1. Introduction
Pain, both acute and chronic, is an unpleasant somatosensory experience that is disturbing to our mental health. Surprisingly, it is also an alarm signal generated by our nervous system to warn us about a danger.
The pain pathway is a bidirectional pathway with both afferent and efferent nerves. After the nociceptor on the peripheral sensory nerve receives noxious stimuli, the signal is transduced and sent to the spinal cord for modulation, during which neurotransmitters such as glutamate and substance P in the central nervous system (CNS), and bradykinin and prostaglandins in the peripheral nervous system mediate the signal. Neurochemical pain initiators are secreted to alleviate the pain signal for the modulation. Those initiators include serotonin, endorphins, enkephalins, and dynorphin, which are all also related to mood regulation[1].
Upon the entry of the pain signal into the descending pathway through the thalamus, descending inhibition molecules will be released in the periaqueductal gray matter (PAG). Similarly, epinephrine, cortisol, and Adrenocorticotropic Hormone (ACTH) ensure that our sensory perception is not overwhelmed by pain. Those molecules are also closely related to stress regulation, and in higher concentration chronically, they may cause depressive disorder.
Therefore, associations between pain and depression exist, and their relationship has been investigated extensively by neuroscientists. Due to the persistent activation of certain brain areas and the secreted molecules, a strong correlation has been found between pain and mental state. Patients suffering from chronic pain are likely to be diagnosed with depression or other mental health problems.
It has been reported that low concentration level of neurotransmitter generated by above-mentioned monoaminergic neurons, such as norepinephrine (NE), 5-hydroxytryptamine (5HT) and Dopamine (DA), can be a possible reason of depression and a series of mental state dysfunction[2].
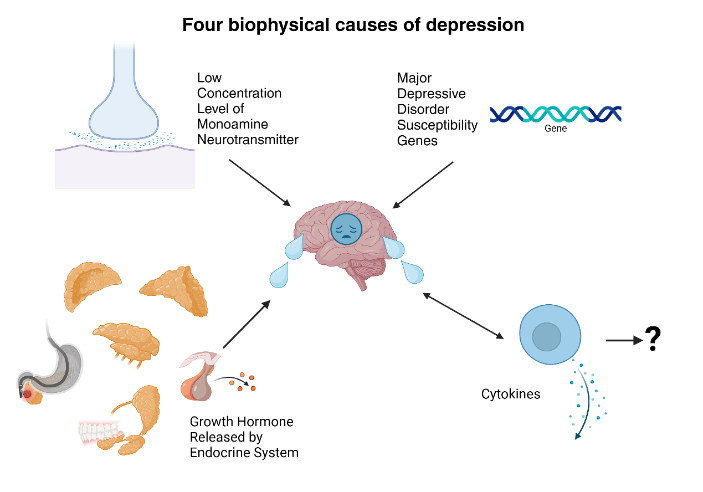
Created with BioRender.com
Figure 1: Four biophysical causes of depression.
Multiple factors cause depression (Figure 1). Apart from the “monoamine hypothesis”, gene also plays an important role in the development of depression. In some cases, the patients who carries MDD (major depressive disorder) susceptibility genes (apolipoprotein E, guanine nucleotide-binding protein (GND3), methylenetetrahydrofolate reductase (MTHFR 677T), dopamine transporter (SLC6A3) and the like) have shown higher possibilities of MDD. But the association has not been fully proved yet[2].
Another biophysical factor involved in depression is the dysfunction of endocrine system. For instance, the hormonal fluctuation of growth hormone (GH) can disturb the NE system, which affect the development of depression[2].
It has been reported that generated cytokines, such as interleukins IL1, IL6 and the like, could result in symptoms similar to MDD. While the specific relation has not been determined yet, great importance has been attached on the association between cytokines and the development of depression[2].
Other factors such as environment factors, family and social relation factors, and personal character factors have certain influence on the development of depression, which are discussed elsewhere [3][4].
The rate of experiencing depression is notable. According to the WHO, there are approximately 280 million patients suffering from depression, which takes up 3.8% of the world's population, including 5% of adults and 5.7% of adults over 60 years old. It is shown that women have a 50% higher tendency to be diagnosed with depression than men, especially women in and after the period of gestation. Depression also has a serious influence on teenagers and people in young adulthood, which has become the leading cause of their suicide[5].
People diagnosed with depression can experience constant depressed feelings and carry a hopeless and pessimistic attitude towards life. Moreover, there are some physical symptoms, such as changes in appetite, insomnia, and pains which are unlikely to respond to treatments[6]. In this case, the patients may be unable to lead a normal life with pressure and despair. When the situation deteriorates, committing suicide would be considered an option to stop the endless physical and mental pain. Depression can bring severe consequences to the patients, their families and society.
Recent statistics show that the relation between pain and depression has attracted a lot of attention in the neuroscience research field. The pain can lead to depression, while depression reduces patients’ pain-perception threshold. Since the consequences of depression are evident, it is urgent to find effective treatment for the disorder. Therefore, as a crucial factor for depressive disorder, deeper understanding of the relation between pain and depression is necessary. This essay will collect the existing information in this field of research, including the mechanism associated with pain and depression and current methods and issues of pain-induced depression treatment, which provides support for further studies.
2. Methods
The research was conducted from scholarly sources including Google scholars, the National Center for Biotechnology Information (NCBI), the Centers for Disease Control and Prevention (CDC), the World Health Organization (WHO), and the National Institutes of Health (NIH). The search was limited to papers published in English over the past 20 years that were focused on the following keywords for research: “pain”, “chronic pain”, “depression”, “depressive syndrome”, “mental health”, “anxiety”. Studies were included within the following criteria: (a) focused on depression and pain in human, (b) were published in peer-reviewed journals. Non-English studies and studies mainly focused on animals were excluded. The figures were created using Biorender.
