1. Introduction
Menstrual health is a crucial aspect of women’s well-being [1]. With menstruation occurring over 400 times in a lifetime, it should be a natural process, not marked by constant pain. However, dysmenorrhea affects 45-95% of women globally [2], impacting daily life and mental health [3]. Dysmenorrhea is classified as primary (PD) or secondary (SD). PD refers to menstrual pain without underlying pelvic disease, while SD results from conditions such as endometriosis [4]. PD is primarily linked to excessive prostaglandins (PGs), which trigger strong uterine contractions and pain [5]. Understanding PG mechanisms can improve menstrual health and pain management.
Despite its prevalence, dysmenorrhea treatment remains limited [6]. Broad-spectrum painkillers, like NSAIDs, are the most used treatment, yet they are not specifically designed for dysmenorrhea [7][8][9][10]. While NSAIDs are the best initial therapy, about 18% of patients report minimal or no relief (NSAID-resistant dysmenorrhea) [11]. Additionally, long-term or high-dose use can cause gastrointestinal, cardiovascular, and organ complications [10][12]. These side effects highlight the need for more targeted, safer therapies for dysmenorrhea.
There has been little progress in developing effective dysmenorrhea treatments [2], reflecting a broader neglect of women's health issues. This research systematically reviews the biological mechanisms behind menstruation and dysmenorrhea, evaluates current treatments, and incorporates first-hand survey data to propose improved management strategies. By increasing public awareness and understanding, we aim to enhance solutions for dysmenorrhea and address unmet women's health needs.
2. What is the menstrual cycle?
The female reproductive system includes the vulva, vagina, uterus, fallopian tubes, and ovaries [13]. The endometrium, lining the uterus, plays a crucial role in the menstrual cycle, a 28-day process preparing the body for pregnancy [13].
The menstrual cycle depends on interactions between the uterus and ovaries. Each month, the ovaries prepare an egg under FSH and LH regulation, while estrogen and progesterone stimulate endometrial thickening for possible implantation [14]. If fertilization does not occur, the egg shrinks and is reabsorbed [14]. As estrogen and progesterone decline, the endometrium sheds, forming menstrual blood [14]. Uterine contractions assist in this process [13].
The endometrium undergoes three phases: proliferative, secretory, and menstrual [14]. Beyond supporting pregnancy, it prevents ectopic implantation, which could be harmful.
3. How PGs affect menstrual pain
Prostaglandins (PGs) regulate uterine contractions necessary for endometrial shedding [15]. However, excessive PG levels contribute to severe menstrual pain [16]. Three primary pathways explain PG-induced dysmenorrhea:
3.1. Pathway 1: Stimulating Uterine Contractions
During menstruation, prostaglandin F2α (PGF2α) and prostaglandin E2 (PGE2) are released as the endometrium sheds. At the luteal phase's end, decreased progesterone and estrogen upregulate collagenases, inflammatory cytokines, and MMPs [17]. MMPs break down the endometrium, releasing phospholipids converted into arachidonic acid by uterine phospholipases. Arachidonic acid is processed by COX-1 and COX-2 into PGH2, which serves as a precursor for prostacyclins, PGE2, PGF2α, and thromboxane A2 (TXA2) [9].
