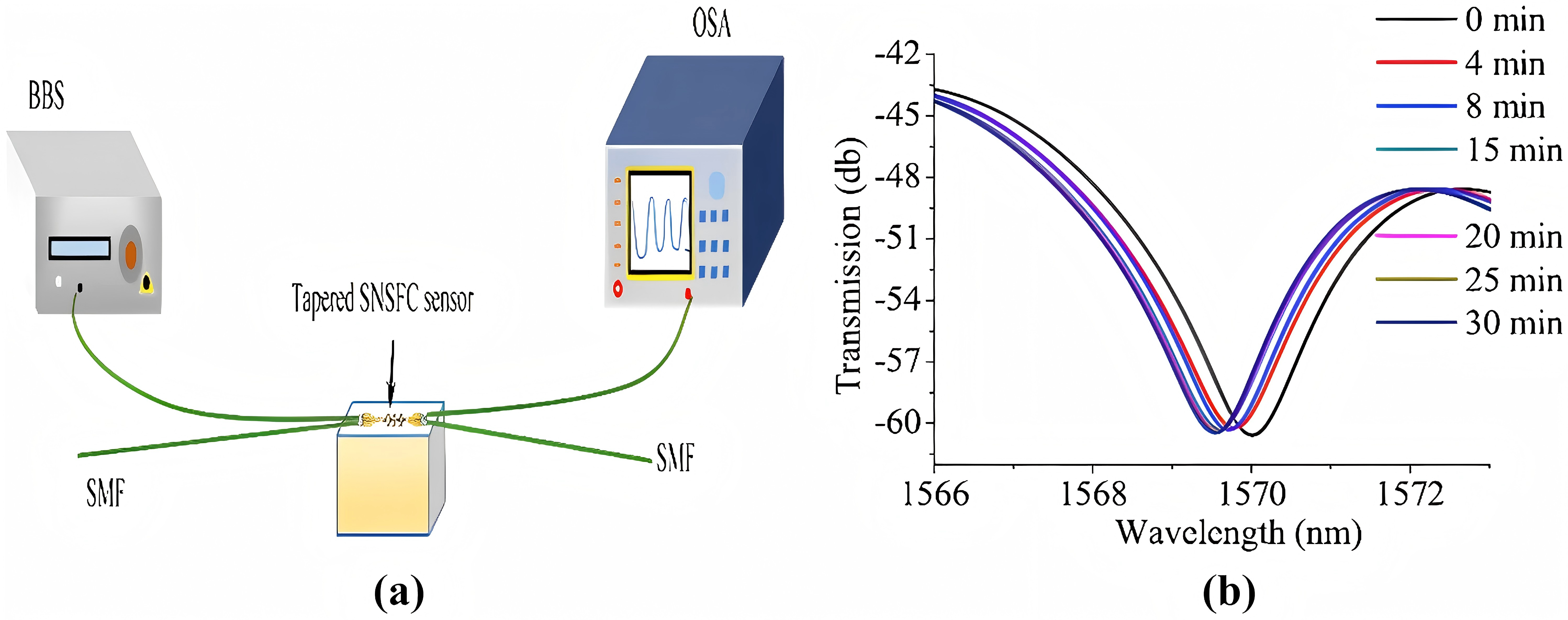
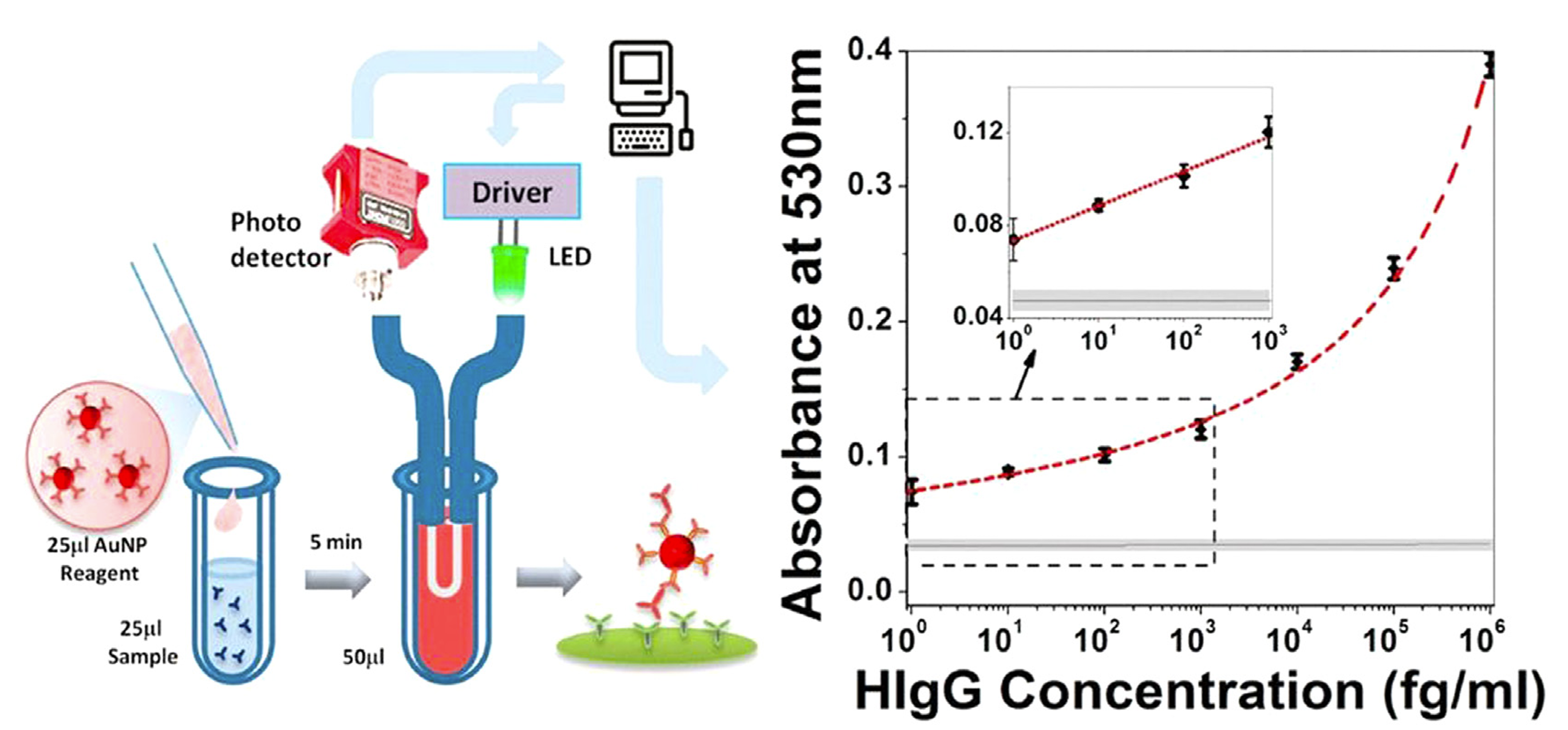
Figure 2 (Left): Schematic SPR chip’s principle [6]
Figure 3 (Middle): Optical setup for acquiring reflection spectra on the plasmonic fiber probe [5]
Figure 4 (Right): U-bent plasmonic fiber optic absorbance biosensor composition [5]
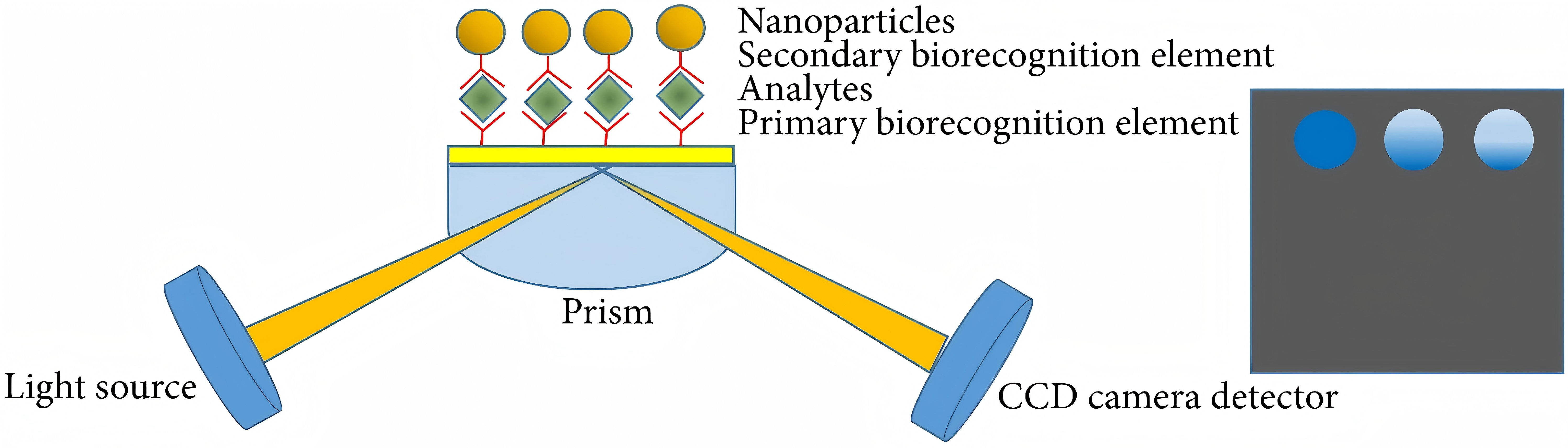
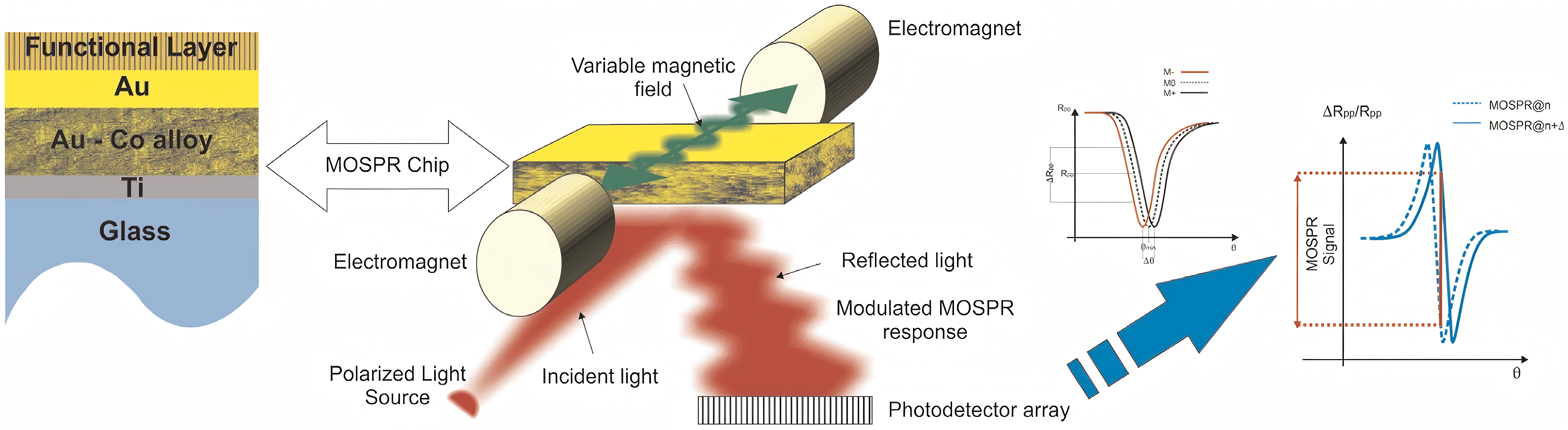
Figure 5 (Left): Experimental setup for SPR imaging [6]
Figure 6 (Right): Principles of MOSPR in the Kretschmann configuration [6]
3.2. Evanescent Wave Fluorescence (EWF) Biosensors
EW generates subsurface fluorescence through excitation and reduces the background signal from large samples. In Figure 7, the diagrammatical EW planar optical waveguide chip directly demonstrates the principle. Made of silica (glass) and plastic fiber optics, they rely on eviscerated nano-thick waves extending from fiber core layer and cladding interface to excite fluorophore adhering its surface, whose architecture includes light sources (LED or laser), full-featured fluorescent fiber optic biosensors (FOB) with target-specific recognition molecules, filters, lenses and detectors (photodiodes) [5]. Due to evapode-wave limited excitation range, the system is protected from the substrate componential influence and interferences beyond reaction surface. They are designed to weaken background noise signals, enhance fluorophore surface signals, monitor unlabeled molecular interactions, and detect refractive index changes.