3. Discussion
The latest studies show little evidence that acute pain itself will cause depressive disorder, but there are some situations where depression has effect on the perception of acute pain. One of them researched much by the scientists is the acute postoperative pain.
There are several published papers during the past 15 years on this topic, but they didn’t stress a significant relationship between preoperative depression and postoperative pain. Despite some statistically significant outcomes, there are also papers that failed to show strong relation between two factors. According to a systematic review of 18 papers in the span of 2008-2016, only fewer than a half of papers reported that they have a p value smaller than 0.05[7].
In a recent study published in 2024, the author mentioned the association between acute postsurgical pain and presurgical depressive disorder. The subjects were reported to have moderate-to-severe acute resting pain (OR 2.87, 95% CI 1.04–7.97) after surgery, if they suffered from depression before. But it is notable that the presurgical depressive disorder does not have an impact on the intensity of pain, and it only affects the severity of pain[8]. Therefore, it is deduced that depression can reduce the pain threshold, which leads to a more severe perception of pain in patients. Nevertheless, the relationship between acute postoperative pain and preoperative depression still needs to be further explored.
Acute pain can be caused by exposure to excessive stimuli, including mechanical information, temperature change, photonic information, and chemical information. The transduced electric current activates a voltage-gated channel on the nociceptors, then produces action potential to release neurotransmitters, for example, glutamate, from the nociceptor terminals, which triggers the sense of pain, even inflammation[1].
The Molecules and neurotransmitters generated in the process of pain perception and signal transmission also have a strong effect on mood regulation. Patients with depression have lower concentration of Serotonin, Endorphins, and Enkephalins, therefore, their brain receives stronger pain signal, which makes their pain threshold decrease. And the experiment results reviewed above can be supported by this causal relationship.
If it remains unattended, acute pain will progress into chronic pain, which becomes a significant cause of depression. Scientists have focused on the transition from acute pain to chronic pain and its prevention. This will be discussed further in this essay.
Chronic pain has been established as a major cause of depression due to their similar neuropathways. Studies show that chronic pain patients are more likely to be diagnosed with depression, and conducted surveys revealed that about 21% of patients in Europe and 35% in the USA community are comorbid major depressive disorder (MDD) patients[9,10]. For example, a study of patients with chronic low back pain (LBP) revealed that they had a significantly higher risk (OR: 1.81, 95% CI: 1.34–2.44) of showing depressive symptoms[11].
Similarly, there is a higher possibility for individuals with MDD to be suffering from chronic pain. A cross-sectional study of 149,611 subjects in the UK revealed that MDD patients were 12.2% more likely to report chronic pain symptoms[12]. As depression and chronic pain share a similar pathophysiological pathway, the areas related to pain modulation, namely the periaqueductal gray, amygdala, and hypothalamus could be affected in the presence of depressive disorder. Therefore, some individuals may experience a stronger perception of pain. Some psychological factors also help to explain a reduced pain threshold, including social isolation, increased threatened feelings, and reduced physical activities[13].
Constant and severe sensory stimuli can lead to chronic pain may happen. A prolonged inflammation with the activation of TNF(Tumor Necrosis Factor)-α and interleukins such as IL1, IL6, and IL1β will induce a series of consequences, for instance, a lower pain threshold and up-regulation of voltage-gated channels and receptors, which can lead to a hyperexcitable state within the spinal cord. During the continuous abnormal pain-generating process, the structure of neurons has been changed to achieve the strengthened ability to transduce signals, which gives rise to the state of “central sensitization”[14].
The low concentration of monoamine neurotransmitters secreted by monoaminergic neurons, such as 5-HT, NE, DA, is one of the factors underlying depression. According to the published studies, subjects were more likely to be diagnosed with depression if the monoamine neurotransmitter concentration decreased[15,16,17,18]. Monoamine neurotransmitters are released in the descending pathway to achieve an analgesic purpose, when chronic pain constantly activates the descending pathway and exhausts the mechanism, the availability of monoamine neurotransmitters will decrease, which result in depressive disorder[19].
Dopamine, which is a major player in the reward pathway, is also affected by chronic pain. The midbrain dopaminergic system can be damaged by chronic pain and exhibit reduced reactivity when stimulated again. With reduced DA concentration, the subject will feel less satisfied and rewarding, thus there stands a higher possibility of depression[18].
BDNF is involved in the pain pathway by binding with receptor kinase B (TrkB) and activating kinase C expression in the spinal cord, which affects hypersensitivity and neuroplasticity in the progression of neuropathic pain[18].
It has been found that the level of BDNF is reduced in depressed patients and suicide victims, along with a downregulated level of TrkB activation, which should result in a volume reduction of prefrontal cortex (PFC) and hippocampus as a typical symptom of MDD patients[20]. According to early studies, exposure to long-term stress can be a major cause of BDNF decrease in PFC and hippocampus, while, further discovered in other studies, the BDNF expression is increased in other brain regions, for instance the nucleus accumbens and amygdala, which contributes to synaptic plasticity as an potential factor of depressive disorder[21].
Experiment on mice has shown that the individual difference of comorbidity is associated with pro-inflammatory cytokines, and reduced levels of BDNF in the PFC in the rodents (tested on spared nerved ligation subjects with and without depression behaviour). But whether such findings can be extended to human is still unconfirmed[22].
In certain cases, immune cells can enter CNS through BBB (brain-blood barrier) and cause the immune response of CNS. Detailed mechanism of such pathological conditions is not discussed in this article, and more information can be found elsewhere[23,24]. In the pro- and anti-inflammatory effects, cytokines are be secreted by microglia and other non-immune cells, such as neurons and astrocytes. Chronic inflammatory process and sustained release of cytokines may disturb several neuronal functions, including impairment of neurotransmitter signaling and release of neurotransmitters, which in turn have influence on the neurocircuit and affect cognition and mood regulation[25].
