1. Introduction
Rabies is an ancient disease [1]. Among them, RABV is the most widespread, has the widest range of hosts, and has the greatest impact on humans [2]. Rabies is an acute zoonotic infectious disease with a fatality rate of 100% [3]. Patients usually die within a week after the onset of the disease [3]. The rabies virus belongs to the Rhabdoviridae family, Lyssavirus genus, and serotype I virus. It is approximately 200 nanometers in length and 70 nanometers in diameter. The rabies virus has an envelope composed of a lipid bilayer outer membrane and a matrix protein M [4]. Currently, the rabies virus can be classified into 17 types. Based on genetic composition and serological cross-reactions, it can be divided into three genetic lineages. Globally, except for Japan, Iceland, and the United Kingdom, rabies virus exists in all other countries. According to the World Health Organization, approximately 60,000 people die from rabies each year, still causing serious global public health problems [3]. About 95% of these cases occur in Asia and Africa, resulting in an economic loss of about $8.6 billion a year [3]. Rabies is mainly transmitted to humans through bites or scratches from virus-carrying animals. The virus can also infect through wounds [5]. For thousands of years, people have tried to treat rabies with the saliva, blood and internal organs of animals, but without the expected results. It was not until the 19th century that the French microbiologist Louis Pasteur conducted a series of meticulous studies and finally developed the Pasteur inactivated vaccine.
When this vaccine was injected into experimental animals, it produced an induced effect. The Pasteur inactivated vaccine also became the world's first approved rabies vaccine [1]. Once humans are infected, one type of spread is centripetal, where the virus enters the peripheral nerves from the entry site through neuromuscular junctions or nerve receptors and travels up the spinal cord to the central nervous system (CNS), causing encephalomyelitis. The other type of spread is centrifugal, where the virus spreads from the CNS to the peripheral nervous system, especially highly innervated organs, ultimately leading to the failure of the entire nervous system and death [4]. The initial clinical manifestations of rabies are often atypical. In the early stage, patients may present with non-specific symptoms such as fever, chest tightness, numbness in limbs, and general discomfort. When the disease progresses to the later stage, symptoms such as mania, excitement, hydrophobia, fear of wind, nausea, vomiting, diarrhea, consciousness disorders, and convulsions will occur [6]. Once bitten by an animal, it is necessary to handle the wound as soon as possible and get vaccinated against rabies at the nearest medical institution within 24 hours. It may cause you to miss the best treatment time [7, 8]. For patients in need of post-exposure prophylaxis, the affected area should be washed immediately with soap and plenty of water for at least 15 minutes and then disinfected with iodophor or alcohol. The patient needs to be vaccinated against rabies as soon as possible, usually with 4 or 5 doses, administered on days 0, 3, 7, 14 (or 28). For those with severe exposure, rabies immunoglobulin (RIG) should be injected around the wound to provide short-term protection. For pre-exposure prophylaxis, patients need to receive 3 doses of rabies vaccine, administered on days 0, 7, and 21 or 28.
In terms of clinical treatment, once the disease occurs, the treatment mainly focuses on supportive care, and there is no specific therapy available. In recent years, with the rapid development of biotechnology, immunology and other fields, significant breakthroughs have been made in the research of new rabies vaccines. And a variety of vaccines have been developed [3]. Regarding the current research status of rabies monoclonal antibody preparations, with the continuous advancement of related studies, some new antigenic epitope regions have been discovered successively. They can be classified as epitope IV and G5 regions, but it remains challenging, suggesting that the classification of epitopes I to III needs to be updated. Rabies is preventable but not curable. It is necessary to increase the immunization rate and the rate of tethering of animals. When bitten by an animal, patients can receive proper treatment and be promptly injected with rabies vaccine and rabies immunoglobulin [6]. With the development of science and technology and the increasing demand for public health, the rabies vaccine market is expected to embrace unprecedented opportunities and lay the foundation for the global elimination of rabies in the future. This article elucidates the pathogenesis of rabies through an examination of the initial viral invasion, its manifestations within the nervous system, clinical presentations, and the pathways of viral transmission.
2. Pathogenesis of rabies
2.1. Virus invasion and initial infection
Rabies virus belongs to the Rhabdoviridae family and the genus Lyssavirus, and it's a type I serum virus. As shown in Figure 1, a complete rabies virus is bullet-shaped [9], about 200 nanometers long and 70 nanometers in diameter [4]. In nature, the rabies virus can infect various warm-blooded means. Among them, canine animals play a very important role in the transmission of human rabies [8].