Each compound has distinct roles: TXA2 promotes vasoconstriction and platelet aggregation [18]; PGE2 induces inflammation and vasodilation, attracting immune cells [19]; PGF2α causes uterine constriction and pain [20]; and prostacyclin promotes vasodilation and inhibits platelet aggregation [19]. PGF2α and PGE2 elevate uterine tone and induce hypercontractility, overstimulating muscles and causing pain [21].
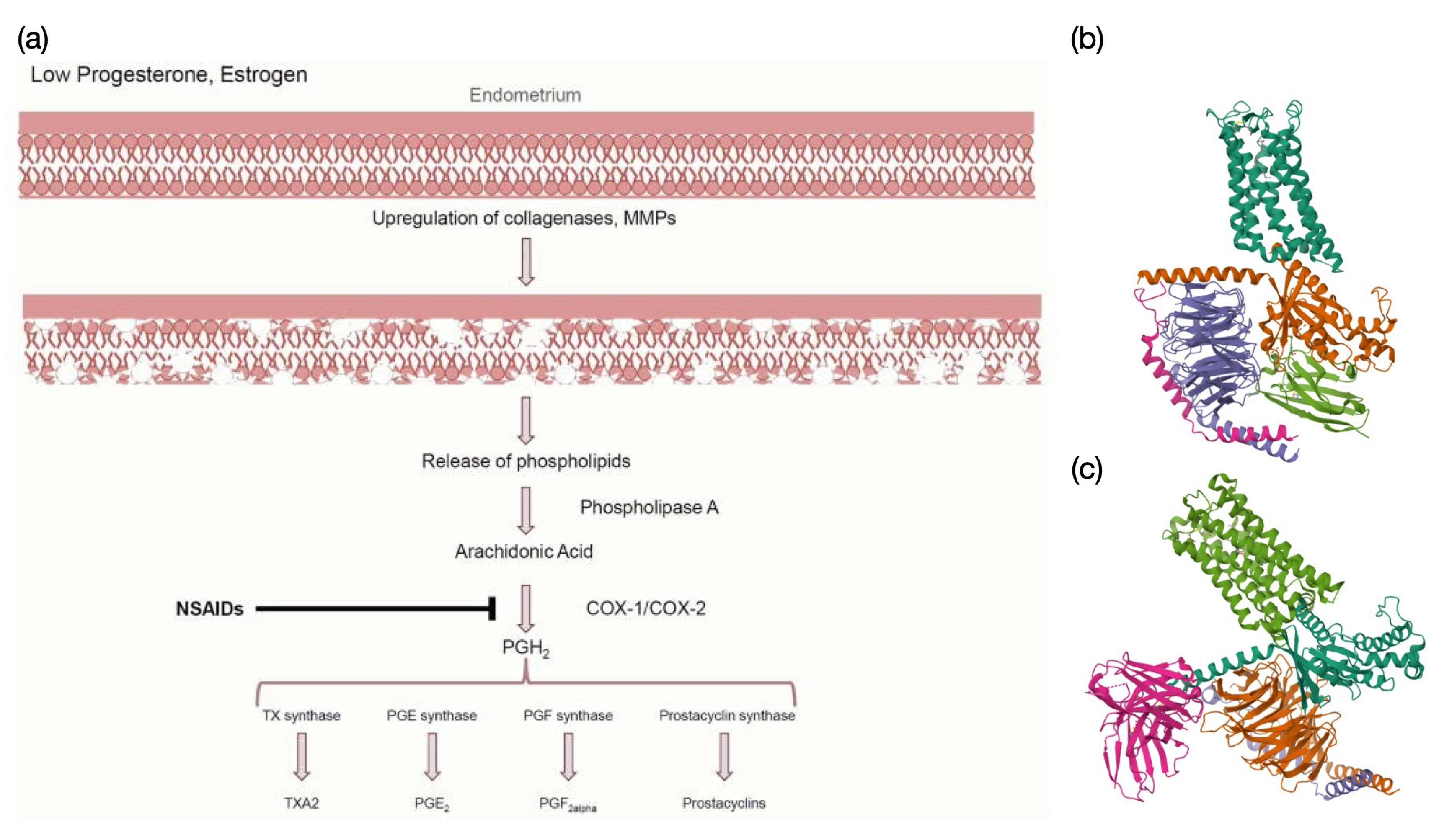
Figure 1: NSAID pathway and prostaglandin receptor structures.
(a) NSAID Pathway: Low progesterone and estrogen levels trigger phospholipid release, leading to arachidonic acid synthesis. COX-1/COX-2 enzymes convert arachidonic acid into PGH2, a precursor for various prostaglandins. NSAIDs inhibit COX enzymes, reducing prostaglandin synthesis and alleviating pain [9]. (b) PGE2-EP2 Receptor Complex: Cryo-EM structure of the PGE2-bound EP2-Gs complex (PDB: 7CX2), a GPCR mediating vasodilation and inflammation [22]. (c) PGF2α -FP Receptor Complex: Cryo-EM structure of the PGF2α -bound FP-Gq complex (PDB: 8XJL), a GPCR involved in smooth muscle contraction and uterine function [20].
3.2. Pathway 2: Reducing Uterine Blood Flow (Ischemia)
Prostaglandins induce vasoconstriction, narrowing blood vessels and causing ischemia in uterine muscles, reducing oxygen supply and leading to dysmenorrhea pain [23]. Since stronger muscle contractions require more oxygen, ischemia worsens fatigue and pain [23].
3.3. Pathway 3: Sensitizing Nerve Endings
Prostaglandins, especially PGF2α, heighten uterine nociceptor sensitivity by lowering the pain perception threshold, amplifying discomfort during contractions, even when contractions are not excessively strong [2].
4. Current Common Ways to Manage Menstrual Pain
Primary dysmenorrhea is managed through nonpharmacologic and pharmacologic methods.
Heat therapy is a preferred option with no side effects [24]. Studies comparing it to ibuprofen and acetaminophen confirm its effectiveness in pain relief [25]. However, for severe symptoms, heat therapy alone may not be sufficient [25]. Exercise and a balanced diet are also recommended, as evidence supports their role in reducing pain intensity [26][27]. Dietary adjustments, such as fish oil and vitamin B1 supplements, can help alleviate symptoms. Omega-3 fatty acids in fish oil inhibit prostaglandin synthesis, reducing pain [27]. Vitamin B1 influences nervous system activity and muscle contraction, improving pain perception and reducing uterine contractions [28]. However, while beneficial for long-term health, exercise and diet provide limited immediate relief.
The most common pharmacologic treatments for dysmenorrhea are NSAIDs and hormonal contraceptives. NSAIDs are the primary choice and have demonstrated effectiveness in placebo-controlled trials [9]. They reduce menstrual pain by inhibiting COX, thereby lowering PGH2 production from arachidonic acid [9]. Despite this, NSAIDs have several limitations:
1. Limited Effectiveness: About 20% of women report no relief from NSAIDs, as seen in a review of 51 clinical trials [9].
2. Partial Pain Relief: Many women find NSAIDs insufficient, alleviating some symptoms but not eliminating discomfort [9]. Additionally, the tendency to remain silent about menstrual pain complicates treatment outcomes [29].
