1. Introduction
Biofilm is an important component of cells, bacteria, and other living organisms. Biofilm is an essential structure for cells to carry out life activities. It not only has the function of independently separating substances inside and outside the cell, but is also closely related to the life activities of cells, playing a key role in material transfer, energy metabolism, signal transduction, cell recognition, cell cancer, and cell movement [1,2].
Biofilm has been a hotspot for novel drug delivery systems due to its natural biocompatibility, immune escape ability and targeting, meanwhile, nano-biomaterials have the advantages of specific targeting, intelligent delivery, high drug loading, low toxicity and penetration, etc. By combining the common advantages of the two, we can construct optimized drug carriers and delivery systems to significantly improve the biofilm permeability and penetration of drugs and enhance the treatment effect The drug carriers and delivery systems are optimized by combining the common advantages of the two.
Nanomedicines usually refer to spherical drug particles with particle size between 1-200 nm prepared using natural or synthetic polymeric materials as carriers, which are loaded with active ingredients based on non-covalent forces (hydrogen bonding interactions, hydrophobic interactions, electrostatic interactions, ligand bonding, and π-π stacking) for active or passive targeting of diseases, and transported across the biobarrier by various mechanisms. Such small-sized nanoparticles can accumulate drugs in tumor tissues by enhancing the high permeability and retention effect (EPR) in solid tumors, which facilitates sustained drug release and reduces distribution in normal tissues [3-6]. It has been used to deliver difficult-to-solve drugs, antitumor drugs, gene drugs, and drugs that need to break the blood-brain barrier, etc.
The carrier adjuvant drug delivery system has good therapeutic effects, but such systems contain a large number of excipients, and so they also pose significant barriers to drug delivery; for example, the drug-carrying capacity of carrier-assisted drug delivery systems is unsatisfactory (usually less than 10%); most of the carriers used for drug delivery Carriers or excipients do not have therapeutic effects per se, and their metabolites may cause short- or long-term systemic toxicity; in addition, some carriers may cause an immune response to the therapeutic agent in the physiological milieu [7, 8].
Adverse reactions caused by accumulation or rejection of carrier materials in vivo still need to be examined by systematic in vivo metabolism dynamics studies. By then, the carrier-free novel nano drug delivery system has attracted the attention of researchers.
Novel carrier-free nanomedicines are nanodelivery systems consisting of single or multiple small molecule drugs or precursor drugs self-assembled without the aid of carrier materials [9].This kind of assembly is the molecules or molecular aggregates through the weak interactions of non-covalent bonds such as hydrogen bonding, van der Waals force, π-π stacking, electrostatic interaction and ligand bonding, and through the additive and synergistic effects to form a stable self-assembly system with a specific structure, especially the intermolecular hydrogen bonding, the self-assembly force is mainly composed of synergistic interactions between the hydrogen bonding or between the hydrogen bonding and the other non-covalent bonding. When molecules are bonded by hydrogen bonding forces, single and multiple hydrogen bonds can be formed, and the stronger the multiplicity of hydrogen bonds, the stronger the bonding energy and stability between molecules [10].
Extracted components of traditional Chinese medicines (TCM) have structural diversity, and some natural TCM molecules have self-assembly properties and can be assembled with other molecules by non-electrostatic forces. The natural small molecules of traditional Chinese medicine with self-assembling properties that have been identified mainly include triterpenes, steroids, glycosides, peptides and anthraquinones. These natural products can self-assemble at the interface of different solvents to form nanoparticles.
Most of the carrier-free nanomedicines are prepared using a bottom-up technique, where the drug is prepared directly into nanocrystals, protecting it from destruction and promoting its selective accumulation in tumors, and for pure nanomedicines, the drug loading can be as high as 100%, avoiding the cumbersome step of preparing an additional carrier, and further avoiding carrier-induced toxicity and immunogenicity [11, 12].
The tetracyclic triterpenes in the ginsenoside skeleton exhibit hydrophobicity, while the sugar groups exhibit hydrophilicity. This amphiphilicity makes ginsenosides show self-assembly tendency in aqueous solution, forming micellar structure. The formation of micelles not only wraps the drug and improves its solubility in water, but also changes its physicochemical properties in solution.
Therefore, our article is developed from the following perspectives: introducing some basic physicochemical properties of ginsenosides, thus pointing out and discussing in depth the special biofilm permeation activity of ginsenosides, which can be developed into the corresponding ginsenosides as carriers or pore-forming pore-forming pore-forming pore to assist drug entry in modern nanoapplications, and finally summarizing the delivery activity of such new nano-drugs that will be possessed in the clinic.
2. Source
Ginsenosides are mainly distributed in plants of the Panax genus and are mainly derived from Panax ginseng C.A. Meyer, known as the "king of all herbs". Ginseng is a perennial herbaceous plant of the Panax genus in the Araliaceae family, distributed in the eastern provinces of China, Russia, and North Korea. Most rare ginsenosides are obtained through transformation or heterologous synthesis of the prototype ginsenosides. The more commonly used prototype ginsenosides in the transformation pathway are ginsenosides Rb1, Re, Rc, Rd, etc. These ingredients mainly come from the roots, leaves, flowers, and buds of ginseng, the roots and leaves of American ginseng, and the roots and leaves of American ginseng [13]. Ginsenoside compound K was initially discovered to be a microbial degradation product of other ginsenosides [14]. Therefore, for a period of time, researchers believed that there was no natural source. It was not until 2006 that Wang et al. found this ingredient in Ginseng fruit that they found a natural source [15]. Li et al. used RP-HPLC-ELSD method to analyze the part with the highest ginsenoside content in the aerial part (stem and leaf) of American ginseng harvested from September to October of that year [16].