The virus binds to peripheral nerve terminals via the nicotinic acetylcholine receptor (nAChR) or the nerve growth factor receptor (p75NTR) at the neuromuscular junction. Following the fusion of the viral envelope glycoprotein (G protein) with the host cell membrane, the nucleocapsid is released into the axon. This invasion process exhibits high selectivity, as the virus propagates exclusively along neural pathways without significant dissemination through the bloodstream. Upon entering the peripheral nerves, the virus undergoes retrograde axonal transport at a rate of 5-100 mm per day, a process dependent on the microtubule network and the active transport mechanism mediated by dynein. During this phase, the virus can temporarily evade immune surveillance as it does not expose surface antigens while propagating within the nerves. Upon reaching the dorsal root ganglia of the spinal cord, the virus initiates exponential replication and rapidly disseminates into the CNS. A critical turning point in the initial infection occurs when the virus breaches the blood-brain barrier and enters the brain parenchyma. At this stage, conformational changes in the viral glycoproteins enhance their binding affinity to CNS cells. The massive viral replication within the brain leads to neuronal dysfunction, manifesting the characteristic clinical symptoms. Notably, infected individuals remain non-contagious until the virus reaches the salivary glands.
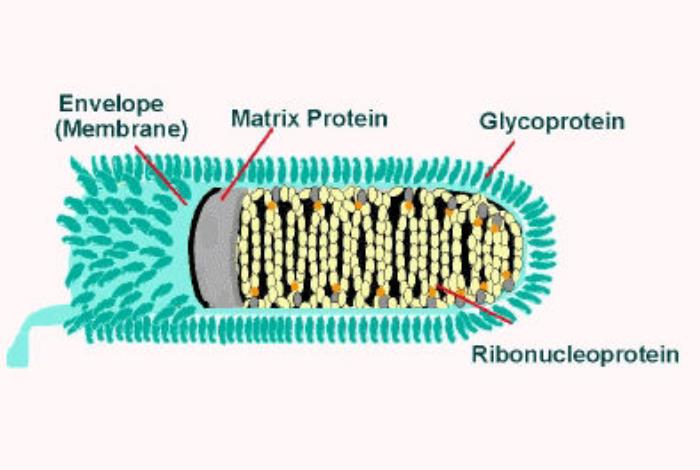
Figure 1: The structure of rabies virus [9].
The duration of the virus's incubation period is determined by the distance between the wound site and the CNS, with facial bites averaging 30 days, while distal extremity bites may exceed 90 days. This phase represents the final opportunity for post-exposure prophylaxis (PEP), as once the virus establishes itself in the CNS, the mortality rate approaches 100%. This unique invasion pathway explains why prompt wound cleansing and vaccination can effectively interrupt the infection process.
2.2. Transmission of the viruses in the nervous system
Upon invasion of sensory or motor nerve endings through specific receptors at the neuromuscular junction (e.g., nAChR), the viral nucleocapsid undergoes retrograde axonal transport toward the spinal cord at a rate of approximately 5-100 mm/day. This process is dependent on the host cell's microtubule network and the active transport system mediated by cytoplasmic dynein. During this phase, the viral genome remains in a quiescent state, avoiding the expression of viral proteins within the axon, thereby evading local immune surveillance. The viral particles are transported as intact nucleocapsids within the axon, rather than through intercellular spread, a unique strategy that ensures stable progression along a single neural pathway.
Upon reaching the dorsal root ganglia of the spinal cord, the virus initiates exponential replication and breaches the blood-brain barrier. The virus first infects spinal neurons, then spreads to the brainstem, cerebellum, and limbic system via anterograde/retrograde transport. Within the CNS, the virus employs a "leap-frog" mode of transmission. It propagates continuously along nerve fibers while simultaneously achieving transneuronal infection through synaptic connections. Regions such as the basal ganglia, hippocampus, and pons, which are rich in acetylcholine receptors, become primary targets. At this stage, conformational changes in viral glycoproteins enhance their ability to fuse with neuronal membranes, leading to neuronal vacuolation and the disappearance of Nissl bodies.