3. Side Effects: As broad-spectrum drugs, NSAIDs can cause gastrointestinal, cardiovascular, liver, kidney, brain, and lung complications [10]. Developing dysmenorrhea-specific treatments may help minimize these risks.
4. Impact on Endometrial Shedding: NSAIDs reduce prostaglandin levels and uterine contractions, potentially affecting proper endometrial shedding [15].
Besides NSAIDs, hormonal contraceptives are another option for menstrual pain relief. However, they have significant side effects, including mood swings, depression, nausea, weight gain, and increased risk of blood clots [30]. Women with a family history or diagnosis of hormone-sensitive cancers are generally advised against using hormonal contraceptives due to their effects on estrogen levels and associated cancer risks [31].
While NSAIDs and hormonal contraceptives are widely used, their limitations highlight the need for more targeted and safer treatment options for dysmenorrhea.
5. New Drug Targets
Although NSAIDs provide relief, they remain ineffective for many patients [10][12]. More targeted therapeutic strategies are needed to better address dysmenorrhea. PGE2 and PGF2α are the prostaglandins most directly linked to dysmenorrhea [5]. Instead of inhibiting their upstream precursor PGH2, as NSAIDs do, directly blocking PGE2 and PGF2α may offer a more effective solution.
We propose two drug design approaches:
5. Low-Affinity Competitive Antagonists: Designing antagonists to compete with PGE2 and PGF2α for their receptors could regulate their activity without completely blocking them [9]. Because these prostaglandins are essential for endometrial shedding and immune functions, antagonists should have low binding affinity to prevent excessive receptor occupation. Slight structural modifications in antagonists may allow partial receptor binding, reducing their activity while maintaining necessary physiological functions (Figure 1, Panel b & c).
6. Selective Inhibition of PGE2 and PGF2α Production: Unlike NSAIDs, which broadly inhibit COX and reduce PGH2 synthesis, selectively targeting the synthesis of PGE2 and PGF2α may provide greater efficacy [9]. While this strategy could enhance dysmenorrhea treatment, it may also disrupt other prostaglandin-dependent physiological processes, requiring careful regulation.
Focusing on PGE2 and PGF2α could offer advantages over NSAIDs, especially for patients unresponsive to traditional treatments [11]. NSAIDs inhibit PGH2 but do not always proportionally reduce PGE2 and PGF2α, potentially leading to inadequate pain relief. A more targeted approach could improve efficacy while minimizing side effects associated with broad-spectrum NSAIDs [10].
In addition to pharmacologic innovations, we advocate integrating lifestyle modifications. Arachidonic acid, a precursor to PGs, is derived from dietary sources and stored in cell membranes [32]. Reducing dietary intake of arachidonic acid-rich foods may lower PG production. Exercise can also modulate PG synthesis by increasing progesterone levels during the luteal phase, reducing phospholipid release from cell membranes [26]. Improved blood circulation from regular exercise ensures adequate uterine blood supply, mitigating muscle contractions induced by ischemia and hypoxia [26].
While lifestyle adjustments may not provide immediate relief, they contribute to long-term symptom reduction, improving overall menstrual health. Further analysis of previous survey data supports a correlation between exercise and reduced menstrual pain severity (Figure 2) [24].
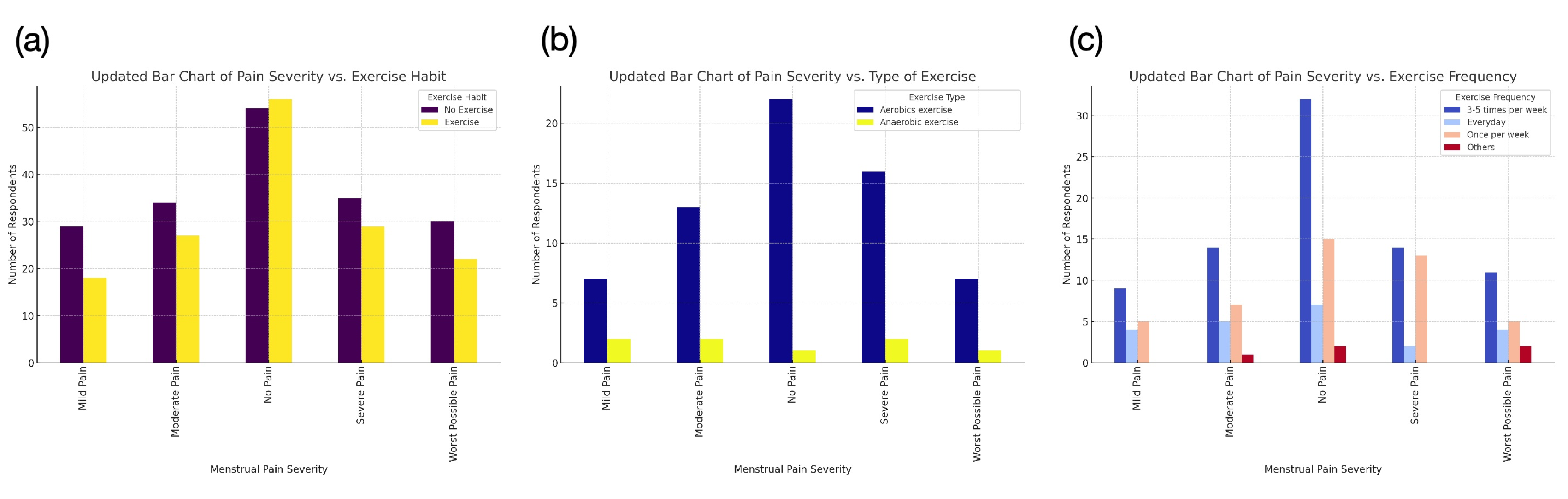
Figure 2: Correlation Between Menstrual Pain Severity and Exercise.