3. Ginsenoside Classification
Ginsenosides can be categorized into dammarane-type, oleanolic acid-type and oxytetrone-type according to the structure of the mother nucleus, dammarane-type and oxytetrone-type belonging to tetracyclic triterpene saponins [17], and oleanolic acid-type belonging to pentacyclic triterpene saponins; Dammarane-type ginsenosides can be categorized into ginseng glycoside and ginseng triprolidin according to the substituents on the C-6 position or not; the two of them both have the chiral carbon atom C-20, and thus can be subdivided into Rand S[18]. The metabolites of different types of ginsenosides can be summarized as prototypic ginsenosides and rare ginsenosides, among which rare ginsenosides are mainly secondary metabolic derivatives obtained by hydrolysis of some of the sugar radicals of the prototypic ginsenosides, which are of low polarity, with strong hydrophobicity and cell penetrability [19].
Table 1: Classification of ginsenosides.
Type of parent nucleus | dammarane | Oleanolic acid type | Octillon type | |
Ginseng Diol | Ginseng triol | |||
fabric | ||||
original form | GinsenosideRa1、Ra2、Ra3、Rb1、Rb2、Rb3、Rc、Rd;Malonyl GinsenosideRb1、Rb2、Rc、Rd | GinsenosideRg1、Rf、Re | GinsenosideR0 | / |
rarity | GinsenosideRh3、Rd2、Rg3、Rh2、Rk1、Rs1、Rs2、Rs4、Rs5、F2、CK、Protopanaxadiol(aPPD);compoundK | GinsenosideRg2、Rg6、Rh1、Rh4、Rk3、F1、F4、20(R)-ginsenosideRh2、Protopanaxatriol(aPPT) | / | GinsenosideRg5、Rg6、Rk1、Rk2、Rk3、Rh3、Rh4、Rh5 |
4. Biofilm permeation properties of ginsenosides
4.1. Lipid rafts are key targets for ginsenoside action
Lipid rafts are cholesterol- and sphingolipid-rich microstructural domains of the plasma membrane that exhibit a relatively compact and ordered structure relative to other regions of the plasma membrane with a thicker liquid-ordered phase. This structure allows lipid rafts to exist relatively stably in the cell membrane and to be distinguished from the surrounding liquid disordered phase. Since ginsenosides have a parent nucleus similar to that of cholesterol, it is likely that ginsenosides are embedded in lipid rafts and thus function like cholesterol in regulating the fluidity of lipid raft membranes [20]. It has been shown that ginsenoside Rh2 is structurally similar to cholesterol and can interact with membrane lipids [21] to disrupt lipid rafts [22]. So it was further investigated that ginsenoside Rh2 treatment reduces cellular cholesterol levels and thus disrupts lipid raft distribution [23]. Ginsenoside Rh2 can be embedded in the plasma membrane, and the hydroxyl group of ginsenoside Rh2 prevents sphingolipids and cholesterol from tightly wrapping into lipid rafts [24]. Ginsenoside Rh2 disrupts the physical and chemical properties of Hela cell lipid rafts, thereby decreasing the membrane hydrophobicity and increasing the membrane fluidity of Hela cells [25].
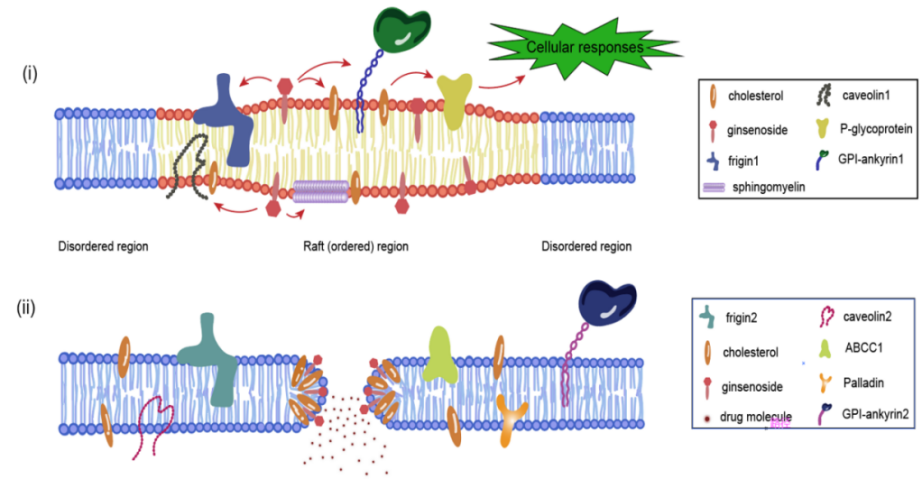
Figure 1: Two modes of action of ginsenosides with biofilms (i) Ginsenosides act on lipid rafts (ii)Ginsenosides replace cholesterol to make biofilms porous
4.2. Constitutive relation
The specific amphiphilic structure of ginsenosides is also key to their membrane-permeable bioactivity. The amphiphilic nature of ginsenosides allows them to adsorb or insert into the phospholipid bilayer, where the sugar portion interacts with the interfacial portion of the membrane, which contains mainly polar headgroups, consisting of sugar residues from a wide range of glycolipids and glycoproteins, with which intramolecular hydrogen bonds are formed, and the steroidal or triterpenoidal saponin element portion interacts with the membrane hydrophobic core [26].Aggregation of phospholipid vesicles is an important phenomenon in cell-cell interactions, and other ginsenosides lacking this sugar moiety exhibit less or no agglutination, an agglutination reaction that occurs only with short or unsaturated fatty acid chains of phosphatidylcholine, but not with phosphatidylethanolamine, phosphatidylserine, or phosphatidic acid. Ginsenoside Rc can interact with the polar head of phosphatidylcholine, ginsenoside Rc can be inserted into the hydrophobic region of lecithin-choline vesicles, the whole process of ginsenoside Rc inserted into the lipid bilayer of the cell membrane, interacting with the fatty acyl group of phospholipids, binding with the polar head of other phosphatidylcholine vesicles, and cross-linking with the other vesicles to show agglutination. Ginsenoside Rb2 can insert into the lipid bilayer and interact with fatty acyl chains of phospholipids, ginsenosides Rb1 and Rd cannot insert into the lipid bilayer [27]. This suggests that the interaction of ginsenoside Rc with phospholipid membranes is influenced not only by the chemical structure of phospholipids, but also by membrane fluidity. Ginsenosides are membrane-active substances that regulate membrane dynamics and transverse organization of domains [28-30]. Relative to the higher polarity ginsenosides, the lower polarity ginsenosides are more likely to disrupt bacterial cell membranes, leading to cell death [31].