The exact pathology by which cytokines affect neuronal functions remains partly unclear. However, blocking peripheral cytokines and blocking BBB is sufficient in antidepressant process[25]. Besides, depressive disorder symptoms have been observed to be aggravated in up to 45% patients receiving inflammatory pain treatments. Major cytokines causing inflammation-induced depression are interleukin (IL), TNF, IFNs and the like[18].
The level of glutamate is crucial in chronic pain development, which activated in the excitatory pathway and transfer pain signals. Constant activation of receptor N-methyl-D-aspartic acid (NMDA) and α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) causes increased pain sensitivity[18]. In contrast, Gamma-Aminobutyric Acid (GABA) is responsible for its inhibition. Studies suggest that Na-K-Cl-cotransporter-1 (NKCC1) and K-Cl-cotransporter-2 (KCC2) can affect GABA transmission. Experiment data showed that higher NKCC1 and KCC2, namely a higher GABA excitatory level, can be a cause of spontaneous depression[26,27].
Some data suggest that glutamate is involved in depressive disorder. A meta-analysis showed that the level of glutamate + glutamine (Glx) is decreased in medial prefrontal cortex (mPFC) by a small size in the depression group compared with the control group. But an elevated glutamate level is also found in other region of the brain, therefore, disturbing the glutamate balance in the brain[28]. The antidepressant effect of NMDA antagonists also proved that glutamate plays a role in mood disorders. Due to the complexity of the association between glutamate and depression, the disorder of glutamate in MDD patients is still unclear[27].
Sexual disparity, where a higher number of women suffering from depressive disorder is larger than that of men, has emphasized on the role of hormone in such differences. The incidence percentage of depression among females has been constantly increasing by an average of 0.39% in the last 30 years[29]. It has become a serious problem.
Chronic noncancer pain (CNCP) is more common in women, such as musculoskeletal pain, migraine headache, temporal mandibular disorder, pelvic pain, and the like[30]. The changes in sex hormones, mainly sex steroid hormones can affect pain perception and threshold. Therefore, women may be more sensitive to pain around the menstrual cycle, making them more susceptible to depression[31].
Studies showed that women have lower pain thresholds and a greater prevalence of pain. It is found that an unstable level of estrogen is the reason why women feel more pain. In contrast, a stable level of estrogen can be protective. The lowest pain threshold during the ovulatory phase, which has a steep decrease in estrogen, proves that the fluctuation of estrogen affects pain occurrence, intensity, and perception[32].
Primate and rodent studies showed that the fluctuation of estrogen is also related to depression during the menstrual cycle, pregnancy and perimenopausal transition[33]. However, the researchers have still not concluded, how exactly are estrogen and other sex hormones related to depression.
Neuroplasticity can provide a linkage between pain with depression. Once acute pain develops into chronic pain, neuroplasticity occurs due to the presence of consistent abnormalities, which results in the emergence of depression. As for patients who suffer from depression, their neuroplasticity also influence their pain perception. The causal relationship is summarized in Table 1.
Table 1: The causal relationship between chronic pain and depression
Abnormality | Mechanism in chronic pain | Mechanism in depression | ||
Monoamine neurotransmitters | • Reduced monoamine neurotransmitter concentration | • Constant activation for an analgesic purpose • Damage to the dopaminergic system | • A possible reason of depression and a series of mental state dysfunction • Less satisfaction and reward | [15] [16] [17] [2] |
BDNF | • Reduced level of BDNF • Downregulation of TrkB activation • Increased activity of nucleus accumbens and amygdala | • Hypersensitivity and neuroplasticity in the progression of neuropathic pain | • A volume reduction of prefrontal cortex (PFC) and hippocampus • Synaptic plasticity as an potential factor of depressive disorder • Comorbidity in human unconfirmed | [18] [20] [21] [22] |
inflammatory factors | • Immune response in the CNS • Sustained release of cytokines | • Chronic inflammatory process | • Neurocircuit changes affecting cognition and mood regulation | [25] [18] |
glutamate | • Disturbed glutamate balance | • Higher NKCC1 and KCC2 affect GABA’s pain sensitivity inhibition function | • A cause of spontaneous depression • Still unclear | [26] [27] [28] |
Sex hormone | • Changes in sex steroid hormones | • Lower pain thresholds and a greater prevalence of pain • CNCP | • Susceptibility to depression • Depression during the menstrual cycle, pregnancy and perimenopausal transition • Still unclear | [30] [31] [32] [33] |
Abbreviations: CNS, central nervous system; BDNF, brain-derived neurotrophic factor; TrkB, kinase B; NKCC1, Na-K-Cl-cotransporter-1; KCC2, K-Cl-cotransporter-2; GABA, Gamma-Aminobutyric Acid; CNCP, Chronic noncancer pain
In the recent 10 years, depression treatment studies have generally shed light on the effect of opioid. The clinical use of opioid for analgesic purpose has been widely accepted, and its mechanism is mainly due to multiple factors: binding to receptors such as mu, kappa and delta, which inhibit the activation of neuropathways and reduces the secretion of certain neurotransmitters, such as glutamate in CNS, to regulate the perception of pain. Meanwhile, opioid can stimulate the release of DA, therefore, the reward system will be affected and the patient will feel a sense of joy. Since DA and other monoamine neurotransmitters are also significant in the pathology of major depressive disorder, opioid is considered to have effect on antidepressant treatment. [18]
Mu receptors are primarily responsible for pain, while kappa receptors are related to emotional and psychic effects, demonstrating a complementary role to perceive pain. The use of kappa opioid receptor (KOR) antagonist in the treatment of MDD has been explored. [34] Tested KOR antagonist can be focused on different situations and symptoms, for instance, Buprenorphine is often used on the middle-aged and the elderly in treatment for refractory depressive disorder, while Tramadol, which control the reuptake of 5-HT and NE, and can be effective in depression treatment related to chronic neuropathic pain. [18]
Nevertheless, a problem of opioid in depression treatment remains to be solved before a wide application, namely the high addiction rate of patients. Additionally, a long-term use of opioid will possibly even result in a prolonged depressive disorder. Therefore, further studies are still required before widespread investment. [18]
Another monoamine related treatment for depression is monoamine oxidase (MAO) inhibitor. Iproniazid, the first successful pharmacological drug for depression, is categorized as a MAO inhibitor. Type A MAO is often involved in depression, for it is responsible for the deaminate of 5-HT, NE and other enzyme activity, which are associated with both pain pathways and depressive disorder. Inhibiting type A MAO can increase the concentration of above chemicals, thus have impact on depression treatment. However, the treatment has been out of practice due to its side effects. In recent years, the potential of MAO inhibitor in chronic pain and chronic pain-induced depression has come into sight again and practiced on patients. [35] [18]
As mentioned previously, NMDA antagonists can act as an antidepressant drug. [36] Composition such as memantine, amantadine, and subtype-selective NMDA (NR2B) receptor blockers have been practiced in MDD patients’ treatment and received satisfying outcomes.