Concerning endocrine disruptor 17 β-estradiol in environmental water sample tests on 200 clinicals, the EWF biosensors successfully distinguished healthy individuals from HIV, syphilis and hepatitis-C-positive individuals characteristically [6]. Moreover, tapered FOB can monitor bacterial growth real-time with a distributed feedback laser, measuring the evanescent light field and cone bacteria on optical throughput. Meanwhile, the electrostatic polylysine interaction can fix bacteria on fibers [7]. In fluorescence detection, a primary (capture) antibody against a specific antigenic target is fixed to the FOB probe [8]. Placed in probational liquids, the probe is treated with a secondary (revealing) antibody. Since the antibody is coupled to a fluorophore, the intensity is proportional to the target antigen concentration. An FOB probe is marked with excitation and emission wavelengths (figure 8). During operation, the incident light is transmitted along the fiber to the fixed sensing layer distally. After interacting with measured object, the emitted light returns to the detector through the same fiber. However, the limitations are that FOB background absorption may affect localization results and limit biosensor stability; Lack of optimized components often leads to response delays and complicated background interference; Detective effect of micromolecules or LMW biomolecules fails.
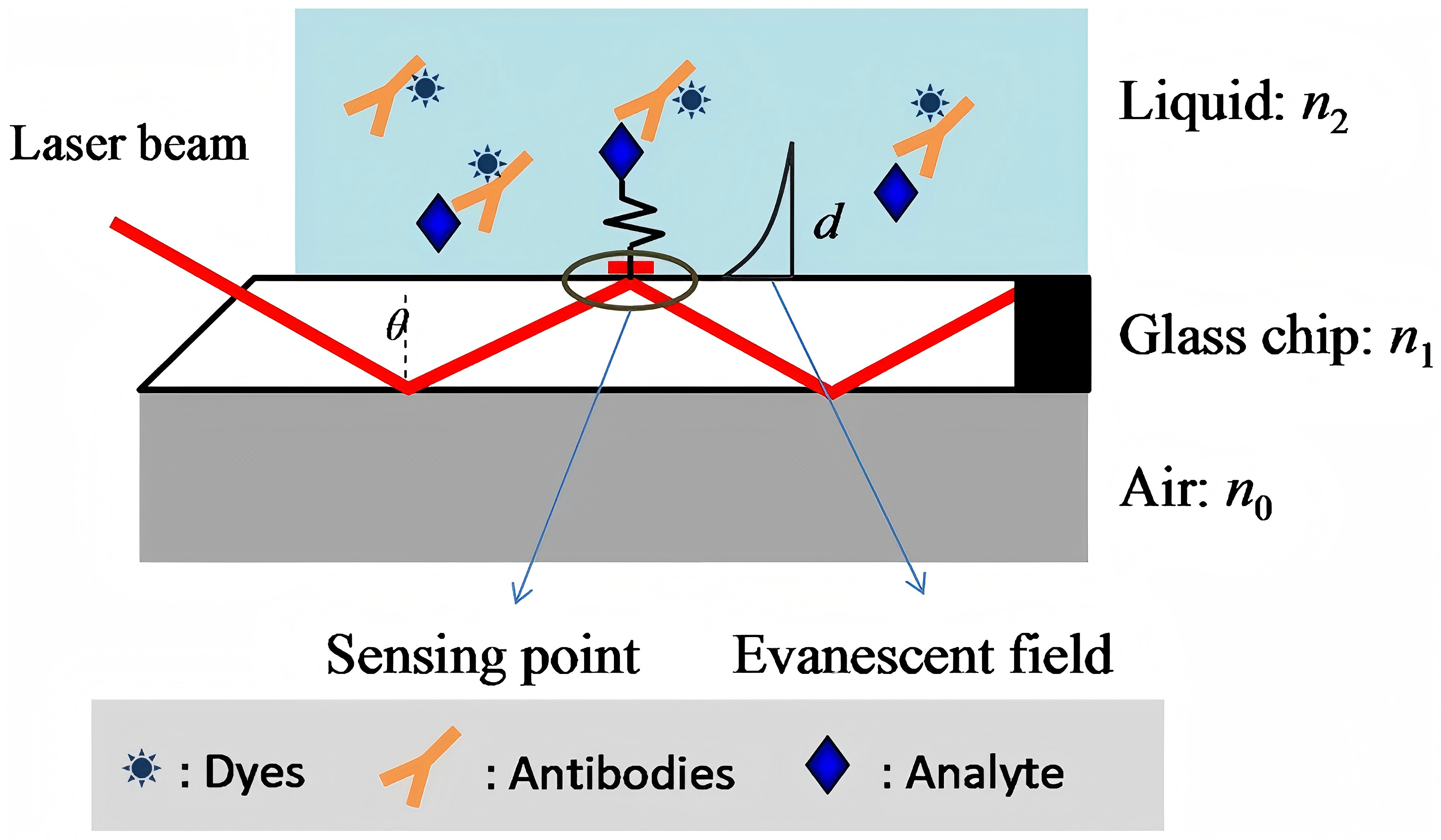
Figure 7: Schematic diagram of EW planner optical waveguide chip [6]
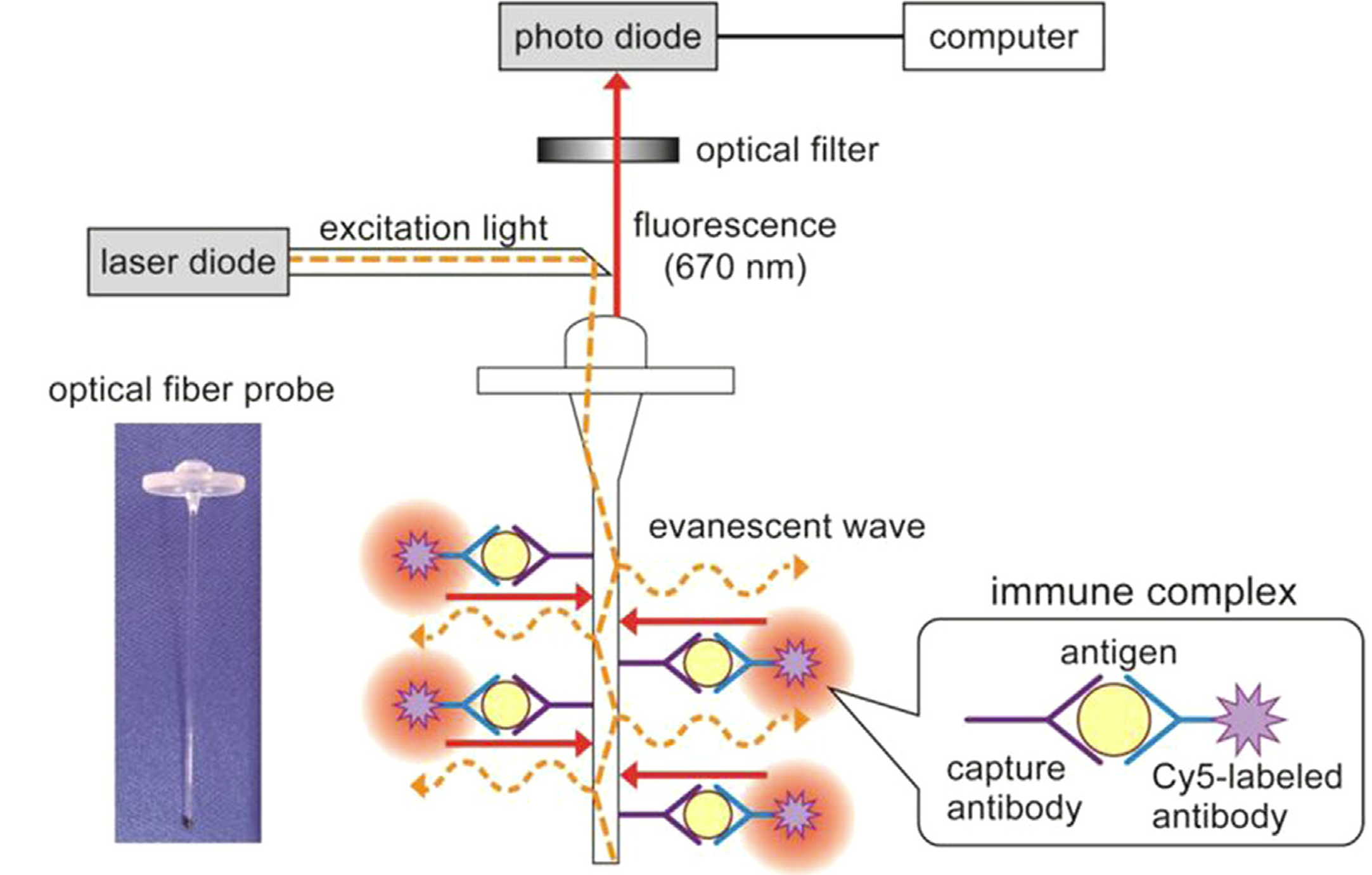
Figure 8: Fluorescent fiber transducer probe with excitation and emission wavelengths [5]
3.3. Ellipsometric Biosensors
Ellipsometric Biosensors can detect influenza virus strains with an ATR-based microarray. Also, detecting serum tumor biomarkers with equilibrium dissociation avidity constants (10 to 100 pM) as distinctive viral receptor profile parameters matters. Their classic components include an ellipsometric parameter quantifier, a sensory cell, incorporating a semi-cylindrical coupled prism to keep the incident light beam below the critical interface angle (Figure 9). The system admits probe solution through an inlet, where a Si-wafer bio-recognition element engages with target analytes, ultimately discarding unwanted substances via an outlet.