5. Ginsenosides as carriers for nanodelivery systems
5.1. Ginsenoside self-assembled micelles
Ginsenoside monomers can be spontaneously assembled into ordered nanostructures under near thermodynamic equilibrium conditions by non-covalent interactions between molecules, which belongs to the spontaneous behavior of ginsenoside molecules in aqueous solution. It is based on the principle of minimum energy and is essentially intermolecular forces [32]. Critical micelle concentration (CMC) is the initial concentration at which a surfactant begins to self-assemble to form micelles, which reflects the surfactant's micellar capacity, and the special amphiphilicity of ginsenosides will cause them to form self-assembled micelles at CMC. The CMC values of different types of ginsenosides are also different and highly variable [33].Dai et al. found that Rg1 is the most likely to form micelles, mainly because Rg1 has the fewest sugar groups and is the most hydrophobic, and it is this strong hydration that will resist the aggregation process, leading to mutual repulsion of charges [34].The solubility of different types of ginsenosides is highly variable, and the hydrophilic groups of ginsenosides are located in the structure of the cell membrane, including the phospholipid polar head and the cholesterol of β-OH. Water solubility has a very important effect on the activity of ginsenosides and the formation of self-assembled micelles. Rare ginsenosides are formed by dehydrating or hydrolyzing off some of the sugar groups of common ginsenosides, and the lower the content of the sugar groups of ginsenosides, the lower the hydrophilicity and the higher the activity. Ginsenosides are surface-active, so they adsorb at the water interface and the nature of the self-assembled micelles formed may be affected by temperature, ionic strength and pH value [35]. Ginsenosides exhibit special elasticity at the ginsenoside-water interface, and the formation of this special highly viscoelastic interfacial layer requires that the saponins have an oleanane-type triterpenoid saponin meta-structure. Triterpenoid saponins can form highly viscoelastic networks through intermolecular hydrogen bonding between neighboring sugar residues, especially at the air/water interface [36]. Dai et al. were the first to find that ginsenoside Rb1 and anticancer drugs such as DHA, BA, and HCPT could self-assemble into appropriately sized and stable micelles; the obtained micelles had a hydrophilic head region that could contact the surrounding aqueous solution and isolate the hydrophobic drug in the center [37]. The interaction between Rb1 and the drug is hydrophobic, but also playing a very important assembly force is the π-π stacking, and late hemolysis experiments also showed that the Nps does not cause adverse reactions, which may also be due to the anti-hemolytic properties of Rb1, so it is possible that the green Nps of ginsenoside Rb1 may hold promise for the delivery of other insoluble drugs [32].
Table 2: A real-life summary of applications of ginsenoside self-assembly for drug delivery
Ginsenoside Type | Drugs delivered | appliance | |
ginsenosideRb1 | Paclitaxel、protopanaxadiol | breast tumor | [38] |
ginsenosideRb1 | diclofenac | In vivo cor-neal penetration and anti-inflammatory | [39] |
ginsenosideRg1 | Doxorubicin | Reduced cardiotoxicity and enhanced anti-tumor activity | [40] |
ginsenosideRg1、Re、Rf、Rb1、Rc、Rb2、Rb3、Rd | insulin | Reduces side effects such as pain, localized tissue necrosis and hypoglycemia | [41] |
ginsenosideRg3、Rb1 | / | Anti-Triple Negative Breast Cancer | [42] |
ginsenosideRb1 | betulinic acid, dihydroartemisinin,hydroxycamptothecine) | antitumor | [37] |
ginsenosideRg1、Re、Rb1 | / | Anti-tumor metastasis | [43] |
ginsenosideRb1 | Doxorubicin、Cypate、gambogic acid | Anti-Breast Cancer | [44] |
ginsenosideRg5 | Paclitaxel | Anti Prostate Cancer | [45] |
5.2. Surface Active Agent
Since ginsenosides contain both hydrophobic and hydrophilic groups in their chemical structure, they are a natural surfactant in their own right [46]. The air-water interface is the most common hydrophobic-hydrophilic interface. Depending on their solubility, some ginsenosides dissolve as monomers, while others accumulate at the air-water interface with their hydrophilic heads toward the water side and hydrophobic tails toward the air side, a behavior that reduces the surface tension of water by decreasing the number of hydrogen bonds per length [47]. Ginsenosides stabilize emulsions (emulsifiers) because they reduce the interfacial energy between different phases (hydrophobic and hydrophilic). Many cosmetics (e.g. creams, lotions, etc.) and milk [48] contain saponins as emulsifiers. Saponins have the potential to stabilize nanosuspensions and nanoemulsions, which are two-phase systems containing very small droplets (<100 nm). The special amphiphilic molecular structure of saponins leads to special surface properties. The adsorbed surface layer exhibits viscoelastic behavior with very high viscosity and elasticity under swelling and shear forces [49-52], This property is attributed to the tight molecular packing at the interface and the strong hydrogen bonding between neighboring sugar groups in the interfacial layer. Saponins can be used as single or complex carrier materials for drugs due to their surface activity, and saponins can encapsulate hydrophobic drugs in a hydrophobic core and act as solubilizers [53]. This property allows them to replace synthetic surfactants in microemulsion delivery systems to promote and stabilize emulsification [54].The combination of the surface activity of the saponin and its biological activity makes it synergistic with the drug and enhances the drug efficacy better than ordinary carrier materials, which is achieved through a mechanism or pathway not contained in ordinary materials [55-58]. Ginsenosides are very promising carrier materials for drug delivery as surfactants with the characteristics of pharmaceutical ingredients and the safety and degradability of natural ingredients [59]. Differences between glycosides and sugar chains lead to the diversity of ginsenoside structures, which further leads to differences in the properties of ginsenosides as surfactants. The most important property of ginsenosides as surfactants is that they can be adsorbed at the water interface. At the air/water and oil/water interfaces, ginsenosides form a strong viscoelastic interfacial film through hydrogen bonding between neighboring sugar residues. Because ginsenosides have a triterpene structure they are most likely to form viscoelastic films, resulting in stabilized foams and emulsions, whereas steroidal saponins are not able to form viscoelastic films and are therefore not suitable for stabilizing foams and emulsions. Glycoside subtypes may also affect interfacial properties, with oleanane-type triterpenoid saponins forming the most stable interfacial network. Shu et al. dispersed astaxanthin in soybean oil as the oil phase and dissolved ginsenoside in high-purity water as the aqueous phase, and successfully prepared astaxanthin-carrying nanoemulsions by high-pressure homogenization.