(a) Exercise Habit vs. Pain Severity: Individuals who exercise regularly report less severe pain, while severe pain groups have more non-exercisers, indicating a possible link between inactivity and higher pain severity. (b) Exercise Type vs. Pain Severity: Aerobic exercise is most common across all pain levels, with the "No Pain" group having the highest aerobic participation. Anaerobic exercise is less frequent and shows no strong correlation with pain levels. (c) Exercise Frequency vs. Pain Severity: Moderate, consistent exercise (3-5 times per week) is most common in the "No Pain" group, suggesting an association between regular exercise and better pain management.
6. Discussion
Dysmenorrhea affects a vast number of women, yet menstrual health remains underprioritized [24]. Research on menstruation is inadequate, and effective dysmenorrhea treatments are still limited.
During this study, I observed significant gaps in menstrual research. For example, there is no standardized definition for menstrual phases; different studies classify them inconsistently, sometimes into three phases and sometimes four, often without clear sources. While the menstrual cycle involves changes in both ovarian and endometrial phases, inconsistent terminology may mislead researchers and hinder progress. This reflects a broader issue: menstrual research lacks a structured framework, emphasizing the need for greater awareness and investment in this field.
This paper has two key objectives. First, to propose a new pharmacological target for dysmenorrhea treatment. While promising, this approach is not the only possibility, as alternative mechanisms need further exploration. Additionally, potential side effects must be addressed. Second, by introducing a new drug target, we hope to stimulate further discussions on menstrual health. Current dysmenorrhea treatments remain inadequate despite the strong demand for pain relief. Many women endure years of menstrual pain without sufficient options. Further analysis of my survey data shows that women, particularly those with severe pain, actively seek better treatments and are willing to try new solutions (Figure 3) [24]. We hope this paper encourages further research and broader therapeutic development. Given that this issue affects approximately 70% of women monthly, it deserves urgent attention [33].
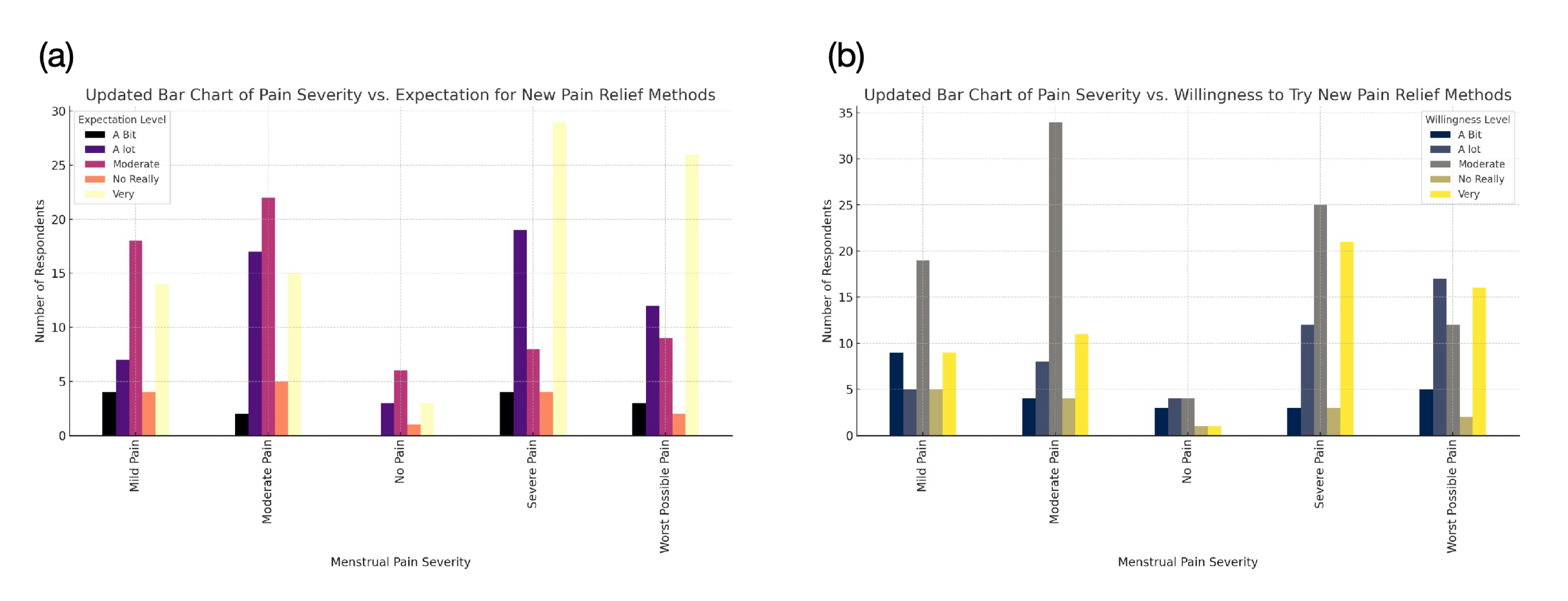
Figure 3: Correlation Between Menstrual Pain Severity and Attitudes Toward New Pain Relief Methods.