Following replication in the CNS, the virus spreads to peripheral organs via anterograde axonal transport through the autonomic nervous system. Infection of the dorsal nucleus of the vagus nerve facilitates viral invasion of the salivary glands via the glossopharyngeal and vagus nerves, where it replicates extensively in acinar epithelial cells and is released into saliva—a critical step in inter-animal transmission. Concurrently, the virus can invade the cornea via the trigeminal nerve and spread to organs such as the adrenal glands via the sacral nerves. This centrifugal dissemination occurs 1-5 days before the onset of clinical symptoms, rendering the host infectious prior to death. Throughout the transmission process, the virus strictly relies on the physiological mechanisms of the host's nervous system.
2.3. Viral invasion of the CNS
It is related to the pathogenesis of glycoprotein, apoptosis, infection and immunity, replication and transcription [4]. The invasion of the rabies virus into the CNS constitutes the pivotal stage in the progression of its lethality, demonstrating unique neurotropism and highly evolved invasion strategies. Upon breaching the blood-brain barrier (BBB), the virus achieves systemic infection of the brain parenchyma through multi-layered molecular mechanisms. Following initial amplification in the dorsal root ganglia of the spinal cord, the virus infiltrates the CNS via two parallel pathways. In the transcellular pathway, viral particles directly infect cerebrovascular endothelial cells, utilizing their endocytic function to penetrate the BBB. The viral glycoprotein (G protein) binds to neural cell adhesion molecules (NCAM) or low-density lipoprotein receptors (LDLR) on the endothelial cell surface, triggering membrane fusion. In the neural pathway, the virus is retrogradely transported along ascending spinal tracts (such as the spinothalamic tract) to the medulla oblongata, bypassing the physical barrier of the BBB. During this process, the virus enhances its fusion efficiency with neuronal membranes by altering the pH-dependent conformation of the G protein (from the pre-fusion to the fusion state). The disruption of the BBB is bidirectional: on one hand, the virus directly damages the tight junctions of endothelial cells; on the other hand, pro-inflammatory factors (such as TNF-α and IL-6) released by infected neurons increase BBB permeability, accelerating viral penetration into the brain parenchyma. Upon entering the brain tissue, the virus preferentially infects neural sources in specific functional regions. Early infection of brainstem nuclei (such as the dorsal vagal nucleus and hypoglossal nucleus) leads to autonomic dysfunction, resulting in hydrophobia and spasms of the swallowing muscles. Invasion of the limbic system (hippocampus and amygdala) causes emotional and behavioral abnormalities, as well as increased aggression. Damage to cerebellar Purkinje cells results in ataxia, while infection of the basal ganglia induces motor control disorders. This regional selectivity is determined by the differential distribution of neuronal surface receptors, where the G protein interacts with metabotropic glutamate receptors on central neurons.
2.4. Clinical manifestation
The clinical manifestations of rabies directly reflect its neuroinvasiveness and immune evasion capabilities, with disease progression exhibiting a highly predictable and fatal course. From the incubation period to the terminal stage, the evolution of symptoms is closely associated with the viral dissemination pathway within the CNS, with the typical disease course divisible into distinct phases. During the incubation period (2-9 days), the duration is directly correlated with the distance from the site of viral entry to the CNS. In cases of head and facial bites, the virus rapidly reaches the brainstem. This phase is asymptomatic, yet the virus undergoes retrograde transport within peripheral nerves, representing the final window for post-exposure prophylaxis (PEP). The prodromal phase (2-10 days) marks the initial viral breach of the blood-brain barrier, characterized by nonspecific symptoms and increased neuronal excitability. Localized abnormalities manifest in 80% of patients, with radiating pain, numbness, or pruritus at the original wound site, indicating dorsal root ganglion infection. Systemic symptoms include low-grade fever (37.8-38.5°C), fatigue, and headache, resembling influenza-like illness. Neurological precursors include anxiety, insomnia, and irritability, potentially accompanied by nocturnal nightmares or hallucinations. This phase is frequently misdiagnosed as anxiety disorder or viral infection, though abnormal wound sensations provide diagnostic clues. The acute neurologic phase (2-7 days) involves widespread viral dissemination throughout the CNS, with clinical presentations divided into two types: encephalitic (furious, 80% of cases) and paralytic. The encephalitic form features autonomic hyperactivity, including sympathetic overactivation (mydriasis, tachycardia, hypersalivation with daily saliva production up to 1L) and thermoregulatory dysfunction (intermittent high fever 39-40°C alternating with abrupt temperature drops). Pathognomonic symptoms include hydrophobia, where swallowing triggers intense pain due to pharyngeal muscle spasms, causing throat spasms and respiratory distress upon sight, sound, or even imagination of water, and aerophobia, where facial airflow stimulation induces neck rigidity and choking sensations.