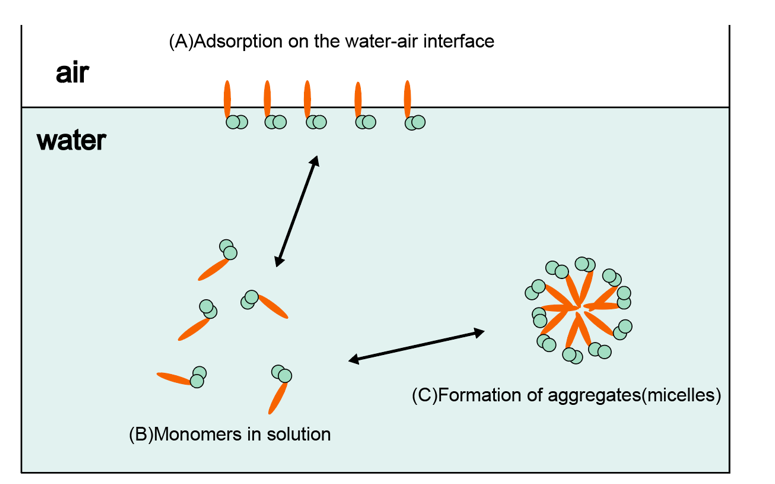
Figure 2: Behavior of ginsenoside in aqueous solution.
6. Encapsulation activity of ginsenosides as carriers
6.1. Reduced toxicity
Conventional chemotherapeutic drugs often cause severe toxicity to normal tissues due to lack of targeting when treating tumors. Combined targeted delivery of ginsenosides as drug carriers can significantly reduce the distribution of chemotherapeutic drugs in non-target tissues, thereby reducing toxicity to normal tissues. For example, ginsenoside liposomes showed lower cardiotoxicity and hepatotoxicity in animal experiments, and have a higher safety profile compared to conventional chemotherapeutic drugs [60].
In addition, the ginsenoside-as-carrier nanodelivery system can further reduce the exposure time of chemotherapeutic drugs in normal tissues by controlling or slowing down the release of the drugs, thus reducing toxicity [61].
6.2. Enhanced immunomodulation
Ginsenosides have significant immunomodulatory effects and can enhance anti-tumor immune responses by regulating the tumor microenvironment (TME), so nanocarriers formed from ginsenosides can further enhance this effect. For example, ginsenoside nanoparticles can enhance the body's immune response by regulating the expression of T cell subsets (e.g., CD4+, CD8+ T cells) and cytokines [62].
In addition, nanocarriers can enhance immunotherapy by improving the tumor microenvironment and reducing the number of immunosuppressive cells (e.g., regulatory T cells, myeloid-derived suppressor cells [63].
6.3. Multifunctional integration
Ginsenoside nanocarriers can not only be used as drug carriers, but can also be integrated with a variety of functions, such as imaging, diagnostics, and therapeutics. For example, ginsenoside nanoparticles can be combined with fluorescent probes or magnetic nanoparticles to achieve drug delivery along with diagnostic imaging [64]. This multifunctional integrated nanocarrier not only improves the therapeutic efficacy of drugs, but also provides a new means for early diagnosis and treatment of tumors [65].
7. Conclusion
For a long time, we have focused on ginsenosides as delivered drugs to study its solubility and bioavailability, etc., while this paper pioneers the prospect of developing ginsenosides as carrier materials applied to drug delivery systems.
This paper is of profound significance for the in-depth understanding of the efficacy and in vivo application of ginsenosides and the investigation of their mechanism of action by elaborating the role of ginsenosides with cell membranes. Although such nanomedicines are still in the early stages of development, ginsenoside carrier delivery systems for diagnostic, therapeutic, and prognostic purposes will need to be evaluated in vivo to assess their efficacy, which gives researchers new ideas for nanomedicines that are worth investigating. Overall, ginsenoside carrier drug delivery systems show great potential in emerging healthcare.
8. Acknowledgment
Fund Project: 2025 National College Students' Innovation and Entrepreneurship Plan Project, Project Number: 202510316087E
References
[1]. T. Inoue, S.-i. Yanagihara, Y. Misono, M. Suzuki, Chemistry and Physics of Lipids, 109 (2001) 117-133.
[2]. M. Paz Sánchez-Migallón, F.J. Aranda, J.C. Gómez-Fernández, Biochimica et Biophysica Acta (BBA) - Biomembranes, 1279 (1996) 251-258.