3.4. Optical Waveguide Interferometric (OWI) Biosensors
OWI Biosensors can analyze cellular processes, responses and detect cellular content redistribution, especially avian influenza. In Figure 10, instrumentation for detecting S.aureus uses a developed FOB that input single-mode fibers (SMFs) to a photic broadband source, and an optical spectrum analyzer (OSA) to determine tapered SNSFC spectral response.
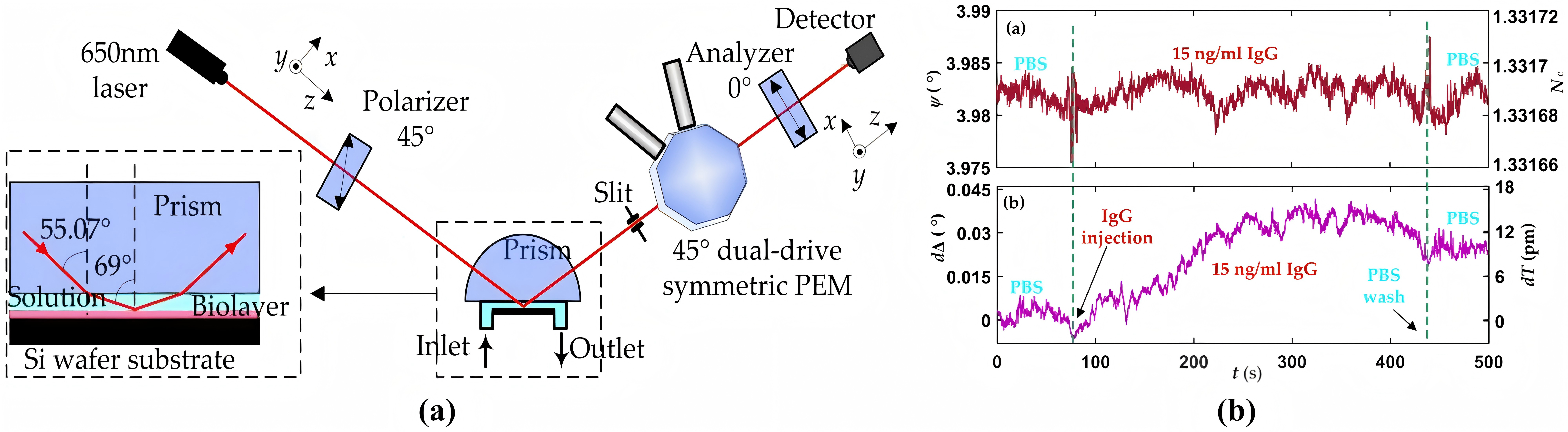
Figure 9: Schematic diagram of Ellipsometric biosensor [6]
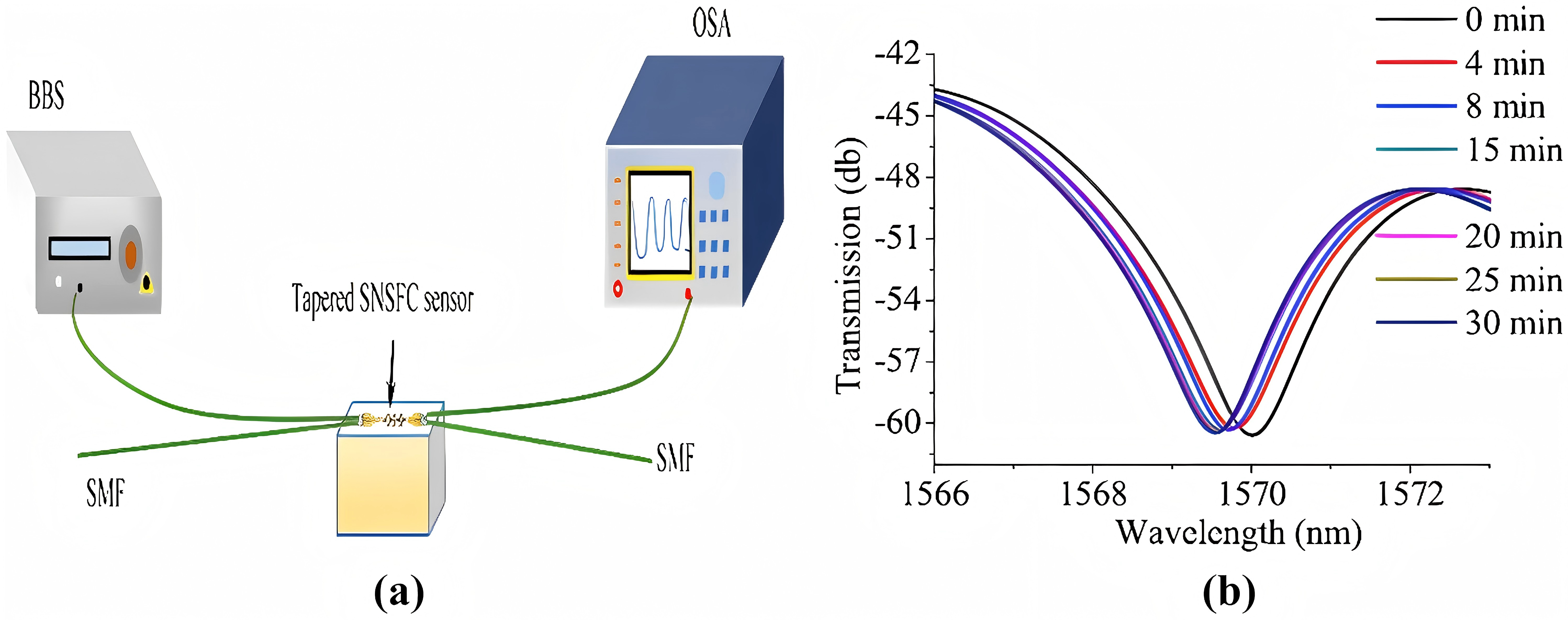
Figure 10: Experimental setup of tapered SNSFC and transmission [6]
3.5. Surface-Enhanced Raman Scattering (SERS) Biosensors
SERS Biosensors harness the interaction between metal surfaces and molecular Raman signal amplification5 about molecular structures. They detect analytes near metallic surfaces, offering prominent sensitivity for tracing samples at approximately 10 nl. In aquatic environments, they can expedite endocrine-disrupting substance detection through sensitive protein biomarker at concentrations as low as 5 pg/ml. The multiplexing SERS microarrays enable simultaneous biomolecule identification, reducing false positive rates and enhancing diagnostic reliability. Furthermore, the integration of miniaturization and microfluidic technology has made SERS substrates cost-effective and portable, facilitating rapid point-of-care (POC) detection.