(a) Expectation for New Treatments: Individuals with severe pain show the highest expectation for new pain relief methods, while those with little to no pain exhibit lower demand. (b) Willingness to Try New Methods: Those experiencing severe pain are most open to trying new treatments, whereas individuals with little or no pain show the least interest. Each color represents a different expectation level, ranging from "No Really" (low expectation) to "Very" (high expectation).
Moreover, we urge women to advocate for better health solutions rather than silently endure pain. Dysmenorrhea's persistence is not a personal failing but rather a shortfall of existing treatment options. By speaking up, women can raise awareness and drive advancements in healthcare and research.
Addressing menstrual pain also requires an interdisciplinary approach. Pain is influenced by multiple factors [2][4][6]. While this paper explores biological treatments, integrating perspectives from neuroscience, psychology, biomedical engineering, and sociology can enhance understanding and solutions. A multidisciplinary approach will be crucial in advancing dysmenorrhea research and improving women’s health outcomes.
References
[1]. Hillard, P. J. A. (2014). Menstruation in adolescents: What's normal, what's not. Annals of the New York Academy of Sciences, 1308(1), 21–29. DOI: 10.1196/annals.1429.022
[2]. Iacovides, S., Avidon, I., & Baker, F. C. (2015). What we know about primary dysmenorrhea today: A critical review. Human Reproduction Update, 21(6), 762–778. DOI: 10.1093/humupd/dmv039
[3]. Chen, C. X., Draucker, C. B., & Carpenter, J. S. (2018). What women say about their dysmenorrhea: A qualitative thematic analysis. BMC Women's Health, 18, Article 47. DOI: 10.1186/s12905-018-0538-8
[4]. Balachandren, N., & Davies, L. (2023). Dysmenorrhea. StatPearls Publishing. Retrieved from https://www.ncbi.nlm.nih.gov/books/NBK560834/
[5]. Christensen, K. (2024). Dysmenorrhea: An update on primary healthcare management. Australian Journal of General Practice, 53(1–2). doi: 10.31128/AJGP/04-23-6815
[6]. Dawood, M. Y. (2006). Primary dysmenorrhea: Advances in pathogenesis and management. Obstetrics & Gynecology, 108(2), 428–441. DOI: 10.1097/01.AOG.0000230214.26638.0c
[7]. Tadesse, M., Kassa, A., Muluneh, A. A., & Altaye, G. (2021). Prevalence of dysmenorrhoea associated risk factors and its relationship with academic performance among graduating female university students in Ethiopia: a cross-sectional study. BMJ Open, 11(3), e043814. doi: 10.1136/bmjopen-2020-043814
[8]. Nie, W., Xu, P., Hao, C., Chen, Y., Yin, Y., & Wang, L. (2020). Efficacy and safety of over-the-counter analgesics for primary dysmenorrhea: A network meta-analysis. Medicine, 99(19), e19881. doi: 10.1097/MD.0000000000019881
[9]. Oladosu, F. A., Tu, F. F., & Hellman, K. M. (2018). Nonsteroidal antiinflammatory drug resistance in dysmenorrhea: Epidemiology, causes, and treatment. American Journal of Obstetrics and Gynecology, 218(4), 390–400. PMCID: PMC5839921 NIHMSID: NIHMS904572 PMID: 28888592
[10]. Bindu, S., Mazumder, S., & Bandyopadhyay, U. (2020). Non-steroidal anti-inflammatory drugs (NSAIDs) and organ damage: A current perspective. Biochem Pharmacol, 180, 114147. https://doi.org/10.1016/j.bcp.2020.114147
[11]. Owen, P. R. (1984). Prostaglandin synthetase inhibitors in the treatment of primary dysmenorrhea. American Journal of Obstetrics and Gynecology, 148(1), 96–103. DOI: 10.1016/s0002-9378(84)80039-3
[12]. Hijos-Mallada, G., Sostres, C., & Gomollón, F. (2022). NSAIDs, gastrointestinal toxicity and inflammatory bowel disease. Gastroenterol Hepatol, 45(3), 215-222. https://doi.org/10.1016/j.gastrohep.2021.06.003 (AINE, toxicidad gastrointestinal y enfermedad inflamatoria intestinal.)