[3]. R.R. Allison, FUTURE ONCOLOGY, 10 (2014) 123-142.
[4]. S.M. Banerjee, A.J. MacRobert, C.A. Mosse, B. Periera, S.G. Bown, M.R.S. Keshtgar, The Breast, 31 (2017) 105-113.
[5]. B. Purushothaman, J. Choi, S. Park, J. Lee, A.A.S. Samson, S. Hong, J.M. Song, JOURNAL OF MATERIALS CHEMISTRY B, 7 (2019) 65-79.
[6]. X.W. Ren, L. Zhang, Y.Y. Zhang, Z.Y. Li, N. Siemers, Z.M. Zhang, Insights Gained from Single-Cell Analysis of Immune Cells in the Tumor Microenvironment, in: W.M. Yokoyama (Ed.) ANNUAL REVIEW OF IMMUNOLOGY2021, pp. 583-609.
[7]. Y.T. Yang, C.T. Chen, H.F. Chien, T. Tsai, Current Nanoscience, 7 (2011) 850-855.
[8]. D. Graça, H. Louro, J. Santos, K. Dias, A.J. Almeida, L. Gonçalves, M.J. Silva, A. Bettencourt, Toxicology Letters, 276 (2017) 129-137.
[9]. X.B. Zhang, N. Li, S.W. Zhang, B.J. Sun, Q. Chen, Z.G. He, C. Luo, J. Sun, Medicinal Research Reviews, 40 (2020) 1754-1775.
[10]. X.B. Zhang, M. Li, Chinese Journal of Organic Chemistry, 29 (2009) 528-535.
[11]. H.X. Lin, M.S. Yang, C. Tian, C.R. Han, J. Song, J.F. Duan, J.X. Jiang, Colloids and Surfaces B-Biointerfaces, 165 (2018) 191-198.
[12]. T. Vasconcelos, S. Marques, B. Sarmento, European Journal of Pharmaceutics and Biopharmaceutics, 123 (2018) 1-8.
[13]. M. Sun, J. Li, J. Ma, Z. Li, K. Li, M. Lu, X. Gong, Journal of Jilin Agricultural University, 45 (2023) 674-684.
[14]. I. Yosioka, T. Sugawara, K. Imai, I. KITAGAWA, Chemical and Pharmaceutical Bulletin, 20 (1972) 2418-2421.
[15]. J. Wang, X. Li, X. Yang, Chinese Traditional and Herbal Drugs, 37 (2006) 1761-1764.
[16]. B. Li, Y. Ren, Z. Yang, Y. Xu, Y. Liu, West China Journal of Pharmaceutical Sciences, 30 (2015) 74-78.
[17]. L.T. Nguyen, A. Fărcaş, S. Socaci, M. Tofana, Z. Diaconeasa, O.L. Pop, L.C. Salanță, Research Gate, 77 (2020).
[18]. Z. Lu, Q. Zheng, X. Zhang, Q. Han, Q. Liang, Chinese Traditional and Herbal Drugs, 54 (2023) 7260-7272.
[19]. M. Li, L. Lin, Y. Wang, J. Shi, C. Wu, C. Zhu, Chinese Traditional and Herbal Drugs, 55 (2024) 688-696.
[20]. A.S. Attele, J.A. Wu, C.S. Yuan, Biochemical Pharmacology, 58 (1999) 1685-1693.
[21]. Y. PY, M. NK, C. YK, L. KW, N. TB, F. DT, Y. HW, W. RN, - Chinese Medicine, (2007) - 6.
[22]. U. Y, H. I, I. N, R. PC, S. Y, N. N, T. T, I. T, H. T, K. T, Journal of Lipid Research, (2005) - 904-912.
[23]. J. Qiu, W. Li, S.H. Feng, M. Wang, Z.Y. He, Genetics and Molecular Research, 13 (2014) 3586-3598.
[24]. T. Ota, K. Fujikawa-yamamoto, Z.P. Zong, M. Yamazaki, S. Odashima, I. Kitagawa, H. Abe, S. Arichi, Cancer research, 47 (1987) 3863-3867.
[25]. J.-S. Yi, H.-J. Choo, B.-R. Cho, H.-M. Kim, Y.-N. Kim, Y.-M. Ham, Y.-G. Ko, Biochemical and Biophysical Research Communications, 385 (2009) 154-159.
[26]. E.A.J. Keukens, T. deVrije, C. vandenBoom, P. deWaard, H.H. Plasman, F. Thiel, V. Chupin, W.M.F. Jongen, B. deKruijff, Biochimica Et Biophysica Acta-Biomembranes, 1240 (1995) 216-228.
[27]. K. Fukuda, H. Utsumi, S. Soda, J. Shoji, A. Hamada, Biochimica et biophysica acta, 900 (1987) 267-274.
[28]. H.-Y. Kwon, E.-H. Kim, S.-W. Kim, S.-N. Kim, J.-D. Park, D.-K. Rhee, Archives of Pharmacal Research, 31 (2008) 171-177.
[29]. E.K. Park, E.J. Lee, S.H. Lee, K.H. Koo, J.Y. Sung, E.H. Hwang, J.H. Park, C.W. Kim, K.C. Jeong, B.K. Park, Y.N. Kim, British Journal of Pharmacology, 160 (2010) 1212-1223.
[30]. J.H. Lorent, J. Quetin-Leclercq, M.-P. Mingeot-Leclercq, Organic & Biomolecular Chemistry, 12 (2014) 8803-8822.
[31]. P. Xue, Y. Yao, X.-s. Yang, J. Feng, G.-x. Ren, Journal of Ginseng Research, 41 (2017) 180-187.