2.5. Host immune response and immune escape
After RABV infection, the host's innate immune system gets activated first. Pattern recognition receptors (like toll-like receptors) recognize the viral RNA, triggering the production of interferons (IFNs) and other cytokines to inhibit viral replication. RABV's tropism for neural tissue limits the infiltration of immune cells, making the innate immune response have limited effects in the early stage, and then the adaptive immune response kicks in. B cells produce specific antibodies that can neutralize free viruses and stop their spread; T cells clear infected cells through cytotoxic effects. However, since the virus mainly exists in the immune-privileged CNS, the role of the adaptive immune response is restricted. RABV evades the immune system's attack through various strategies. First off, the virus spreads through synapses between neurons, avoiding exposure to the extracellular environment and thus evading antibody neutralization. Second, proteins encoded by RABV (such as the P protein) can inhibit the interferon signaling pathway, weakening the host's antiviral response. Besides, the low replication rate and concealment of the virus in the CNS further reduce the recognition efficiency of the immune system. These mechanisms work together, allowing RABV to keep spreading in the host and ultimately causing a fatal infection [10].
2.6. The virus spreads to peripheral tissues
RABV gets into the host's subcutaneous tissue or muscles through the saliva of infected animals. The virus particles bind to specific receptors on local nerve endings (like the neural cell adhesion molecule NCAM and p75 neurotrophin receptor) and enter the peripheral neurons. In the early stage of infection, the virus replicates at a low level in the local tissue. There usually aren't any obvious clinical symptoms during this stage, but the virus has already started to spread to the surrounding nerves. RABV spreads along the peripheral nerves to the spinal cord and brain through the retrograde axonal transport mechanism. The virus uses the neuron's microtubule network and motor proteins (such as dynein) for transportation, and this process is relatively slow, usually taking several days to weeks. During this time, the virus can spread to adjacent ganglia and nerve fibers, but it hasn't caused significant inflammation or an immune response yet. While spreading to the CNS, RABV can also spread from the CNS to peripheral tissues through anterograde axonal transport, infecting the salivary glands, skin, and other organs. This process allows the virus to be excreted through saliva and spread further to other hosts. The replication and spread of the virus in peripheral tissues are crucial for its transmission and pathogenesis, and also provide potential targets for disease diagnosis, prevention, and control. Overall, RABV finally spreads to the CNS and other peripheral tissues through the initial infection of peripheral tissues and neural transmission. This process is the core of its pathogenic mechanism.
3. Conclusion
This paper provides an in-depth analysis of the pathogenesis of rabies, and provide a detailed discussion on several existing infection mechanisms. The virus is primarily transmitted through the saliva of infected animals via bites and other means, utilizing receptors in muscle tissue to invade the peripheral nervous system, with surface glycoproteins playing a crucial role in binding. Upon entering the nervous system, the virus undergoes retrograde axonal transport to the CNS, a slow process that evades immune surveillance. Within the CNS, the virus rapidly replicates, leading to neuronal dysfunction and death, and triggering inflammatory responses. Pathological changes manifest as neuronal degeneration, with symptoms ranging from fever to hydrophobia and spasms, culminating in coma and death. Although the virus elicits an immune response, it achieves immune evasion by inhibiting interferons and other mechanisms. The study of immune system response also helps to understand the harm caused by viral infection. In the later stages of infection, the virus spreads to the salivary glands to facilitate transmission. Despite extensive research on its infection mechanisms, issues such as the regulation of viral transmission and the development of effective treatments remain unresolved. Future research should focus on the molecular interactions between the virus and the host, as well as the development of new vaccines and therapeutics. In addition, the study of the mechanism of rabies infection can provide a reference for a deeper understanding of the body's response to rabies virus infection. This can also provide design ideas for developing new treatment methods or developing new treatment methods and means targeting specific infection pathways.
References
[1]. Bastos, V., Pacheco, V., Rodrigues, É. D., Moraes, C. N., Nóbile, A. L., Fonseca, D. L. M., ... & Cabral‐Marques, O. (2023). Neuroimmunology of rabies: New insights into an ancient disease. Journal of Medical Virology, 95(10), e29042.
[2]. Davis, B. M., Rall, G. F., & Schnell, M. J. (2015). Everything you always wanted to know about rabies virus (but were afraid to ask). Annual review of virology, 2(1), 451-471.