SERS have demonstrated exceptional sensitivity in early cancer diagnosis through circulating miRNA detection (miRNA-21 and miRNA-155) [9]. However, limitations are that SERS substrate-preparation requires meticulous control over hot spot distribution; Matrix effects in biological samples introduce background interference and necessitate robust signal standardization protocols. Optimized surface modifications and signal amplification techniques are vital to balance sensitivity and specificity in complex samples. High optical field intensity strength tracing analyte molecules and long-range MgF2 dielectric buffer layer enhances uniformity and stability. Figure 11 and 12 illustrate SERS analysis principles and configurations. To obtain a pristine Raman signal from bacteria-cultural substrate, a minimal spectral interference substrate is selected. Subsequent fine-tuning experimental parameters realize Raman spectral data acquisition; Post-acquisition, spectral processing eliminates background noise and stray peaks integrally. Focus on data and multivariate analysis, deep learning technologies are leveraged for spectral data classification. Figure 13 depicts SERS resonance spectroscopy with an unmodified surface affinity strategy (SAS), where plasma inks containing alcohol-based solvents, NPs, Raman-active molecules are placed on a nano-metal ring-substrate. The approach exploits the SERS-effect and amplifies Raman scattering molecular signals adsorbed on metal nanostructure surfaces without nanostructural modifications. Conversely, Figure 14 showcases SERS with a functionalized SAS. Sulfur-containing organic molecules are bound to metal NPs surfaces through a self-assembled monolayer. Representative monolayer pattern arrangement enhances SERS substrate selectivity and sensitivity via sulfur-metal bonding and functionalization.
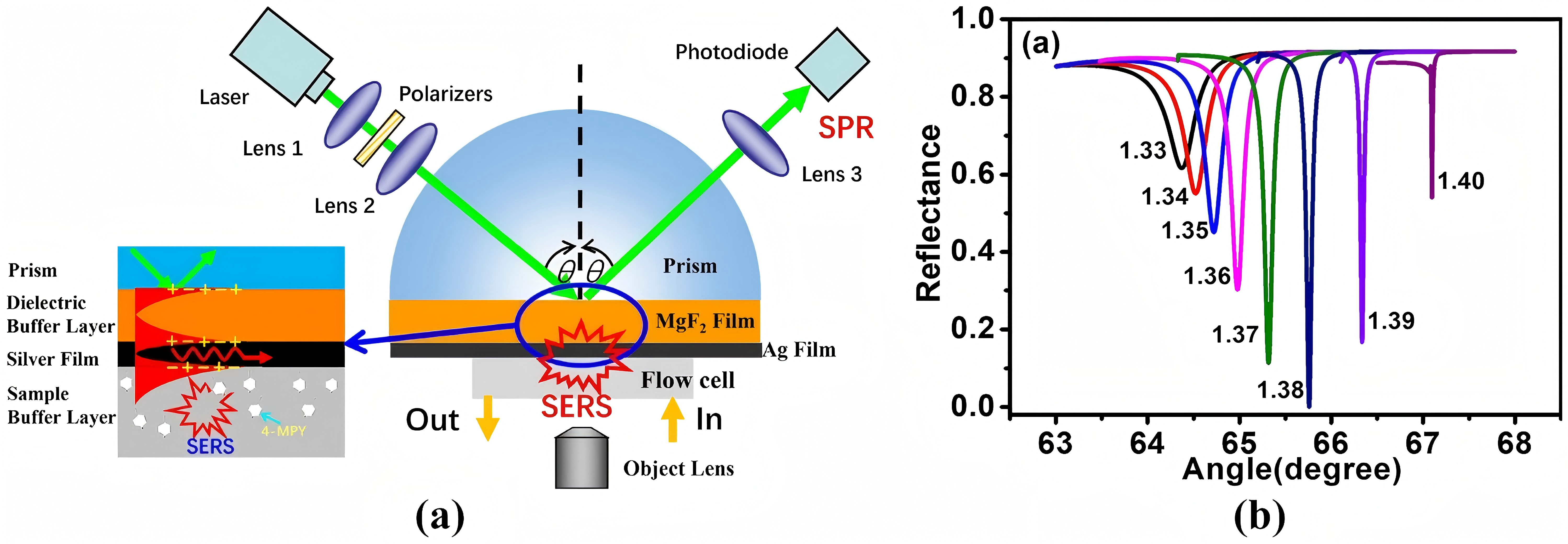
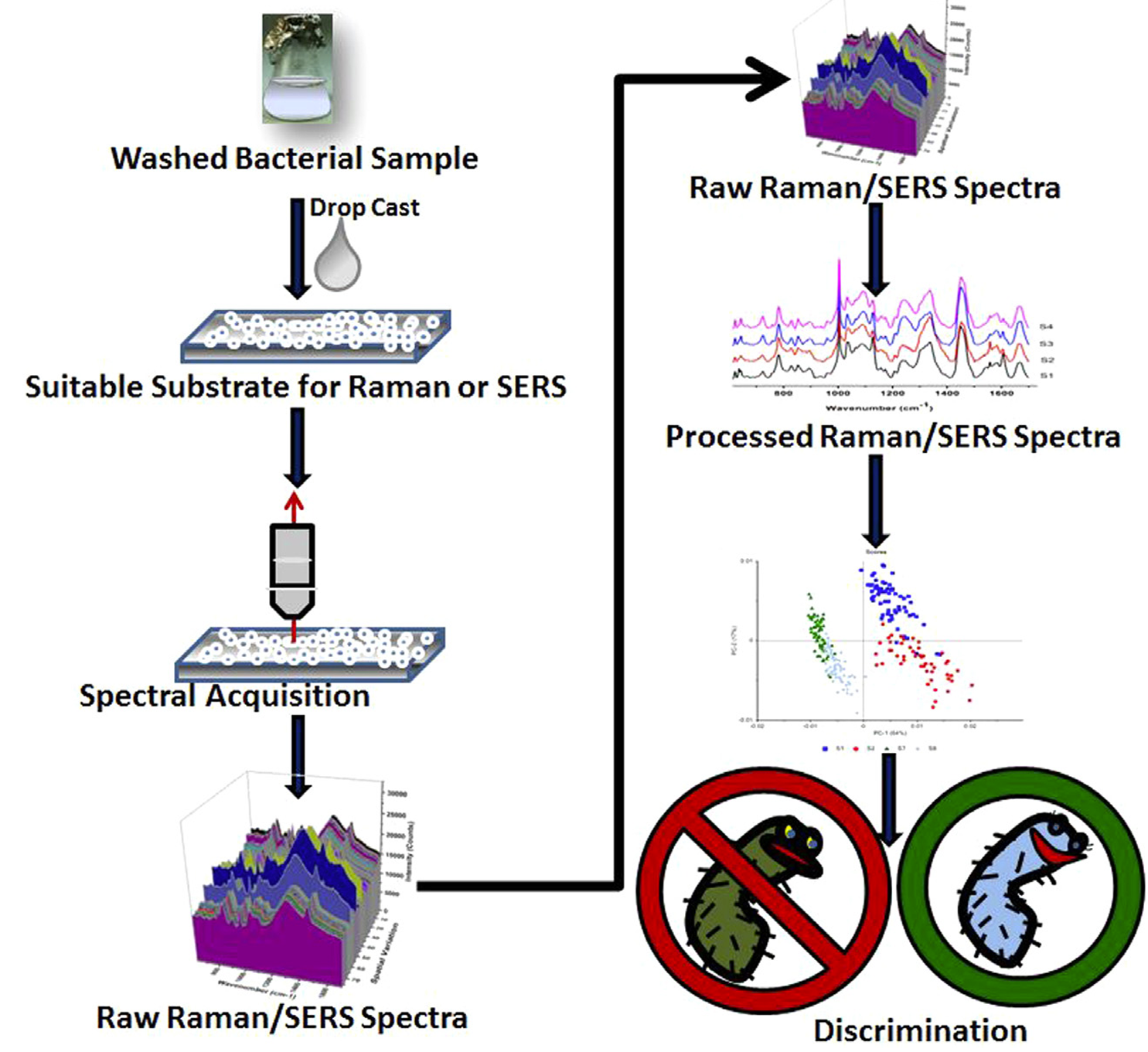
Figure 11 (Left): SERS detection using LRSPR sensor [6]
Figure 12: (Right). Schematic depicting the process of obtaining Raman and SERS experiments [5]
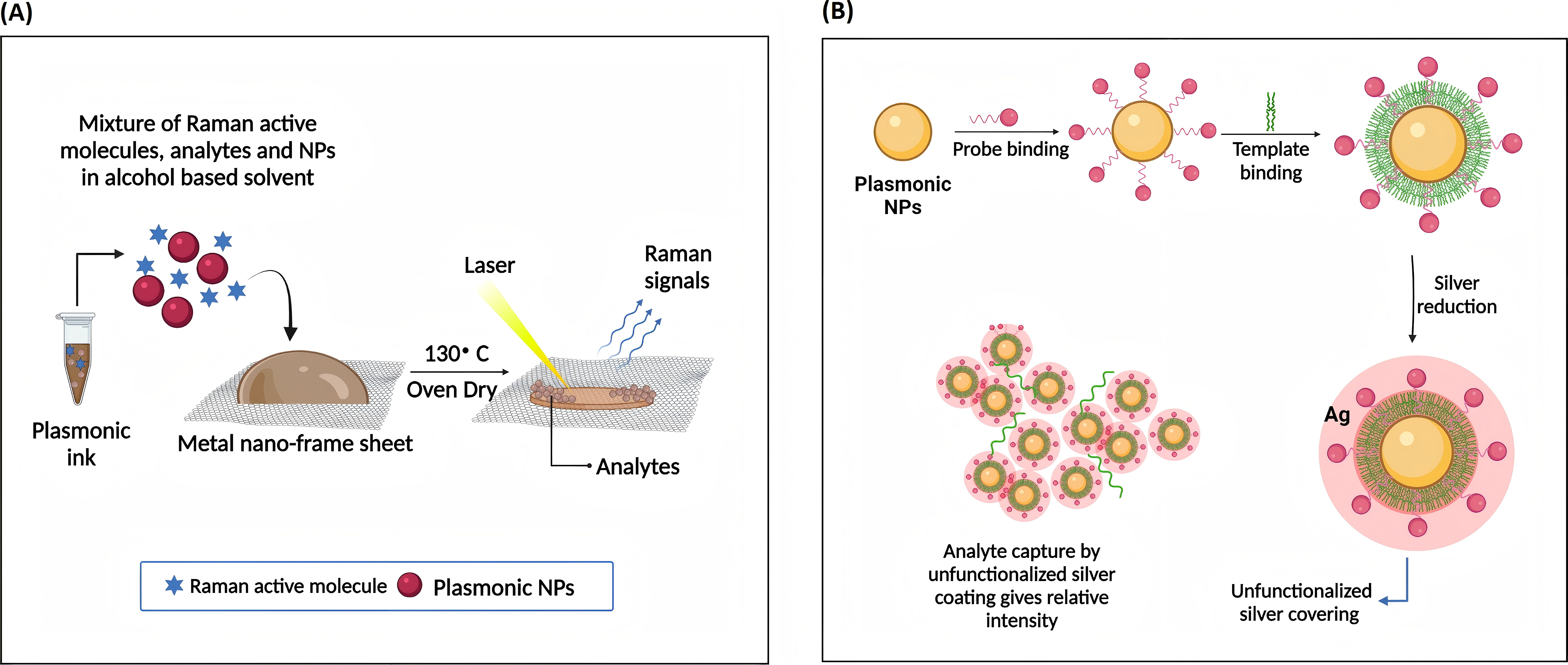
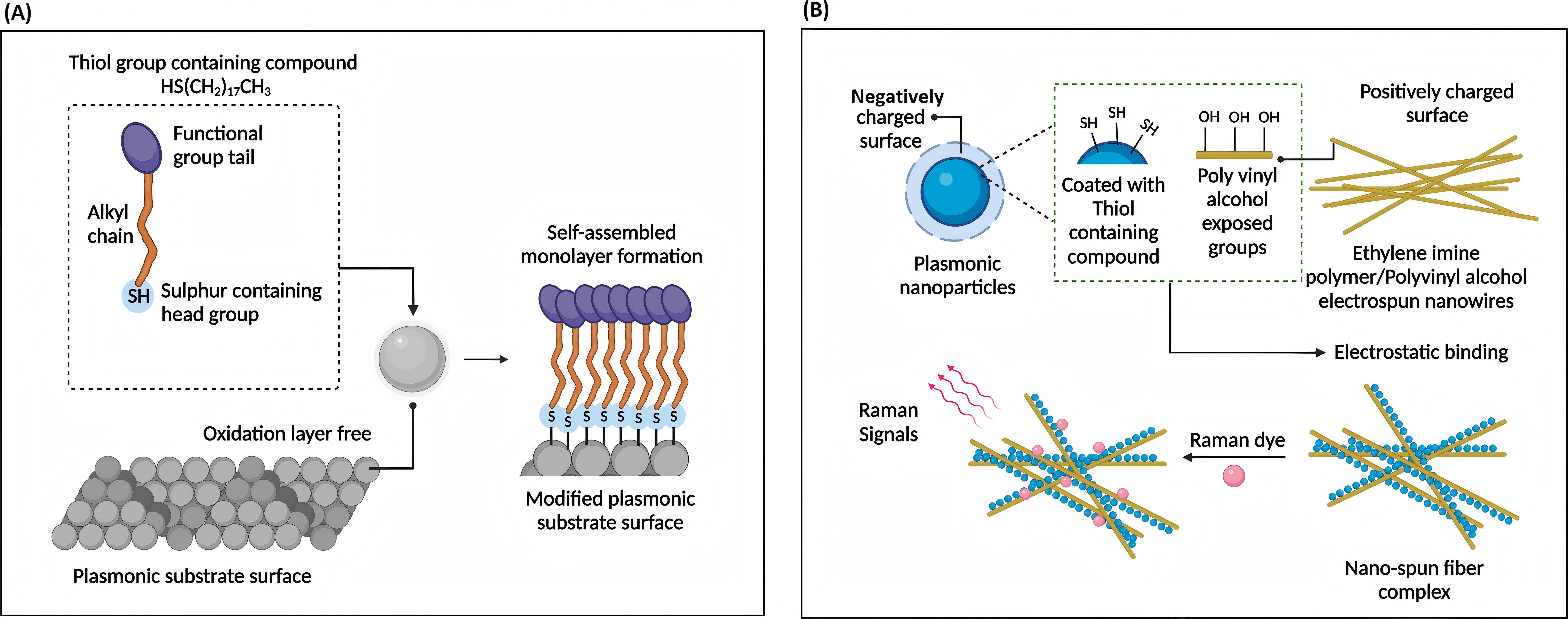
Figure 13 (Left): Unmodified surface affinity strategies of SERS for analyte detection [9]