[13]. Pepin, É., Dehboneh, S. S., Raguema, N., Esfandarani, M. T., & Lavoie, J. L. (2017). Role of the Renin-Angiotensin System in Healthy and Pathological Pregnancies. IntechOpen. DOI: 10.5772/66748
[14]. Haroun, H. S. W. (2016). Reproductive cycles in females. MOJ Womens Health, 2(2), 62–63. DOI: 10.15406/mojwh.2016.02.00028
[15]. Ylikorkala, O., & Dawood, M. Y. (1978). New concepts in dysmenorrhea. American Journal of Obstetrics and Gynecology, 130(7), 833–847. DOI: 10.1016/0002-9378(78)90019-4
[16]. Rosenwaks, Z., & Seegar-Jones, G. (1980). Menstrual pain: Its origin and pathogenesis. The Journal of Reproductive Medicine, 25(4 Suppl), 207–212. PMID: 7001019
[17]. Maia H, Maltez A, Studard E, Zausner B, Athayde C, Coutinho E. Effect of the menstrual cycle and oral contraceptives on cyclooxygenase-2 expression in the endometrium. Gynecol Endocrinol. 2005;21(1):57–61. doi: 10.1080/09513590500099602
[18]. Funk, C. D. (2023). Thromboxane A2. In StatPearls. StatPearls Publishing. Retrieved from https://www.ncbi.nlm.nih.gov/books/NBK539817/
[19]. Nakanishi, M., & Rosenberg, D. W. (2013). Multifaceted roles of PGE₂in inflammation and cancer. Seminars in Immunopathology, 35(2), 123–137. PMCID: PMC3568185 NIHMSID: NIHMS409312 PMID: 22996682
[20]. Li, X., Zhang, X., Wen, X., Zhang, D., Qu, C., Miao, X., Zhang, W., Zhang, R., Liu, G., Xiao, P., Sun, J.-P., & Gong, W. (2024). Structural basis for ligand recognition and activation of the prostanoid receptors. Cell Reports, 43(3), 113893. https://doi.org/10.1016/j.celrep.2024.113893
[21]. Word, R. A., Kamm, K. E., & Casey, M. L. (1992). Contractile effects of prostaglandins, oxytocin, and endothelin-1 in human myometrium in vitro: Refractoriness of myometrial tissue of pregnant women to prostaglandins E2 and F2 alpha. The Journal of Clinical Endocrinology & Metabolism, 75(4), 1027–1032. DOI: 10.1210/jcem.75.4.1400867
[22]. Qu, C., et al. (2021). Ligand recognition, unconventional activation, and G protein coupling of the prostaglandin E2 receptor EP2 subtype. Science Advances, 7, eabf1268. https://doi.org/10.1126/sciadv.abf1268
[23]. Dawood, M. Y. (1981). Dysmenorrhoea and prostaglandins: pharmacological and therapeutic considerations. Drugs, 22(1), 42–56. DOI: 10.2165/00003495-198122010-00003
[24]. Zhang, R. (2024). Breaking Myths and Improving Awareness: Insights from a Survey on Menstrual Pain and Health. Theoretical and Natural Science (TNS), 61, 163–172. DOI: 10.54254/2753-8818/61/20241513
[25]. Akin, M. D., Price, W., Rodriguez, G. Jr., Erasala, G., Hurley, G., & Smith, R. P. (2004). Continuous, low-level, topical heat wrap therapy as compared to acetaminophen for primary dysmenorrhea. Journal of Reproductive Medicine, 49(9), 739–745. PMID: 15493566
[26]. Matthewman, G., Lee, A., Kaur, J. G., & Daley, A. J. (2018). Physical activity for primary dysmenorrhea: A systematic review and meta-analysis of randomized controlled trials. American Journal of Obstetrics and Gynecology, 219(3), 255.e1–255.e20. DOI: 10.1016/j.ajog.2018.04.001
[27]. Hosseinlou, A., Alinejad, V., Alinejad, M., & Aghakhani, N. (2014). The effects of fish oil capsules and vitamin B1 tablets on duration and severity of dysmenorrhea in students of high school in Urmia-Iran. Global Journal of Health Science, 6(7 Spec No), 124–129. doi: 10.5539/gjhs.v6n7p124
[28]. Matsas, A., Sachinidis, A., Lamprinou, M., Stamoula, E., & Christopoulos, P. (2023). Vitamin effects in primary dysmenorrhea. Life, 13(6), 1308. https://doi.org/10.3390/life13061308
[29]. Wiggleton-Little, J. (2024). "Just" a painful period: A philosophical perspective review of the dismissal of menstrual pain. Women's Health, 20, 17455057241255646. PMCID: PMC11113068 PMID: 38773901
[30]. Mukanga, B., Mwila, N., Nyirenda, H. T., & Daka, V. (2023). Perspectives on the side effects of hormonal contraceptives among women of reproductive age in Kitwe district of Zambia: A qualitative explorative study. BMC Women's Health, 23, Article 436. PMCID: PMC10439553 PMID: 37596577
[31]. Torres-de la Roche, L. A., Acevedo-Mesa, A., Lizarazo, I. L., Devassy, R., Becker, S., Krentel, H., & De Wilde, R. L. (2023). Hormonal contraception and the risk of breast cancer in women of reproductive age: A meta-analysis. Cancers, 15(23), 5624. PMCID: PMC10705112 PMID: 38067328
[32]. Smith, R. P. (2018). The role of prostaglandins in dysmenorrhea and menorrhagia. In Dysmenorrhea and Menorrhagia (pp. 75–88). Springer. Retrieved from https://link.springer.com/chapter/10.1007/978-3-319-71964-1_6
[33]. Ju, H., Jones, M., & Mishra, G. (2014). The prevalence and risk factors of dysmenorrhea. Epidemiologic Reviews, 36(1), 104–113. DOI: 10.1093/epirev/mxt009
Cite this article
Zhang,R. (2025). Mechanisms and Management of Dysmenorrhea: Current Understanding and Novel Therapeutic Directions. Theoretical and Natural Science,99,28-35.