[3]. Pan, W., Tao, M., Zhou, C., et al. (2024) Rabies Knowledge Mini-Lecture. In Proceedings of the 2024 Academic Conference on Animal Injury Prevention and Treatment, Wenzhou Medical University Affiliated Fifth Hospital (Lishui Central Hospital), 1.
[4]. Pan, W., Tao, M., Zhou, C., et al. (2024) Rabies Knowledge Mini-Lecture. In Proceedings of the 2024 Academic Conference on Animal Injury Prevention and Treatment, Wenzhou Medical University Affiliated Fifth Hospital (Lishui Central Hospital), 1.
[5]. Huang, J. (2024, December 23). What You Need to Know About Rabies. Gansu Science and Technology News, 8.
[6]. Qu, Z., Ye, L., Chen, P., et al. (2022) Clinical and Epidemiological Characteristics of 18 Rabies Cases. Journal of Medical Pest Control, 38(3), 274-276.
[7]. Bao, J. (2024, August 1). Urgent Need to Prevent Rabies. Health Times, 1. DOI: 10.28034 n.cnki.nbjsb.2024.000214.
[8]. Li Zhijun, Gao Yanchun, Zhang Zuopeng, et al. (2023) Epidemiological Characteristics and Prevention Strategies of Rabies. Journal of Guangzhou Medical University, 51(6), 75-80.
[9]. CDC Public Image Library. Available at: https://phil.cdc.gov
[10]. Lin, Y. (2019) Epidemiological and Clinical Analysis of Rabies Cases. Chinese Community Doctors, 35(14), 169+171.
Cite this article
Muhetaer,M. (2025). Analysis of Pathogenesis and Mechanism of Rabies. Theoretical and Natural Science,93,39-44.
Data availability
The datasets used and/or analyzed during the current study will be available from the authors upon reasonable request.
Disclaimer/Publisher's Note
The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of EWA Publishing and/or the editor(s). EWA Publishing and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
About volume
Volume title: Proceedings of the 3rd International Conference on Environmental Geoscience and Earth Ecology
© 2024 by the author(s). Licensee EWA Publishing, Oxford, UK. This article is an open access article distributed under the terms and
conditions of the Creative Commons Attribution (CC BY) license. Authors who
publish this series agree to the following terms:
1. Authors retain copyright and grant the series right of first publication with the work simultaneously licensed under a Creative Commons
Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this
series.
2. Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the series's published
version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial
publication in this series.
3. Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and
during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See
Open access policy for details).
References
[1]. Bastos, V., Pacheco, V., Rodrigues, É. D., Moraes, C. N., Nóbile, A. L., Fonseca, D. L. M., ... & Cabral‐Marques, O. (2023). Neuroimmunology of rabies: New insights into an ancient disease. Journal of Medical Virology, 95(10), e29042.
[2]. Davis, B. M., Rall, G. F., & Schnell, M. J. (2015). Everything you always wanted to know about rabies virus (but were afraid to ask). Annual review of virology, 2(1), 451-471.
[3]. Pan, W., Tao, M., Zhou, C., et al. (2024) Rabies Knowledge Mini-Lecture. In Proceedings of the 2024 Academic Conference on Animal Injury Prevention and Treatment, Wenzhou Medical University Affiliated Fifth Hospital (Lishui Central Hospital), 1.
[4]. Pan, W., Tao, M., Zhou, C., et al. (2024) Rabies Knowledge Mini-Lecture. In Proceedings of the 2024 Academic Conference on Animal Injury Prevention and Treatment, Wenzhou Medical University Affiliated Fifth Hospital (Lishui Central Hospital), 1.
[5]. Huang, J. (2024, December 23). What You Need to Know About Rabies. Gansu Science and Technology News, 8.
[6]. Qu, Z., Ye, L., Chen, P., et al. (2022) Clinical and Epidemiological Characteristics of 18 Rabies Cases. Journal of Medical Pest Control, 38(3), 274-276.
[7]. Bao, J. (2024, August 1). Urgent Need to Prevent Rabies. Health Times, 1. DOI: 10.28034 n.cnki.nbjsb.2024.000214.
[8]. Li Zhijun, Gao Yanchun, Zhang Zuopeng, et al. (2023) Epidemiological Characteristics and Prevention Strategies of Rabies. Journal of Guangzhou Medical University, 51(6), 75-80.
[9]. CDC Public Image Library. Available at: https://phil.cdc.gov
[10]. Lin, Y. (2019) Epidemiological and Clinical Analysis of Rabies Cases. Chinese Community Doctors, 35(14), 169+171.