Figure 14 (Right): Functionalized affinity strategies of SERS [9]
3.6. Photonic Crystal Cavity (PCC) Biosensors
PCC Biosensors have wide usage in measurement, monitoring, and parameter control (temperature, pressure, curvature, refractive index, torsion, and electric field). Nevertheless, PCC resonance properties are extremely sensitive to manufacturing errors (a 1% fluctuation in vesicle radius results in a 15 dB/mm attenuation). To reduce coupling loss, crystal waves are introduced bipartitedly while the typical waveguide cross-section is less than 1 μm wide and 500 nm thick [10]. Considering silicon-based PCC sensitivity to external temperature effects, photofluid technology and thermal-optical coefficient liquid utilization are made to minimize or eradicate device temperature reliance through non-temperature-sensitive PCC dimension design. However, photofluid penetration may reduce PCC structural design flexibility.
In the fiber cladding part, the four-ring air hole design allows lights polarized to its core region. Sensitized by the evanescent light field, the sensing solid-state core mechanism [6] enables interaction between the air hole and the analyte on small refractive index change detection. SMFs and coupler direct input light waves to the photonic crystal fiber (PCF). Medium changes in refractive index affect OSA light intensity and fiber light signal modulation (Figure 15). In terms of pressure measurement, the shoulder-coupled PCC is a suspended silicon bridge structure with a 500 nm lattice constant, a 180nm hole radius, and an initial 640nm defect length (Figure 16) [10]. Caused by pressure and cavity defect length variation, the longitudinal air hole deformation leads to PCC resonance wavelength shift. Rather than the output resonance behaviors, deformed PCC hollow hole structure mainly depends on the relative air hole position deviation. Annular PCC are applied in a qualified hexagonal air-hole array. Compared with shoulder-coupled PCC, the annular PCC minimum observable force and strain are 75.7nN and 0.0023% [10]. In Figure 17, two-ring PCC integrated into a silicon cantilever beam shows that the minimum detectable force at different connection positions reaches 7.58nN and a circular silicon film sensors with integrated tricyclic PCC sensitivity is 0.847 μN.