However, the comorbidity of chronic pain caused by glutamate concentration disorder and depression remains to be further explored due to the complexity of the relationship between glutamate and MDD occurrence. Therefore, specific medical treatment has rarely been clinically practiced on chronic pain-induced MDD patients.
Additionally, some studies showed that both endogenous and dietary glutamate can influence depression. Different studies support the effectiveness of the gut-brain axis on CNS diseases, including mood regulation dysfunction and disorder. Their relationship and the potential therapy will not be discussed in this article. Readers can find detailed information elsewhere [27]
BDNF and inflammatory factors can be potential target of Opioids. [18] Using NMDA receptor antagonist ketamine is effective in upregulating the level of BDNF expression, and therefore realizing antidepressant treatment. [37] As for BDNF, latest related studies are mainly focused on its role as Biomarker in MDD treatment. [38] But further experiments need to be conducted to confirm the effect.
There have been a few widely accepted inflammatory antidepressant drugs, for example, Nonsteroidal Anti-inflammatory Drugs, Cytokine modulators, Omega-3 polyunsaturated fatty acids, Minocycline, Statins, and Probiotics and prebiotics. These drugs have analgesic effect, which contributes to the similar even superior outcome compared with that of first-tier antidepressant drugs in the market. [39] While there is also evidence of the causal relationship between pain and depression, a reverse effect might occur in patients with non-inflammatory conditions taking inflammatory drugs, which still requires deeper investigation in drug itself and an improvement in diagnose. [39]
Apart from the medical therapy, psychotherapy also plays an important role in pain-induced depression treatment. For the patients suffering from both pain and depression, Interpersonal Psychotherapy for Depression and Pain (IPT-P) should be effective. It has 8 sessions and integrates the traditional pain management process. Therapists should be aware of the role which pain plays in depression, and adjust the traditional way of cognitive behavioral therapy accordingly. [40]
Current treatments for pain-induced depression still rely mainly on opioid or opioid-related medications. Due to the complexity of depression, the relationship between depression and pain caused by the abnormality of certain chemicals remains to be explored. In addition, existing widely-used opioid analgesics can cause addiction, which can cause additional health and social care burden. Furthermore, there is a lack of clinical studies investigating the association between acute pain and depression. Since the distinction between acute and chronic pain is not clear, current studies focus mostly on the transition from acute to chronic pain and its prevention. Elucidating the causal relationship between acute pain and depression can shed light on the mechanism of such conditions and provide insight into new treatments for pain-induced depression.
4. Conclusion
There is a complicated causal relationship between pain and depression. Depression can arise due to neurotransmitters in the pain pathway, which can also affect the severity of acute pain after the surgery. Progression of acute pain into chronic pain involve multiple factors that contribute to the comorbidity of depression and pain, such as monoamine transmitters, BDNF, inflammatory factors, glutamate and hormones. They have a bidirectional influence, although some has not been clearly identified yet, on the occurrence of pain-induced depression.
While some medical treatments to treat pain-induced depression have been developed, most of them are specific to chronic pain, since the biological pathway of acute pain-induced depression remains to be further explored. Current medicine for pain-induced depression still mainly relies on opioids. However, novel treatments such as inflammatory antidepressant drugs have recently appeared. Besides, psychotherapy plays an important role in pain-induced depression treatment.
Future studies are required to investigate developing specific treatments for chronic pain-induced depression, but also to investigating acute pain-induced depression and its biological pathway, so that we can treat the patients better.
References
[1]. Allan I. Basbaum, Diana M. Bautista, Grégory Scherrer, David Julius, Cellular and Molecular Mechanisms of Pain, Cell, Volume 139, Issue 2, 2009, Pages 267-284, ISSN 0092-8674, https://doi.org/10.1016/j.cell.2009.09.028.
[2]. Emmanuel Jesulola, Peter Micalos, Ian J. Baguley, Understanding the pathophysiology of depression: From monoamines to the neurogenesis hypothesis model - are we there yet?, Behavioural Brain Research, Volume 341, 2018, Pages 79-90, ISSN 0166-4328, https://doi.org/10.1016/j.bbr.2017.12.025.