Data availability
The datasets used and/or analyzed during the current study will be available from the authors upon reasonable request.
Disclaimer/Publisher's Note
The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of EWA Publishing and/or the editor(s). EWA Publishing and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
About volume
Volume title: Proceedings of the 5th International Conference on Biological Engineering and Medical Science
© 2024 by the author(s). Licensee EWA Publishing, Oxford, UK. This article is an open access article distributed under the terms and
conditions of the Creative Commons Attribution (CC BY) license. Authors who
publish this series agree to the following terms:
1. Authors retain copyright and grant the series right of first publication with the work simultaneously licensed under a Creative Commons
Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this
series.
2. Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the series's published
version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial
publication in this series.
3. Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and
during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See
Open access policy for details).
References
[1]. Hillard, P. J. A. (2014). Menstruation in adolescents: What's normal, what's not. Annals of the New York Academy of Sciences, 1308(1), 21–29. DOI: 10.1196/annals.1429.022
[2]. Iacovides, S., Avidon, I., & Baker, F. C. (2015). What we know about primary dysmenorrhea today: A critical review. Human Reproduction Update, 21(6), 762–778. DOI: 10.1093/humupd/dmv039
[3]. Chen, C. X., Draucker, C. B., & Carpenter, J. S. (2018). What women say about their dysmenorrhea: A qualitative thematic analysis. BMC Women's Health, 18, Article 47. DOI: 10.1186/s12905-018-0538-8
[4]. Balachandren, N., & Davies, L. (2023). Dysmenorrhea. StatPearls Publishing. Retrieved from https://www.ncbi.nlm.nih.gov/books/NBK560834/
[5]. Christensen, K. (2024). Dysmenorrhea: An update on primary healthcare management. Australian Journal of General Practice, 53(1–2). doi: 10.31128/AJGP/04-23-6815
[6]. Dawood, M. Y. (2006). Primary dysmenorrhea: Advances in pathogenesis and management. Obstetrics & Gynecology, 108(2), 428–441. DOI: 10.1097/01.AOG.0000230214.26638.0c
[7]. Tadesse, M., Kassa, A., Muluneh, A. A., & Altaye, G. (2021). Prevalence of dysmenorrhoea associated risk factors and its relationship with academic performance among graduating female university students in Ethiopia: a cross-sectional study. BMJ Open, 11(3), e043814. doi: 10.1136/bmjopen-2020-043814
[8]. Nie, W., Xu, P., Hao, C., Chen, Y., Yin, Y., & Wang, L. (2020). Efficacy and safety of over-the-counter analgesics for primary dysmenorrhea: A network meta-analysis. Medicine, 99(19), e19881. doi: 10.1097/MD.0000000000019881
[9]. Oladosu, F. A., Tu, F. F., & Hellman, K. M. (2018). Nonsteroidal antiinflammatory drug resistance in dysmenorrhea: Epidemiology, causes, and treatment. American Journal of Obstetrics and Gynecology, 218(4), 390–400. PMCID: PMC5839921 NIHMSID: NIHMS904572 PMID: 28888592
[10]. Bindu, S., Mazumder, S., & Bandyopadhyay, U. (2020). Non-steroidal anti-inflammatory drugs (NSAIDs) and organ damage: A current perspective. Biochem Pharmacol, 180, 114147. https://doi.org/10.1016/j.bcp.2020.114147
[11]. Owen, P. R. (1984). Prostaglandin synthetase inhibitors in the treatment of primary dysmenorrhea. American Journal of Obstetrics and Gynecology, 148(1), 96–103. DOI: 10.1016/s0002-9378(84)80039-3
[12]. Hijos-Mallada, G., Sostres, C., & Gomollón, F. (2022). NSAIDs, gastrointestinal toxicity and inflammatory bowel disease. Gastroenterol Hepatol, 45(3), 215-222. https://doi.org/10.1016/j.gastrohep.2021.06.003 (AINE, toxicidad gastrointestinal y enfermedad inflamatoria intestinal.)