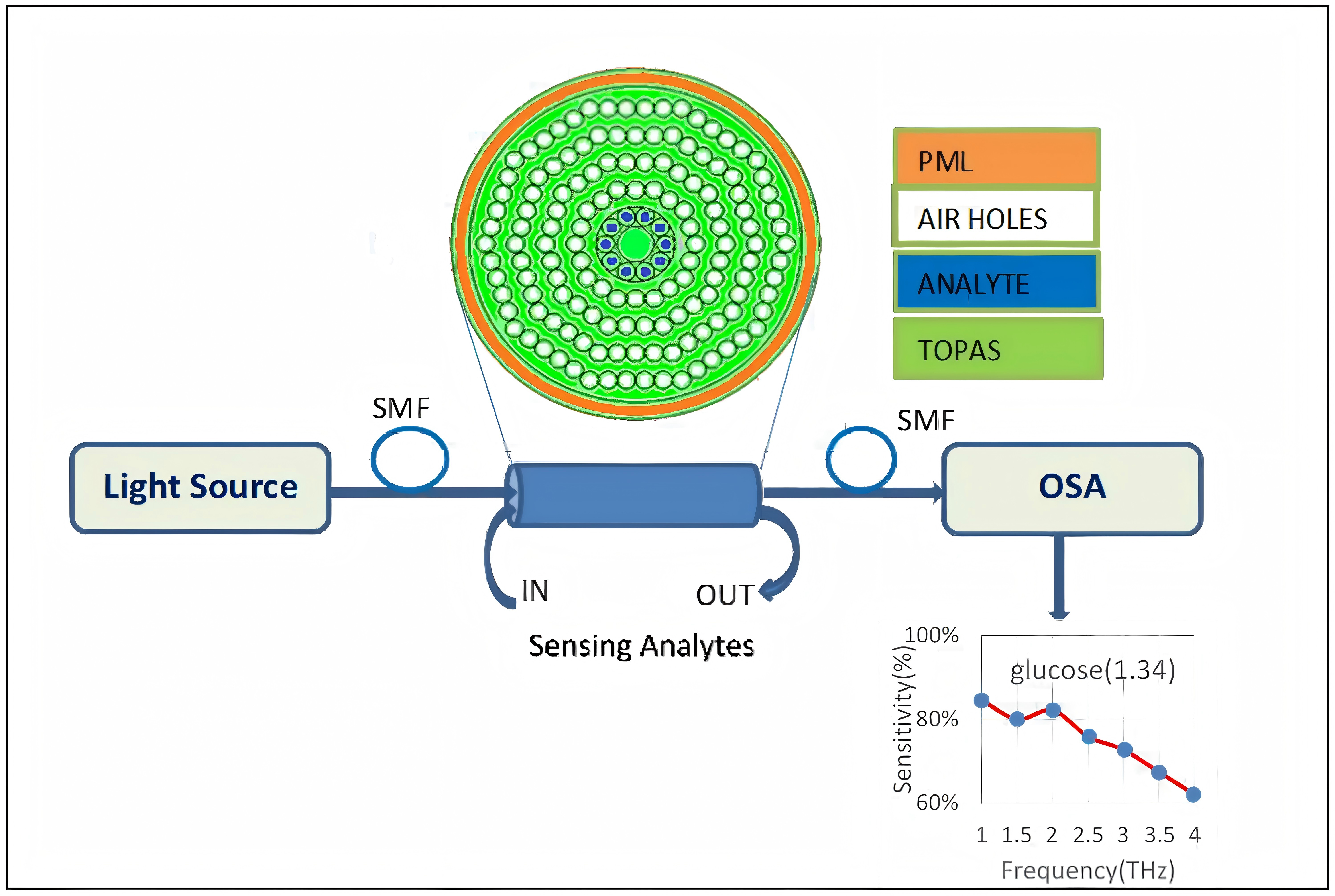
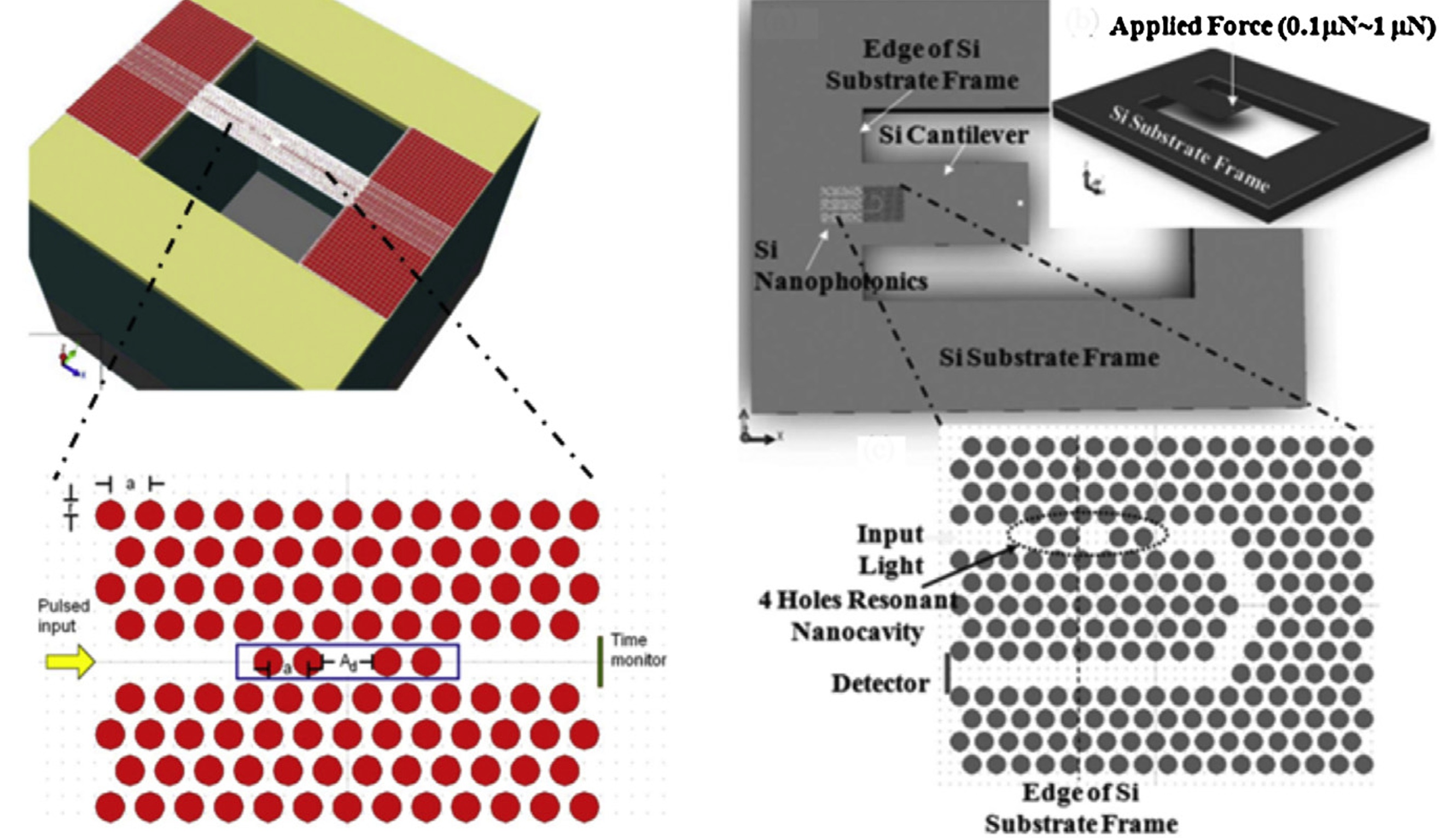
Figure 15 (Left): Schematic of PCF-based refractive index sensor [6]
Figure 16 (Right): Shoulder-coupled PCC structure for mechanical sensing [10]
3.7. Colorimetric Light (CL) Biosensors
Based on viral particle antibody recognition, RNA extraction and amplification, CL Biosensors simplify operation procedure and enable infectious viral particles detection without inactive viral remnant interference. When the viral load is high, the solution color changes from red to purple macroscopicly [11]. For example, gold NPs form nanolayers after binding with SARS-CoV-2 virus, lead to solution spectrum redshift and color variation [12]. Critically, the innovations are antibody functionalization technology simplified operation and virus particles specific binding (Figure 18). They accelerate simple-field operation and home inspection. However, there are still uncertainty in highly dilute viral sample sensitive detection. Faced with production scale challenges, strategies like PIT technology that increase antibody fixed density affected by antibody binding-site steric hindrance take effect.
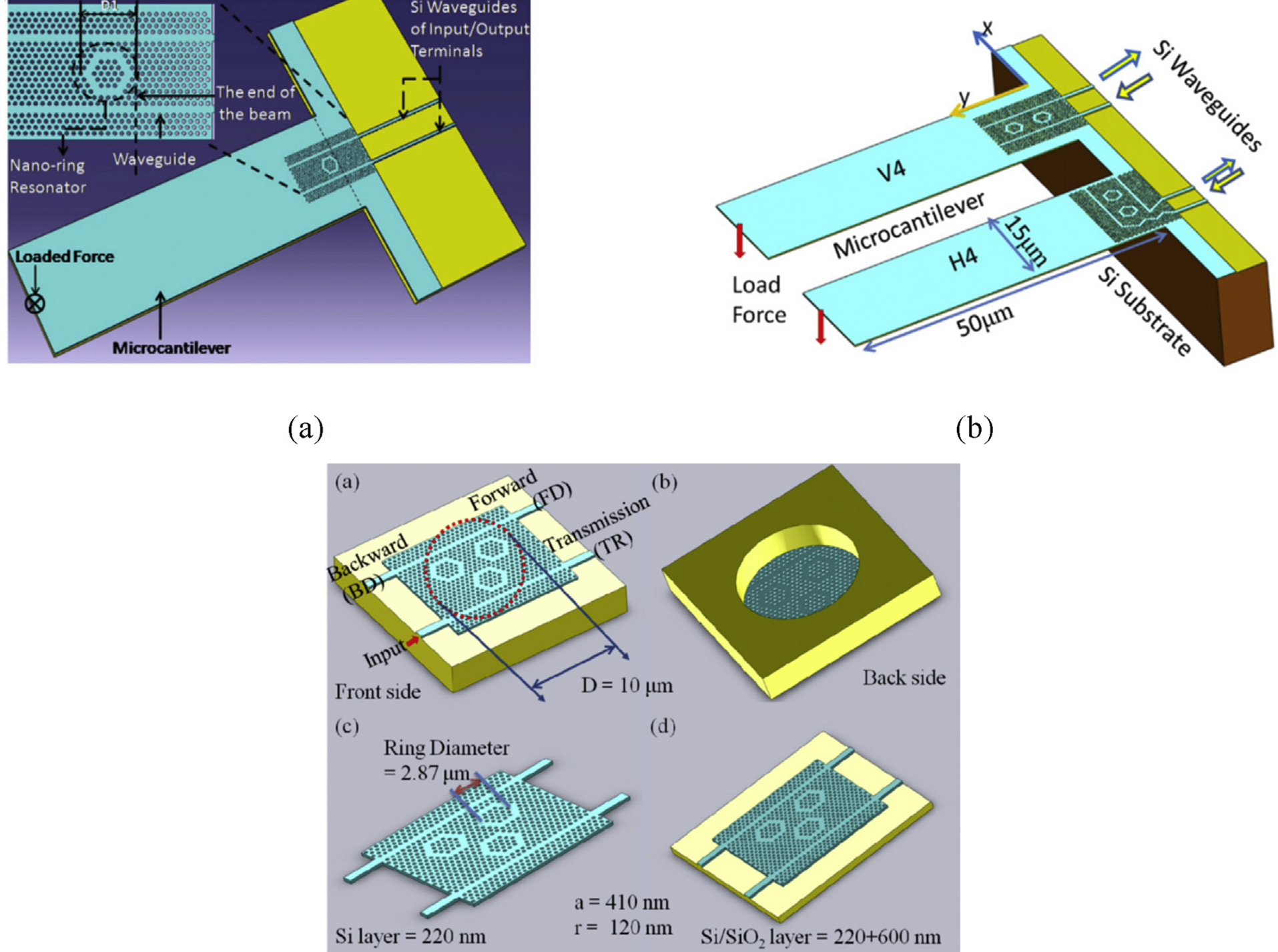
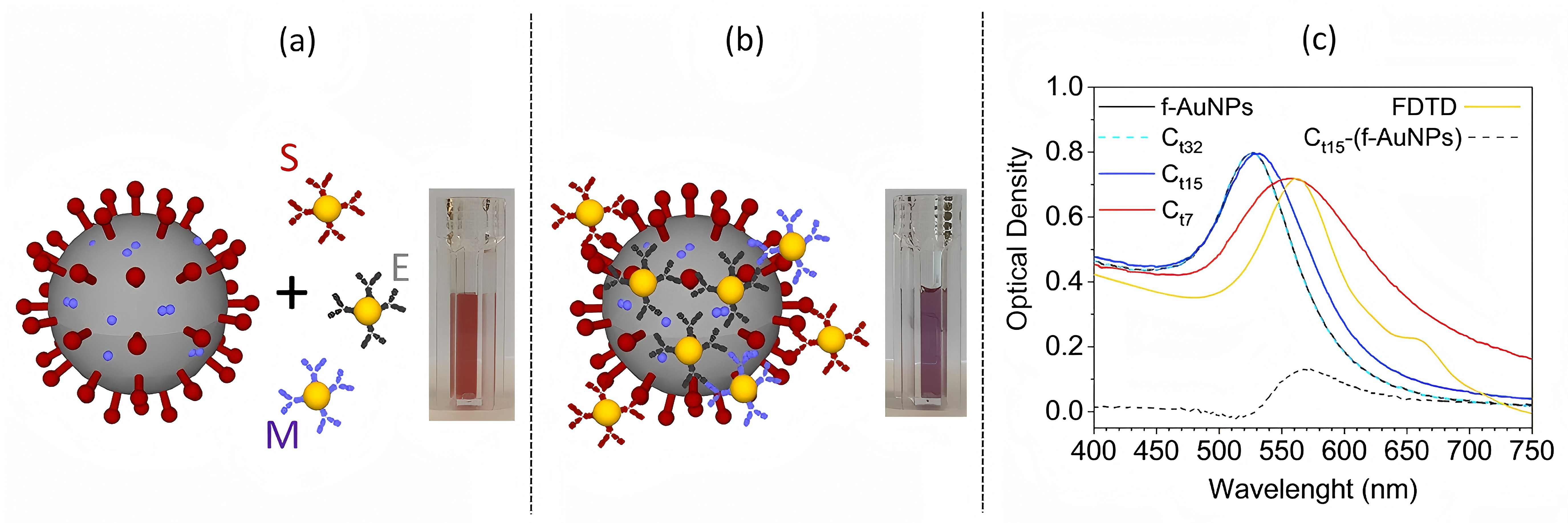
Figure 17: Structure of ring PCC (dual, triple) for mechanical sensing [10]
Figure 18: Sketch of the SARS-CoV-2 and functionalized AuNPs [11]
3.8. Portable Optical Biosensors (POB)
Rapidly and accurately, POB are the leading innovative trend in biomedical detection, point-of-care (POC) diagnosis real-time on medical decisions. Monitoring physiological indicators, wearable POB enable personalized and preventive treatment. In complex situations like sports or low-light environments, data accuracy, reliability assurance, and daily occupant comfort count. Another category is LOC Biosensors. In POC disease diagnostic, they can detect biomarkers in physiological fluids such as blood, urine, saliva; they can flexibly evaluate factors ranging from cell structure to gene, protein expression regulation [13]. Furthermore, tattoo optical biosensors use POB reading devices like smartphone cameras to achieve non-invasive biomarker measurement [14]. Their advantages are continuous monitoring, realizing simultaneous multiple biomarker detection. In diabetes management, glucose monitoring can reflect blood sugar control, reduce hypoglycemic events, and improve living quality [15]. Studies have shown that optimized, implantable fluorescence biosensors perform accurate clinical biomolecular analysis, customized intervention, and treatment strategies. Smartphones, as an integral part of life, also provide platform innovation. Compactly, LMW, low-costly, smartphone-integrated optical biosensors (SIOB) lower operational disturbance and reshape POC diagnostic landscapes. However, SIOB need to intensify reliability and user-friendliness, generating reliable data in real-world scenarios.
Driven by technological advances and innovative materials, they explore new material incorporation such as MXene into optical biosensors to overcome the existing material limitations [16]. Technically, the integration of AI and Internet of Things (IoT) have brought broader development of POB, which can effectively monitor, detect, initiate system actions to enhance patient care... Moreover, applying deep-learning neural network technologies like IoMT, FOB capable of detecting SARS-CoV-2 transmissively have been developed and connected to edge hardware devices. Innovations facilitate long-term analyte monitoring, chronic disease prevention and reliable data provision. Whereas, POB still face many challenges. How to ensure data privacy, improve data storage capacity, optimize energy supply, and expand application scope are current research focus.
4. Discussion
Among multifarious optical biosensors, SPR Biosensors excel in real-time, label-free biomolecular interaction detection. Their sensitivity to refractive index changes enables HMW analyte recognition, standardizing ligand-receptor SAS. However, they are limited by susceptibility to non-specific binding and an inability to detect LMW molecules. EWF Biosensors effectively detect bacteria and monitor pollutants through selective near-surface excitation [17]. Nevertheless, they remain constrained by background signal interference and suboptimal property in complex biological matrices. SERS Biosensors harness localized SPR to amplify Raman signals, achieve ultra-high sensitivity at picogram-level detection limits while invaluably for multiplex biomarker detection. Meanwhile, the non-uniform hot-spot distribution on metallic substrates and susceptibility to matrix effects present formidable challenges. PCC Biosensors offer exceptional sensitivity to minute refractive index changes for mechanical sensing and environmental monitoring. Their reliance on precise fabrication yet limits widespread deployment. Emerging platforms like CL Biosensors and POB further diversify them. Suitable