[3]. Kendler, Kenneth S et al. “The impact of environmental experiences on symptoms of anxiety and depression across the life span.” Psychological science vol. 22,10 (2011): 1343-52. doi:10.1177/0956797611417255
[4]. Rautio, Nina et al. “Living environment and its relationship to depressive mood: A systematic review.” The International journal of social psychiatry vol. 64,1 (2018): 92-103. doi:10.1177/0020764017744582
[5]. https://www.who.int/news-room/fact-sheets/detail/depression
[6]. https://www.nimh.nih.gov/health/topics/depression
[7]. Anali Dadgostar, Mark Bigder, Nahid Punjani, Svjetlana Lozo, Vickramjit Chahal, Alexander Kavanagh, Does preoperative depression predict post-operative surgical pain: A systematic review, International Journal of Surgery, Volume 41, 2017, Pages 162-173, ISSN 1743-9191, https://doi.org/10.1016/j.ijsu.2017.03.061.
[8]. Liu, Q. R., Dai, Y. C., Ji, M. H., Liu, P. M., Dong, Y. Y., & Yang, J. J. (2024). Risk Factors for Acute Postsurgical Pain: A Narrative Review. Journal of Pain Research, 17, 1793–1804. https://doi.org/10.2147/JPR.S462112
[9]. Breivik, H. , Collett, B. , Ventafridda, V. , Cohen, R. , & Gallacher, D. (2006). Survey of chronic pain in Europe: Prevalence, impact on daily life, and treatment. Euro- pean Journal of Pain, 10 (4), 287 -287 .
[10]. Miller, L. R. , & Cano, A. (2009). Comorbid chronic pain and depression: who is at risk? The Journal of Pain, 10 (6), 619–627 .
[11]. Matt Fernandez, Lucia Colodro-Conde, Jan Hartvigsen, Manuela L. Ferreira, Kathryn M. Refshauge, Marina B. Pinheiro, Juan R. Ordoñana, Paulo H. Ferreira, Chronic low back pain and the risk of depression or anxiety symptoms: insights from a longitudinal twin study, The Spine Journal, Volume 17, Issue 7, 2017, Pages 905-912, ISSN 1529-9430, https://doi.org/10.1016/j.spinee.2017.02.009.
[12]. Nicholl, B.I., Mackay, D., Cullen, B. et al. Chronic multisite pain in major depression and bipolar disorder: cross-sectional study of 149,611 participants in UK Biobank. BMC Psychiatry 14, 350 (2014). https://doi.org/10.1186/s12888-014-0350-4
[13]. de Heer, Eric W et al. “The association of depression and anxiety with pain: a study from NESDA.” PloS one vol. 9,10 e106907. 15 Oct. 2014, doi:10.1371/journal.pone.0106907
[14]. A Feizerfan, G Sheh, Transition from acute to chronic pain, Continuing Education in Anaesthesia Critical Care & Pain, Volume 15, Issue 2, April 2015, Pages 98–102, https://doi.org/10.1093/bjaceaccp/mku044.
[15]. Cleare, Anthony et al. “Evidence-based guidelines for treating depressive disorders with antidepressants: A revision of the 2008 British Association for Psychopharmacology guidelines.” Journal of psychopharmacology (Oxford, England) vol. 29,5 (2015): 459-525. doi:10.1177/0269881115581093
[16]. Cipriani, Andrea et al. “Comparative efficacy and acceptability of 12 new-generation antidepressants: a multiple-treatments meta-analysis.” Lancet (London, England) vol. 373,9665 (2009): 746-58. doi:10.1016/S0140-6736(09)60046-5
[17]. Doboszewska, Urszula et al. “Zinc in the Monoaminergic Theory of Depression: Its Relationship to Neural Plasticity.” Neural plasticity vol. 2017 (2017): 3682752. doi:10.1155/2017/3682752
[18]. Sheng, Jiyao et al. “The Link between Depression and Chronic Pain: Neural Mechanisms in the Brain.” Neural plasticity vol. 2017 (2017): 9724371. doi:10.1155/2017/9724371
[19]. Bee, Lucy A, and Anthony H Dickenson. “The importance of the descending monoamine system for the pain experience and its treatment.” F1000 medicine reports vol. 1 83. 29 Oct. 2009, doi:10.3410/M1-83
[20]. Eero Castrén, Masami Kojima, Brain-derived neurotrophic factor in mood disorders and antidepressant treatments, Neurobiology of Disease, Volume 97, Part B, 2017, Pages 119-126, ISSN 0969-9961, https://doi.org/10.1016/j.nbd.2016.07.010.