[32]. J. Zhu, Z. Zhang, R. Wang, K. Zhong, K. Zhang, N. Zhang, W. Liu, F. Feng, W. Qu, Acs Applied Nano Materials, 5 (2022) 3146-3169.
[33]. S. Boettcher, S. Drusch, Food Biophysics, 11 (2016) 91-100.
[34]. X. Dai, X. Shi, Q. Yin, H. Ding, Y. Qiao, Journal of Colloid and Interface Science, 396 (2013) 165-172.
[35]. S. Mitra, S.R. Dungan, Journal of Agricultural and Food Chemistry, 45 (1997) 1587-1595.
[36]. S. Boettcher, S. Drusch, Advances in Colloid and Interface Science, 243 (2017) 105-113.
[37]. L. Dai, K. Liu, C. Si, L. Wang, J. Liu, J. He, J. Lei, Journal of Materials Chemistry B, 4 (2016) 529-538.
[38]. L. Lu, H. Ao, J. Fu, M. Li, Y. Guo, Y. Guo, M. Han, R. Shi, X. Wang, Biomedicine & Pharmacotherapy, 163 (2023).
[39]. M. Li, J. Lan, X. Li, M. Xin, H. Wang, F. Zhang, X. Lu, Z. Zhuang, X. Wu, Drug Delivery, 26 (2019) 481-489.
[40]. C. Li, X. Gou, H. Gao, Nanomedicine, 16 (2021) 2587-2604.
[41]. J.-J. Zou, J.-Q. Le, B.-C. Zhang, M.-Y. Yang, J.-L. Jiang, J.-F. Lin, P.-Y. Wu, C. Li, L. Chen, J.-W. Shao, International Journal of Pharmaceutics, 605 (2021).
[42]. Z. S, W. J, A. X, W. Z, Z. X, Z. Y, Frontiers in Bioengineering and Biotechnology. , 10 (2022) - 945472.
[43]. X.-R. Tan, K.-K. Feng, J.-Q. Le, J.-W. Shen, J.-W. Shao, J.-W. Shao, Journal of Industrial and Engineering Chemistry, 116 (2022) 303-309.
[44]. Z. Luo, J. An, W. Shi, C. Li, H. Gao, Nanotechnology, 32 (2021).
[45]. F. Gao, Z. Ma, X. Luo, Y. Wang, X. Liu, M. Tang, J. Chen, L. Tu, D. Ouyang, J. Zheng, C. Li, Molecular Pharmaceutics, 21 (2024) 3502-3512.
[46]. M.T.M.G. Rosa, E.K. Silva, D.T. Santos, A.J. Petenate, M.A.A. Meireles, Journal of Food Engineering, 168 (2016) 68-78.
[47]. J.N. Israelachvili, Intermolecular and surface forces, Academic press2011.
[48]. S. R, M. K, T. S, D. ND, S. S, P. E, Langmuir. , 27 (2011) - 12486-12498.
[49]. R. Stanimirova, K. Marinova, S. Tcholakova, N. Denkov, S. Stoyanov, E. Pelan, Langmuir, 27 (2011) 12486-12498.
[50]. K. Golemanov, S. Tcholakova, N. Denkov, E. Pelan, S. Stoyanov, Langmuir, 28 (2012) 12071-12084.
[51]. K. Golemanov, S. Tcholakova, N. Denkov, E. Pelan, S. Stoyanov, Soft Matter, 9 (2013) 5738-5752.
[52]. N. Pagureva, S. Tcholakova, K. Golemanov, N. Denkov, E. Pelan, S. Stoyanov, Colloids Surfaces A: Physicochemical Engineering Aspects, 491 (2016) 18-28.
[53]. Z. Vinarov, D. Radeva, V. Katev, S. Tcholakova, N. Denkov, Indian J. Pharm. Sci, 80 (2018) 10.4172.
[54]. S. E, Canadian Journal of Physiology and Pharmacology, 103 (1968) - 487-490.
[55]. T. Tolstikova, M. Khvostov, A. Bryzgalov, Mini Reviews in Medicinal Chemistry, 9 (2009) 1317-1328.
[56]. B. Sapra, S. Jain, A. Tiwary, Drug delivery, 15 (2008) 443-454.
[57]. O.Y. Selyutina, N.E. Polyakov, D.V. Korneev, B.N. Zaitsev, Drug Delivery, 23 (2016) 858-865.
[58]. P. S, L. Z, Z. L, L. W, O. Id, L. C, M. DJ, O. Id, Food Function. , (2018) - 1829-1839.
[59]. L. Y, L. Z, Z. Q, S. M, Q. Q, S. Y, Y. J, L. L, D. X, S. X, International Journal of Pharmaceutics. , 603 (2021) - 120709.
[60]. W. X, Z. W, S. Q, W. Y, T. Y, L. Z, W. X, S. L, L. C, L. J, Signal Transduction and Targeted Therapy. , (2021) - 33.
[61]. X. Jin, J. Zhou, Z. Zhang, H. Lv, Artificial Cells Nanomedicine and Biotechnology, 46 (2018) S931-S942.
[62]. Y.Q. Cui, P. Yang, P. Sun, Y.D. Yan, G.Y. Jin, J.S. Quan, Indian Journal of Pharmaceutical Sciences, 82 (2020) 149-156.
[63]. L. Yang, Z. Zhang, J. Hou, X. Jin, Z. Ke, D. Liu, M. Du, X. Jia, H. Lv, International Journal of Nanomedicine, 12 (2017) 7653-7667.
[64]. M. Zheng, C. Yue, Y. Ma, P. Gong, P. Zhao, C. Zheng, Z. Sheng, P. Zhang, Z. Wang, L. Cai, Acs Nano, 7 (2013) 2056-2067.
[65]. W. Fan, W. Bu, B. Shen, Q. He, Z. Cui, Y. Liu, X. Zheng, K. Zhao, J. Shi, Advanced Materials, 27 (2015) 4155-4161.