for rapid viral diagnostics, the former distinguish samples simply and visually; The latter encompass implantable devices and redefine real-time monitoring. The integration with microfluidics and smartphones enhances accessibility, though challenges remain in energy efficiency, sensor miniaturization, and data accuracy under variable conditions. Current trends indicate that POB are tailored for real-time POC diagnostics and SIOB to facilitate user-friendly interfaces [18]. Technological diversification has led to increased adoption of hybrid techniques, combining SPR with nanomaterials, expanding into multi-analyte detection. To minimize interference in biological matrices, addressing non-specific binding, sample complexity, standardized protocols are essential. AI technology is imperative in data processing, decision systems, signal transportation, biorecognition element investigation, and material innovation [19]. Multiplex technologies advance in optical biosensing, enabling multiple analytes simultaneous detection with strengths in high-throughput screening, molecular marker profiling, and biological processes reading [20].
Challenges persist in mitigating non-specific binding, interference with biological sample complexity, technology optimization for scale application. Interdisciplinary collaboration is necessary to improve biosensor surface detection stability and reliability. In terms of data processing and output, the combination of graphical display and intelligent analysis is a crucial technological direction, improving data readability, enhancing multi-functional integration and real-time monitoring. Looking ahead, the integration of AI and IoT technologies promises to facilitate optical biosensor intelligence and functionality. Innovations in nanotechnology and quantum materials will elevate sensitivity and enlarge to more intricate diagnostic areas. Miniaturizing devices and incorporating wireless communication will further align with personalized medicine and environmental sustainability. Emphasizing multi-functional integration, combining photonic and electrochemical sensors empower comprehensive monitoring and broader systemic applications. Developing standardized protocols and robust signal processing techniques will address performance bottlenecks in distinguishing specific signals from complex biological matrices. By closing critical gaps in detection sensitivity, portability, and real-time analysis, optical biosensors can reshape bioanalytical methodologies, enhance diagnostic precision and democratize healthcare on accessible, user-friendly platforms.
5. Conclusion
The exploration of optical biosensors signifies a remarkable intersection of optical science and biomedical engineering, establishing transformative devices in modern diagnostics and environmental monitoring. The study elucidates theoretical foundations, technological advancements, and practical applications of various optical biosensors, including SPR, EWF, and SERS. Particularly in biomedical Engineering, they offer entirely new possibilities for early disease diagnosis and precision treatment. Through highly sensitive and specific biomolecular detection methods, they can capture early biomarkers of major diseases, realize high-resolution molecular interaction analysis and drive targeted therapy development. Especially in drug screening, the biosensors inject new impetus into drug development by efficiently evaluating affinity between ligands and receptors. However, limitations such as non-specific binding and matrix interference persist, necessitating further refinement and integration with nanotechnology and artificial intelligence. Prospects for optical biosensors are promising, with an emphasis on enhancing portability and real-time monitoring capabilities like POBs, wearable devices, and LOC systems. Integration of AI and IoT technologies is expected to revolutionize data processing and multi-analyte detection. Ultimately, by fostering technological innovation and multidisciplinary collaboration, optical biosensors will undoubtedly exert significant impacts on future scientific and societal advancements, ensuring their relevance as indispensable tools in biomedicine, environmental science, and beyond.
References
[1]. Naresh V, Lee N. Review on Biosensors and Recent Development of Nanostructured Materials-Enabled Biosensors. Sensors. 2021; 21:1109. doi.10.3390/s21041109.
[2]. Zhang H, Lv J, Jia Z. Efficient fluorescence resonance energy transfer between quantum dots and gold nanoparticles based on porous silicon photonic crystal for DNA detection. Sensors (Switzerland). 2017; 17:1078. doi.10.3390/s17051078.
[3]. Kaur B, Kumar S, Kaushik B.K. Recent advancements in optical biosensors for cancer detection. Biosensors and Bioelectronics. 2022; 197:113805. doi.10.1016/j.bios.2021.113805.
[4]. Garzón V, Pinacho D. G, Bustos R.H et al. Optical biosensors for therapeutic drug monitoring. Biosensors. 2019; 9:132. doi.10.3390/bios9040132.