[21]. Duman, Ronald S et al. “Role of BDNF in the pathophysiology and treatment of depression: Activity-dependent effects distinguish rapid-acting antidepressants.” The European journal of neuroscience vol. 53,1 (2021): 126-139. doi:10.1111/ejn.14630
[22]. Xie, ZM., Wang, XM., Xu, N. et al. Alterations in the inflammatory cytokines and brain-derived neurotrophic factor contribute to depression-like phenotype after spared nerve injury: improvement by ketamine. Sci Rep 7, 3124 (2017). https://doi.org/10.1038/s41598-017-03590-3
[23]. Ousman SS, and Kubes P (2012). Immune surveillance in the central nervous system. Nat. Neurosci. 15, 1096–1101. https://www.nature.com/articles/nn.3161
[24]. Ransohoff RM, and Engelhardt B (2012). The anatomical and cellular basis of immune surveillance in the central nervous system. Nat. Rev. Immunol. 12, 623–635. https://www.nature.com/articles/nri3265
[25]. Beurel, Eléonore et al. “The Bidirectional Relationship of Depression and Inflammation: Double Trouble.” Neuron vol. 107,2 (2020): 234-256. doi:10.1016/j.neuron.2020.06.002
[26]. Mazzitelli M, Palazzo E, Maione S and Neugebauer V (2018) Group II Metabotropic Glutamate Receptors: Role in Pain Mechanisms and Pain Modulation. Front. Mol. Neurosci. 11:383. doi: 10.3389/fnmol.2018.00383
[27]. Onaolapo, Adejoke Yetunde, and Olakunle James Onaolapo. “Glutamate and depression: Reflecting a deepening knowledge of the gut and brain effects of a ubiquitous molecule.” World journal of psychiatry vol. 11,7 297-315. 19 Jul. 2021, doi:10.5498/wjp.v11.i7.297
[28]. Moriguchi, S., Takamiya, A., Noda, Y. et al. Glutamatergic neurometabolite levels in major depressive disorder: a systematic review and meta-analysis of proton magnetic resonance spectroscopy studies. Mol Psychiatry 24, 952–964 (2019). https://doi.org/10.1038/s41380-018-0252-9
[29]. Gao, Peng et al. “Global, regional, and national trends in incidence of depression among women, 1990-2019: An analysis of the global burden of disease study.” Psychiatry research vol. 331 (2024): 115668. doi:10.1016/j.psychres.2023.115668
[30]. Hassan, Samah et al. “Ovarian hormones and chronic pain: A comprehensive review.” Pain vol. 155,12 (2014): 2448-2460. doi:10.1016/j.pain.2014.08.027
[31]. Vincent, Katy, and Irene Tracey. “Hormones and their Interaction with the Pain Experience.” Reviews in pain vol. 2,2 (2008): 20-4. doi:10.1177/204946370800200206
[32]. Athnaiel, Onella et al. “The Role of Sex Hormones in Pain-Related Conditions.” International journal of molecular sciences vol. 24,3 1866. 18 Jan. 2023, doi:10.3390/ijms24031866
[33]. Albert, Paul R. “Why is depression more prevalent in women?.” Journal of psychiatry & neuroscience : JPN vol. 40,4 (2015): 219-21. doi:10.1503/jpn.150205
[34]. Li, Wei et al. “Major Depressive Disorder and Kappa Opioid Receptor Antagonists.” Translational perioperative and pain medicine vol. 1,2 (2016): 4-16. Li, Wei et al. “Major Depressive Disorder and Kappa Opioid Receptor Antagonists.” Translational perioperative and pain medicine vol. 1,2 (2016): 4-16.
[35]. Hillhouse, Todd M, and Joseph H Porter. “A brief history of the development of antidepressant drugs: from monoamines to glutamate.” Experimental and clinical psychopharmacology vol. 23,1 (2015): 1-21. doi:10.1037/a0038550
[36]. Trullas, R, and P Skolnick. “Functional antagonists at the NMDA receptor complex exhibit antidepressant actions.” European journal of pharmacology vol. 185,1 (1990): 1-10. doi:10.1016/0014-2999(90)90204-j
[37]. Duman RS, Deyama S, Fogaça MV. Role of BDNF in the pathophysiology and treatment of depression: Activity-dependent effects distinguish rapid-acting antidepressants. Eur J Neurosci. 2021; 53: 126–139. https://doi.org/10.1111/ejn.14630
[38]. Zelada MI, Garrido V, Liberona A, Jones N, Zúñiga K, Silva H, Nieto RR. Brain-Derived Neurotrophic Factor (BDNF) as a Predictor of Treatment Response in Major Depressive Disorder (MDD): A Systematic Review. International Journal of Molecular Sciences. 2023; 24(19):14810. https://doi.org/10.3390/ijms241914810
[39]. Yishu Yin, Ting Ju, Deyong Zeng, Fangyuan Duan, Yuanbing Zhu, Junlian Liu, Yongzhi Li, Weihong Lu, “Inflamed” depression: A review of the interactions between depression and inflammation and current anti-inflammatory strategies for depression, Pharmacological Research, Volume 207, 2024, 107322, ISSN 1043-6618, https://doi.org/10.1016/j.phrs.2024.107322.
[40]. Poleshuck, Ellen L et al. “Interpersonal Psychotherapy for Co-occurring Depression and Chronic Pain.” Professional psychology, research and practice vol. 41,4 (2010): 312-318. doi:10.1037/a0019924
[41]. Allegri, M et al. “Acute and chronic pain: where we are and where we have to go.” Minerva anestesiologica vol. 78,2 (2012): 222-35.
[42]. Michael Costigan, Clifford J. Woolf, Pain: Molecular mechanisms, The Journal of Pain, Volume 1, Issue 3, Supplement, 2000, Pages 35-44, ISSN 1526-5900, https://doi.org/10.1054/jpai.2000.9818.
[43]. Basbaum, Allan I et al. “Cellular and molecular mechanisms of pain.” Cell vol. 139,2 (2009): 267-84. doi:10.1016/j.cell.2009.09.028
Cite this article
Su,Y. (2025). Investigating the Association Between Pain and Depression. Theoretical and Natural Science,82,172-181.
Data availability
The datasets used and/or analyzed during the current study will be available from the authors upon reasonable request.
Disclaimer/Publisher's Note
The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of EWA Publishing and/or the editor(s). EWA Publishing and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
About volume
Volume title: Proceedings of the 3rd International Conference on Modern Medicine and Global Health
© 2024 by the author(s). Licensee EWA Publishing, Oxford, UK. This article is an open access article distributed under the terms and
conditions of the Creative Commons Attribution (CC BY) license. Authors who
publish this series agree to the following terms:
1. Authors retain copyright and grant the series right of first publication with the work simultaneously licensed under a Creative Commons
Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this
series.
2. Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the series's published
version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial
publication in this series.
3. Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and
during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See
Open access policy for details).
References
[1]. Allan I. Basbaum, Diana M. Bautista, Grégory Scherrer, David Julius, Cellular and Molecular Mechanisms of Pain, Cell, Volume 139, Issue 2, 2009, Pages 267-284, ISSN 0092-8674, https://doi.org/10.1016/j.cell.2009.09.028.
[2]. Emmanuel Jesulola, Peter Micalos, Ian J. Baguley, Understanding the pathophysiology of depression: From monoamines to the neurogenesis hypothesis model - are we there yet?, Behavioural Brain Research, Volume 341, 2018, Pages 79-90, ISSN 0166-4328, https://doi.org/10.1016/j.bbr.2017.12.025.
[3]. Kendler, Kenneth S et al. “The impact of environmental experiences on symptoms of anxiety and depression across the life span.” Psychological science vol. 22,10 (2011): 1343-52. doi:10.1177/0956797611417255
[4]. Rautio, Nina et al. “Living environment and its relationship to depressive mood: A systematic review.” The International journal of social psychiatry vol. 64,1 (2018): 92-103. doi:10.1177/0020764017744582
[5]. https://www.who.int/news-room/fact-sheets/detail/depression
[6]. https://www.nimh.nih.gov/health/topics/depression
[7]. Anali Dadgostar, Mark Bigder, Nahid Punjani, Svjetlana Lozo, Vickramjit Chahal, Alexander Kavanagh, Does preoperative depression predict post-operative surgical pain: A systematic review, International Journal of Surgery, Volume 41, 2017, Pages 162-173, ISSN 1743-9191, https://doi.org/10.1016/j.ijsu.2017.03.061.
[8]. Liu, Q. R., Dai, Y. C., Ji, M. H., Liu, P. M., Dong, Y. Y., & Yang, J. J. (2024). Risk Factors for Acute Postsurgical Pain: A Narrative Review. Journal of Pain Research, 17, 1793–1804. https://doi.org/10.2147/JPR.S462112
[9]. Breivik, H. , Collett, B. , Ventafridda, V. , Cohen, R. , & Gallacher, D. (2006). Survey of chronic pain in Europe: Prevalence, impact on daily life, and treatment. Euro- pean Journal of Pain, 10 (4), 287 -287 .
[10]. Miller, L. R. , & Cano, A. (2009). Comorbid chronic pain and depression: who is at risk? The Journal of Pain, 10 (6), 619–627 .
[11]. Matt Fernandez, Lucia Colodro-Conde, Jan Hartvigsen, Manuela L. Ferreira, Kathryn M. Refshauge, Marina B. Pinheiro, Juan R. Ordoñana, Paulo H. Ferreira, Chronic low back pain and the risk of depression or anxiety symptoms: insights from a longitudinal twin study, The Spine Journal, Volume 17, Issue 7, 2017, Pages 905-912, ISSN 1529-9430, https://doi.org/10.1016/j.spinee.2017.02.009.
[12]. Nicholl, B.I., Mackay, D., Cullen, B. et al. Chronic multisite pain in major depression and bipolar disorder: cross-sectional study of 149,611 participants in UK Biobank. BMC Psychiatry 14, 350 (2014). https://doi.org/10.1186/s12888-014-0350-4
[13]. de Heer, Eric W et al. “The association of depression and anxiety with pain: a study from NESDA.” PloS one vol. 9,10 e106907. 15 Oct. 2014, doi:10.1371/journal.pone.0106907
[14]. A Feizerfan, G Sheh, Transition from acute to chronic pain, Continuing Education in Anaesthesia Critical Care & Pain, Volume 15, Issue 2, April 2015, Pages 98–102, https://doi.org/10.1093/bjaceaccp/mku044.
[15]. Cleare, Anthony et al. “Evidence-based guidelines for treating depressive disorders with antidepressants: A revision of the 2008 British Association for Psychopharmacology guidelines.” Journal of psychopharmacology (Oxford, England) vol. 29,5 (2015): 459-525. doi:10.1177/0269881115581093
[16]. Cipriani, Andrea et al. “Comparative efficacy and acceptability of 12 new-generation antidepressants: a multiple-treatments meta-analysis.” Lancet (London, England) vol. 373,9665 (2009): 746-58. doi:10.1016/S0140-6736(09)60046-5
[17]. Doboszewska, Urszula et al. “Zinc in the Monoaminergic Theory of Depression: Its Relationship to Neural Plasticity.” Neural plasticity vol. 2017 (2017): 3682752. doi:10.1155/2017/3682752
[18]. Sheng, Jiyao et al. “The Link between Depression and Chronic Pain: Neural Mechanisms in the Brain.” Neural plasticity vol. 2017 (2017): 9724371. doi:10.1155/2017/9724371
[19]. Bee, Lucy A, and Anthony H Dickenson. “The importance of the descending monoamine system for the pain experience and its treatment.” F1000 medicine reports vol. 1 83. 29 Oct. 2009, doi:10.3410/M1-83
[20]. Eero Castrén, Masami Kojima, Brain-derived neurotrophic factor in mood disorders and antidepressant treatments, Neurobiology of Disease, Volume 97, Part B, 2017, Pages 119-126, ISSN 0969-9961, https://doi.org/10.1016/j.nbd.2016.07.010.