Cite this article
Feng,J.;Qin,Y.;Li,X.;Zhang,A.;Zhang,J. (2025). A New Modern Interpretation of Ginsenosides: From Biofilm Permeation Profiling to Nanocarrier Applications. Theoretical and Natural Science,99,85-94.
Data availability
The datasets used and/or analyzed during the current study will be available from the authors upon reasonable request.
Disclaimer/Publisher's Note
The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of EWA Publishing and/or the editor(s). EWA Publishing and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
About volume
Volume title: Proceedings of the 5th International Conference on Biological Engineering and Medical Science
© 2024 by the author(s). Licensee EWA Publishing, Oxford, UK. This article is an open access article distributed under the terms and
conditions of the Creative Commons Attribution (CC BY) license. Authors who
publish this series agree to the following terms:
1. Authors retain copyright and grant the series right of first publication with the work simultaneously licensed under a Creative Commons
Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this
series.
2. Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the series's published
version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial
publication in this series.
3. Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and
during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See
Open access policy for details).
References
[1]. T. Inoue, S.-i. Yanagihara, Y. Misono, M. Suzuki, Chemistry and Physics of Lipids, 109 (2001) 117-133.
[2]. M. Paz Sánchez-Migallón, F.J. Aranda, J.C. Gómez-Fernández, Biochimica et Biophysica Acta (BBA) - Biomembranes, 1279 (1996) 251-258.
[3]. R.R. Allison, FUTURE ONCOLOGY, 10 (2014) 123-142.
[4]. S.M. Banerjee, A.J. MacRobert, C.A. Mosse, B. Periera, S.G. Bown, M.R.S. Keshtgar, The Breast, 31 (2017) 105-113.
[5]. B. Purushothaman, J. Choi, S. Park, J. Lee, A.A.S. Samson, S. Hong, J.M. Song, JOURNAL OF MATERIALS CHEMISTRY B, 7 (2019) 65-79.
[6]. X.W. Ren, L. Zhang, Y.Y. Zhang, Z.Y. Li, N. Siemers, Z.M. Zhang, Insights Gained from Single-Cell Analysis of Immune Cells in the Tumor Microenvironment, in: W.M. Yokoyama (Ed.) ANNUAL REVIEW OF IMMUNOLOGY2021, pp. 583-609.
[7]. Y.T. Yang, C.T. Chen, H.F. Chien, T. Tsai, Current Nanoscience, 7 (2011) 850-855.
[8]. D. Graça, H. Louro, J. Santos, K. Dias, A.J. Almeida, L. Gonçalves, M.J. Silva, A. Bettencourt, Toxicology Letters, 276 (2017) 129-137.
[9]. X.B. Zhang, N. Li, S.W. Zhang, B.J. Sun, Q. Chen, Z.G. He, C. Luo, J. Sun, Medicinal Research Reviews, 40 (2020) 1754-1775.
[10]. X.B. Zhang, M. Li, Chinese Journal of Organic Chemistry, 29 (2009) 528-535.
[11]. H.X. Lin, M.S. Yang, C. Tian, C.R. Han, J. Song, J.F. Duan, J.X. Jiang, Colloids and Surfaces B-Biointerfaces, 165 (2018) 191-198.
[12]. T. Vasconcelos, S. Marques, B. Sarmento, European Journal of Pharmaceutics and Biopharmaceutics, 123 (2018) 1-8.
[13]. M. Sun, J. Li, J. Ma, Z. Li, K. Li, M. Lu, X. Gong, Journal of Jilin Agricultural University, 45 (2023) 674-684.
[14]. I. Yosioka, T. Sugawara, K. Imai, I. KITAGAWA, Chemical and Pharmaceutical Bulletin, 20 (1972) 2418-2421.
[15]. J. Wang, X. Li, X. Yang, Chinese Traditional and Herbal Drugs, 37 (2006) 1761-1764.
[16]. B. Li, Y. Ren, Z. Yang, Y. Xu, Y. Liu, West China Journal of Pharmaceutical Sciences, 30 (2015) 74-78.
[17]. L.T. Nguyen, A. Fărcaş, S. Socaci, M. Tofana, Z. Diaconeasa, O.L. Pop, L.C. Salanță, Research Gate, 77 (2020).
[18]. Z. Lu, Q. Zheng, X. Zhang, Q. Han, Q. Liang, Chinese Traditional and Herbal Drugs, 54 (2023) 7260-7272.
[19]. M. Li, L. Lin, Y. Wang, J. Shi, C. Wu, C. Zhu, Chinese Traditional and Herbal Drugs, 55 (2024) 688-696.
[20]. A.S. Attele, J.A. Wu, C.S. Yuan, Biochemical Pharmacology, 58 (1999) 1685-1693.
[21]. Y. PY, M. NK, C. YK, L. KW, N. TB, F. DT, Y. HW, W. RN, - Chinese Medicine, (2007) - 6.
[22]. U. Y, H. I, I. N, R. PC, S. Y, N. N, T. T, I. T, H. T, K. T, Journal of Lipid Research, (2005) - 904-912.
[23]. J. Qiu, W. Li, S.H. Feng, M. Wang, Z.Y. He, Genetics and Molecular Research, 13 (2014) 3586-3598.
[24]. T. Ota, K. Fujikawa-yamamoto, Z.P. Zong, M. Yamazaki, S. Odashima, I. Kitagawa, H. Abe, S. Arichi, Cancer research, 47 (1987) 3863-3867.
[25]. J.-S. Yi, H.-J. Choo, B.-R. Cho, H.-M. Kim, Y.-N. Kim, Y.-M. Ham, Y.-G. Ko, Biochemical and Biophysical Research Communications, 385 (2009) 154-159.