[13]. Pepin, É., Dehboneh, S. S., Raguema, N., Esfandarani, M. T., & Lavoie, J. L. (2017). Role of the Renin-Angiotensin System in Healthy and Pathological Pregnancies. IntechOpen. DOI: 10.5772/66748
[14]. Haroun, H. S. W. (2016). Reproductive cycles in females. MOJ Womens Health, 2(2), 62–63. DOI: 10.15406/mojwh.2016.02.00028
[15]. Ylikorkala, O., & Dawood, M. Y. (1978). New concepts in dysmenorrhea. American Journal of Obstetrics and Gynecology, 130(7), 833–847. DOI: 10.1016/0002-9378(78)90019-4
[16]. Rosenwaks, Z., & Seegar-Jones, G. (1980). Menstrual pain: Its origin and pathogenesis. The Journal of Reproductive Medicine, 25(4 Suppl), 207–212. PMID: 7001019
[17]. Maia H, Maltez A, Studard E, Zausner B, Athayde C, Coutinho E. Effect of the menstrual cycle and oral contraceptives on cyclooxygenase-2 expression in the endometrium. Gynecol Endocrinol. 2005;21(1):57–61. doi: 10.1080/09513590500099602
[18]. Funk, C. D. (2023). Thromboxane A2. In StatPearls. StatPearls Publishing. Retrieved from https://www.ncbi.nlm.nih.gov/books/NBK539817/
[19]. Nakanishi, M., & Rosenberg, D. W. (2013). Multifaceted roles of PGE₂in inflammation and cancer. Seminars in Immunopathology, 35(2), 123–137. PMCID: PMC3568185 NIHMSID: NIHMS409312 PMID: 22996682
[20]. Li, X., Zhang, X., Wen, X., Zhang, D., Qu, C., Miao, X., Zhang, W., Zhang, R., Liu, G., Xiao, P., Sun, J.-P., & Gong, W. (2024). Structural basis for ligand recognition and activation of the prostanoid receptors. Cell Reports, 43(3), 113893. https://doi.org/10.1016/j.celrep.2024.113893
[21]. Word, R. A., Kamm, K. E., & Casey, M. L. (1992). Contractile effects of prostaglandins, oxytocin, and endothelin-1 in human myometrium in vitro: Refractoriness of myometrial tissue of pregnant women to prostaglandins E2 and F2 alpha. The Journal of Clinical Endocrinology & Metabolism, 75(4), 1027–1032. DOI: 10.1210/jcem.75.4.1400867
[22]. Qu, C., et al. (2021). Ligand recognition, unconventional activation, and G protein coupling of the prostaglandin E2 receptor EP2 subtype. Science Advances, 7, eabf1268. https://doi.org/10.1126/sciadv.abf1268
[23]. Dawood, M. Y. (1981). Dysmenorrhoea and prostaglandins: pharmacological and therapeutic considerations. Drugs, 22(1), 42–56. DOI: 10.2165/00003495-198122010-00003
[24]. Zhang, R. (2024). Breaking Myths and Improving Awareness: Insights from a Survey on Menstrual Pain and Health. Theoretical and Natural Science (TNS), 61, 163–172. DOI: 10.54254/2753-8818/61/20241513
[25]. Akin, M. D., Price, W., Rodriguez, G. Jr., Erasala, G., Hurley, G., & Smith, R. P. (2004). Continuous, low-level, topical heat wrap therapy as compared to acetaminophen for primary dysmenorrhea. Journal of Reproductive Medicine, 49(9), 739–745. PMID: 15493566
[26]. Matthewman, G., Lee, A., Kaur, J. G., & Daley, A. J. (2018). Physical activity for primary dysmenorrhea: A systematic review and meta-analysis of randomized controlled trials. American Journal of Obstetrics and Gynecology, 219(3), 255.e1–255.e20. DOI: 10.1016/j.ajog.2018.04.001
[27]. Hosseinlou, A., Alinejad, V., Alinejad, M., & Aghakhani, N. (2014). The effects of fish oil capsules and vitamin B1 tablets on duration and severity of dysmenorrhea in students of high school in Urmia-Iran. Global Journal of Health Science, 6(7 Spec No), 124–129. doi: 10.5539/gjhs.v6n7p124
[28]. Matsas, A., Sachinidis, A., Lamprinou, M., Stamoula, E., & Christopoulos, P. (2023). Vitamin effects in primary dysmenorrhea. Life, 13(6), 1308. https://doi.org/10.3390/life13061308
[29]. Wiggleton-Little, J. (2024). "Just" a painful period: A philosophical perspective review of the dismissal of menstrual pain. Women's Health, 20, 17455057241255646. PMCID: PMC11113068 PMID: 38773901
[30]. Mukanga, B., Mwila, N., Nyirenda, H. T., & Daka, V. (2023). Perspectives on the side effects of hormonal contraceptives among women of reproductive age in Kitwe district of Zambia: A qualitative explorative study. BMC Women's Health, 23, Article 436. PMCID: PMC10439553 PMID: 37596577
[31]. Torres-de la Roche, L. A., Acevedo-Mesa, A., Lizarazo, I. L., Devassy, R., Becker, S., Krentel, H., & De Wilde, R. L. (2023). Hormonal contraception and the risk of breast cancer in women of reproductive age: A meta-analysis. Cancers, 15(23), 5624. PMCID: PMC10705112 PMID: 38067328
[32]. Smith, R. P. (2018). The role of prostaglandins in dysmenorrhea and menorrhagia. In Dysmenorrhea and Menorrhagia (pp. 75–88). Springer. Retrieved from https://link.springer.com/chapter/10.1007/978-3-319-71964-1_6
[33]. Ju, H., Jones, M., & Mishra, G. (2014). The prevalence and risk factors of dysmenorrhea. Epidemiologic Reviews, 36(1), 104–113. DOI: 10.1093/epirev/mxt009