[21]. Duman, Ronald S et al. “Role of BDNF in the pathophysiology and treatment of depression: Activity-dependent effects distinguish rapid-acting antidepressants.” The European journal of neuroscience vol. 53,1 (2021): 126-139. doi:10.1111/ejn.14630
[22]. Xie, ZM., Wang, XM., Xu, N. et al. Alterations in the inflammatory cytokines and brain-derived neurotrophic factor contribute to depression-like phenotype after spared nerve injury: improvement by ketamine. Sci Rep 7, 3124 (2017). https://doi.org/10.1038/s41598-017-03590-3
[23]. Ousman SS, and Kubes P (2012). Immune surveillance in the central nervous system. Nat. Neurosci. 15, 1096–1101. https://www.nature.com/articles/nn.3161
[24]. Ransohoff RM, and Engelhardt B (2012). The anatomical and cellular basis of immune surveillance in the central nervous system. Nat. Rev. Immunol. 12, 623–635. https://www.nature.com/articles/nri3265
[25]. Beurel, Eléonore et al. “The Bidirectional Relationship of Depression and Inflammation: Double Trouble.” Neuron vol. 107,2 (2020): 234-256. doi:10.1016/j.neuron.2020.06.002
[26]. Mazzitelli M, Palazzo E, Maione S and Neugebauer V (2018) Group II Metabotropic Glutamate Receptors: Role in Pain Mechanisms and Pain Modulation. Front. Mol. Neurosci. 11:383. doi: 10.3389/fnmol.2018.00383
[27]. Onaolapo, Adejoke Yetunde, and Olakunle James Onaolapo. “Glutamate and depression: Reflecting a deepening knowledge of the gut and brain effects of a ubiquitous molecule.” World journal of psychiatry vol. 11,7 297-315. 19 Jul. 2021, doi:10.5498/wjp.v11.i7.297
[28]. Moriguchi, S., Takamiya, A., Noda, Y. et al. Glutamatergic neurometabolite levels in major depressive disorder: a systematic review and meta-analysis of proton magnetic resonance spectroscopy studies. Mol Psychiatry 24, 952–964 (2019). https://doi.org/10.1038/s41380-018-0252-9
[29]. Gao, Peng et al. “Global, regional, and national trends in incidence of depression among women, 1990-2019: An analysis of the global burden of disease study.” Psychiatry research vol. 331 (2024): 115668. doi:10.1016/j.psychres.2023.115668
[30]. Hassan, Samah et al. “Ovarian hormones and chronic pain: A comprehensive review.” Pain vol. 155,12 (2014): 2448-2460. doi:10.1016/j.pain.2014.08.027
[31]. Vincent, Katy, and Irene Tracey. “Hormones and their Interaction with the Pain Experience.” Reviews in pain vol. 2,2 (2008): 20-4. doi:10.1177/204946370800200206
[32]. Athnaiel, Onella et al. “The Role of Sex Hormones in Pain-Related Conditions.” International journal of molecular sciences vol. 24,3 1866. 18 Jan. 2023, doi:10.3390/ijms24031866
[33]. Albert, Paul R. “Why is depression more prevalent in women?.” Journal of psychiatry & neuroscience : JPN vol. 40,4 (2015): 219-21. doi:10.1503/jpn.150205
[34]. Li, Wei et al. “Major Depressive Disorder and Kappa Opioid Receptor Antagonists.” Translational perioperative and pain medicine vol. 1,2 (2016): 4-16. Li, Wei et al. “Major Depressive Disorder and Kappa Opioid Receptor Antagonists.” Translational perioperative and pain medicine vol. 1,2 (2016): 4-16.
[35]. Hillhouse, Todd M, and Joseph H Porter. “A brief history of the development of antidepressant drugs: from monoamines to glutamate.” Experimental and clinical psychopharmacology vol. 23,1 (2015): 1-21. doi:10.1037/a0038550
[36]. Trullas, R, and P Skolnick. “Functional antagonists at the NMDA receptor complex exhibit antidepressant actions.” European journal of pharmacology vol. 185,1 (1990): 1-10. doi:10.1016/0014-2999(90)90204-j
[37]. Duman RS, Deyama S, Fogaça MV. Role of BDNF in the pathophysiology and treatment of depression: Activity-dependent effects distinguish rapid-acting antidepressants. Eur J Neurosci. 2021; 53: 126–139. https://doi.org/10.1111/ejn.14630
[38]. Zelada MI, Garrido V, Liberona A, Jones N, Zúñiga K, Silva H, Nieto RR. Brain-Derived Neurotrophic Factor (BDNF) as a Predictor of Treatment Response in Major Depressive Disorder (MDD): A Systematic Review. International Journal of Molecular Sciences. 2023; 24(19):14810. https://doi.org/10.3390/ijms241914810
[39]. Yishu Yin, Ting Ju, Deyong Zeng, Fangyuan Duan, Yuanbing Zhu, Junlian Liu, Yongzhi Li, Weihong Lu, “Inflamed” depression: A review of the interactions between depression and inflammation and current anti-inflammatory strategies for depression, Pharmacological Research, Volume 207, 2024, 107322, ISSN 1043-6618, https://doi.org/10.1016/j.phrs.2024.107322.
[40]. Poleshuck, Ellen L et al. “Interpersonal Psychotherapy for Co-occurring Depression and Chronic Pain.” Professional psychology, research and practice vol. 41,4 (2010): 312-318. doi:10.1037/a0019924
[41]. Allegri, M et al. “Acute and chronic pain: where we are and where we have to go.” Minerva anestesiologica vol. 78,2 (2012): 222-35.
[42]. Michael Costigan, Clifford J. Woolf, Pain: Molecular mechanisms, The Journal of Pain, Volume 1, Issue 3, Supplement, 2000, Pages 35-44, ISSN 1526-5900, https://doi.org/10.1054/jpai.2000.9818.
[43]. Basbaum, Allan I et al. “Cellular and molecular mechanisms of pain.” Cell vol. 139,2 (2009): 267-84. doi:10.1016/j.cell.2009.09.028