[5]. Nirgund J, Purana N.K, Selvakumar D et al. Nanobiosensors for detection of bacteria: an overview of fiber-optics and Raman spectroscopy based biosensors. Handbook of Microbial Nanotechnology. 2022:91-132. doi.10.1016/B978-0-12-823426-6.00023-1.
[6]. Uniyal A, Srivastava G, Pal A et al. Recent Advances in Optical Biosensors for Sensing Applications: a Review. Plasmonics. 2023; 18:735-750. doi.10.1007/s11468-023-01803-2.
[7]. Zibaii M.I, Kazemi A, Latifi H et al. Measuring bacterial growth by refractive index tapered fiber optic biosensor. Journal of Photochemistry and Photobiology B: Biology. 2010;101(3):313-320. doi.10.1016/j.jphotobiol.2010.07.017.
[8]. Yoo S.M, Lee S.Y. Optical Biosensors for the Detection of Pathogenic Microorganisms. Trends in Biotechnology. 2016;34(1):7-25. doi.10.1016/j.tibtech.2015.09.012.
[9]. Chauhan P, Bhargava A, Kumari R et al. Surface-enhanced Raman scattering biosensors for detection of oncomiRs in breast cancer. Drug Discovery Today. 2022;27(8):2121-2136. doi.10.1016/j.drudis.2022.04.016.
[10]. Zhang Y, Zhao Y, Lv R. A review for optical sensors based on photonic crystal cavities. Sensors and Actuators A: Physical. 2015; 233:374-389. doi.10.1016/j.sna.2015.07.025.
[11]. Ventura B.D, Cennamo M, Minopoli A et al. Colorimetric Test for Fast Detection of SARS-CoV-2 in Nasal and Throat Swabs. ACS Sensors. 2020;5(10):3043-3048. doi.10.1021/acssensors.0c01742.
[12]. Xu M, Li Y, Lin C, et al. Recent Advances of Representative Optical Biosensors for Rapid and Sensitive Diagnostics of SARS-CoV-2. Biosensors. 2022; 12:862. doi.10.3390/bios12100862.
[13]. ]Torrijos-Morán L, Lisboa B.D, Soler M et al. Integrated optical bimodal waveguide biosensors: Principles and applications. Results in Optics. 2022; 9:100285. doi.10.1016/j.rio.2022.100285.
[14]. Gauglitz G. Critical assessment of relevant methods in the field of biosensors with direct optical detection based on fibers and waveguides using plasmonic, resonance, and interference effects. Analytical and Bioanalytical Chemistry. 2020; 412:3317-3349. doi.10.1007/s00216-020-02581-0.
[15]. Yang C, Wang Z, XIAO K et al. Portable optical fiber biosensors integrated with smartphone: technologies, applications, and challenges [Invited]. Biomedical Optics Express. 2024;15(3):1630. doi.10.1364/BOE.517534.
[16]. Rasheed S, Kanwal T, Ahmad N, et al. Advances and challenges in portable optical biosensors for onsite detection and point-of-care diagnostics. TrAC Trends in Analytical Chemistry. 2024; 173:117640. doi.10.1016/j.trac.2024.117640.
[17]. Long F, Zhu A, Shi H. Recent Advances in Optical Biosensors for Environmental Monitoring and Early Warning. Sensors. 2013; 13:13928-13948. doi.10.3390/s131013928.
[18]. Damborský P, Švitel J, Katrlík J. Optical biosensors. Essays in Biochemistry. 2016;60 (1):91-100. doi.10.1042/EBC20150010.
[19]. Irkham I, Ibrahim A.U, Pwavodi P.C, et al. Smart Graphene-Based Electrochemical Nanobiosensor for Clinical Diagnosis: Review. Sensors. 2023; 23:2240. doi.10.3390/s23042240.
[20]. Pakchin P.S, Fathi F, Samadi H et al. Recent advances in receptor-based optical biosensors for the detection of multiplex biomarkers. Talanta. 2025; 281:126852. doi.10.1016/j.talanta.2024.126852.
Cite this article
Li,Z. (2025). Advances in Optical Biosensors: Bridging Optical Science and Biomedical Engineering with Biological Recognition Elements. Theoretical and Natural Science,82,162-171.
Data availability
The datasets used and/or analyzed during the current study will be available from the authors upon reasonable request.
Disclaimer/Publisher's Note
The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of EWA Publishing and/or the editor(s). EWA Publishing and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
About volume
Volume title: Proceedings of the 3rd International Conference on Modern Medicine and Global Health
© 2024 by the author(s). Licensee EWA Publishing, Oxford, UK. This article is an open access article distributed under the terms and
conditions of the Creative Commons Attribution (CC BY) license. Authors who
publish this series agree to the following terms:
1. Authors retain copyright and grant the series right of first publication with the work simultaneously licensed under a Creative Commons
Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this
series.
2. Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the series's published
version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial
publication in this series.
3. Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and
during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See
Open access policy for details).
References
[1]. Naresh V, Lee N. Review on Biosensors and Recent Development of Nanostructured Materials-Enabled Biosensors. Sensors. 2021; 21:1109. doi.10.3390/s21041109.
[2]. Zhang H, Lv J, Jia Z. Efficient fluorescence resonance energy transfer between quantum dots and gold nanoparticles based on porous silicon photonic crystal for DNA detection. Sensors (Switzerland). 2017; 17:1078. doi.10.3390/s17051078.
[3]. Kaur B, Kumar S, Kaushik B.K. Recent advancements in optical biosensors for cancer detection. Biosensors and Bioelectronics. 2022; 197:113805. doi.10.1016/j.bios.2021.113805.
[4]. Garzón V, Pinacho D. G, Bustos R.H et al. Optical biosensors for therapeutic drug monitoring. Biosensors. 2019; 9:132. doi.10.3390/bios9040132.
[5]. Nirgund J, Purana N.K, Selvakumar D et al. Nanobiosensors for detection of bacteria: an overview of fiber-optics and Raman spectroscopy based biosensors. Handbook of Microbial Nanotechnology. 2022:91-132. doi.10.1016/B978-0-12-823426-6.00023-1.
[6]. Uniyal A, Srivastava G, Pal A et al. Recent Advances in Optical Biosensors for Sensing Applications: a Review. Plasmonics. 2023; 18:735-750. doi.10.1007/s11468-023-01803-2.
[7]. Zibaii M.I, Kazemi A, Latifi H et al. Measuring bacterial growth by refractive index tapered fiber optic biosensor. Journal of Photochemistry and Photobiology B: Biology. 2010;101(3):313-320. doi.10.1016/j.jphotobiol.2010.07.017.
[8]. Yoo S.M, Lee S.Y. Optical Biosensors for the Detection of Pathogenic Microorganisms. Trends in Biotechnology. 2016;34(1):7-25. doi.10.1016/j.tibtech.2015.09.012.
[9]. Chauhan P, Bhargava A, Kumari R et al. Surface-enhanced Raman scattering biosensors for detection of oncomiRs in breast cancer. Drug Discovery Today. 2022;27(8):2121-2136. doi.10.1016/j.drudis.2022.04.016.
[10]. Zhang Y, Zhao Y, Lv R. A review for optical sensors based on photonic crystal cavities. Sensors and Actuators A: Physical. 2015; 233:374-389. doi.10.1016/j.sna.2015.07.025.
[11]. Ventura B.D, Cennamo M, Minopoli A et al. Colorimetric Test for Fast Detection of SARS-CoV-2 in Nasal and Throat Swabs. ACS Sensors. 2020;5(10):3043-3048. doi.10.1021/acssensors.0c01742.
[12]. Xu M, Li Y, Lin C, et al. Recent Advances of Representative Optical Biosensors for Rapid and Sensitive Diagnostics of SARS-CoV-2. Biosensors. 2022; 12:862. doi.10.3390/bios12100862.
[13]. ]Torrijos-Morán L, Lisboa B.D, Soler M et al. Integrated optical bimodal waveguide biosensors: Principles and applications. Results in Optics. 2022; 9:100285. doi.10.1016/j.rio.2022.100285.
[14]. Gauglitz G. Critical assessment of relevant methods in the field of biosensors with direct optical detection based on fibers and waveguides using plasmonic, resonance, and interference effects. Analytical and Bioanalytical Chemistry. 2020; 412:3317-3349. doi.10.1007/s00216-020-02581-0.
[15]. Yang C, Wang Z, XIAO K et al. Portable optical fiber biosensors integrated with smartphone: technologies, applications, and challenges [Invited]. Biomedical Optics Express. 2024;15(3):1630. doi.10.1364/BOE.517534.
[16]. Rasheed S, Kanwal T, Ahmad N, et al. Advances and challenges in portable optical biosensors for onsite detection and point-of-care diagnostics. TrAC Trends in Analytical Chemistry. 2024; 173:117640. doi.10.1016/j.trac.2024.117640.
[17]. Long F, Zhu A, Shi H. Recent Advances in Optical Biosensors for Environmental Monitoring and Early Warning. Sensors. 2013; 13:13928-13948. doi.10.3390/s131013928.
[18]. Damborský P, Švitel J, Katrlík J. Optical biosensors. Essays in Biochemistry. 2016;60 (1):91-100. doi.10.1042/EBC20150010.
[19]. Irkham I, Ibrahim A.U, Pwavodi P.C, et al. Smart Graphene-Based Electrochemical Nanobiosensor for Clinical Diagnosis: Review. Sensors. 2023; 23:2240. doi.10.3390/s23042240.
[20]. Pakchin P.S, Fathi F, Samadi H et al. Recent advances in receptor-based optical biosensors for the detection of multiplex biomarkers. Talanta. 2025; 281:126852. doi.10.1016/j.talanta.2024.126852.