[26]. E.A.J. Keukens, T. deVrije, C. vandenBoom, P. deWaard, H.H. Plasman, F. Thiel, V. Chupin, W.M.F. Jongen, B. deKruijff, Biochimica Et Biophysica Acta-Biomembranes, 1240 (1995) 216-228.
[27]. K. Fukuda, H. Utsumi, S. Soda, J. Shoji, A. Hamada, Biochimica et biophysica acta, 900 (1987) 267-274.
[28]. H.-Y. Kwon, E.-H. Kim, S.-W. Kim, S.-N. Kim, J.-D. Park, D.-K. Rhee, Archives of Pharmacal Research, 31 (2008) 171-177.
[29]. E.K. Park, E.J. Lee, S.H. Lee, K.H. Koo, J.Y. Sung, E.H. Hwang, J.H. Park, C.W. Kim, K.C. Jeong, B.K. Park, Y.N. Kim, British Journal of Pharmacology, 160 (2010) 1212-1223.
[30]. J.H. Lorent, J. Quetin-Leclercq, M.-P. Mingeot-Leclercq, Organic & Biomolecular Chemistry, 12 (2014) 8803-8822.
[31]. P. Xue, Y. Yao, X.-s. Yang, J. Feng, G.-x. Ren, Journal of Ginseng Research, 41 (2017) 180-187.
[32]. J. Zhu, Z. Zhang, R. Wang, K. Zhong, K. Zhang, N. Zhang, W. Liu, F. Feng, W. Qu, Acs Applied Nano Materials, 5 (2022) 3146-3169.
[33]. S. Boettcher, S. Drusch, Food Biophysics, 11 (2016) 91-100.
[34]. X. Dai, X. Shi, Q. Yin, H. Ding, Y. Qiao, Journal of Colloid and Interface Science, 396 (2013) 165-172.
[35]. S. Mitra, S.R. Dungan, Journal of Agricultural and Food Chemistry, 45 (1997) 1587-1595.
[36]. S. Boettcher, S. Drusch, Advances in Colloid and Interface Science, 243 (2017) 105-113.
[37]. L. Dai, K. Liu, C. Si, L. Wang, J. Liu, J. He, J. Lei, Journal of Materials Chemistry B, 4 (2016) 529-538.
[38]. L. Lu, H. Ao, J. Fu, M. Li, Y. Guo, Y. Guo, M. Han, R. Shi, X. Wang, Biomedicine & Pharmacotherapy, 163 (2023).
[39]. M. Li, J. Lan, X. Li, M. Xin, H. Wang, F. Zhang, X. Lu, Z. Zhuang, X. Wu, Drug Delivery, 26 (2019) 481-489.
[40]. C. Li, X. Gou, H. Gao, Nanomedicine, 16 (2021) 2587-2604.
[41]. J.-J. Zou, J.-Q. Le, B.-C. Zhang, M.-Y. Yang, J.-L. Jiang, J.-F. Lin, P.-Y. Wu, C. Li, L. Chen, J.-W. Shao, International Journal of Pharmaceutics, 605 (2021).
[42]. Z. S, W. J, A. X, W. Z, Z. X, Z. Y, Frontiers in Bioengineering and Biotechnology. , 10 (2022) - 945472.
[43]. X.-R. Tan, K.-K. Feng, J.-Q. Le, J.-W. Shen, J.-W. Shao, J.-W. Shao, Journal of Industrial and Engineering Chemistry, 116 (2022) 303-309.
[44]. Z. Luo, J. An, W. Shi, C. Li, H. Gao, Nanotechnology, 32 (2021).
[45]. F. Gao, Z. Ma, X. Luo, Y. Wang, X. Liu, M. Tang, J. Chen, L. Tu, D. Ouyang, J. Zheng, C. Li, Molecular Pharmaceutics, 21 (2024) 3502-3512.
[46]. M.T.M.G. Rosa, E.K. Silva, D.T. Santos, A.J. Petenate, M.A.A. Meireles, Journal of Food Engineering, 168 (2016) 68-78.
[47]. J.N. Israelachvili, Intermolecular and surface forces, Academic press2011.
[48]. S. R, M. K, T. S, D. ND, S. S, P. E, Langmuir. , 27 (2011) - 12486-12498.
[49]. R. Stanimirova, K. Marinova, S. Tcholakova, N. Denkov, S. Stoyanov, E. Pelan, Langmuir, 27 (2011) 12486-12498.
[50]. K. Golemanov, S. Tcholakova, N. Denkov, E. Pelan, S. Stoyanov, Langmuir, 28 (2012) 12071-12084.
[51]. K. Golemanov, S. Tcholakova, N. Denkov, E. Pelan, S. Stoyanov, Soft Matter, 9 (2013) 5738-5752.
[52]. N. Pagureva, S. Tcholakova, K. Golemanov, N. Denkov, E. Pelan, S. Stoyanov, Colloids Surfaces A: Physicochemical Engineering Aspects, 491 (2016) 18-28.
[53]. Z. Vinarov, D. Radeva, V. Katev, S. Tcholakova, N. Denkov, Indian J. Pharm. Sci, 80 (2018) 10.4172.
[54]. S. E, Canadian Journal of Physiology and Pharmacology, 103 (1968) - 487-490.
[55]. T. Tolstikova, M. Khvostov, A. Bryzgalov, Mini Reviews in Medicinal Chemistry, 9 (2009) 1317-1328.
[56]. B. Sapra, S. Jain, A. Tiwary, Drug delivery, 15 (2008) 443-454.
[57]. O.Y. Selyutina, N.E. Polyakov, D.V. Korneev, B.N. Zaitsev, Drug Delivery, 23 (2016) 858-865.
[58]. P. S, L. Z, Z. L, L. W, O. Id, L. C, M. DJ, O. Id, Food Function. , (2018) - 1829-1839.
[59]. L. Y, L. Z, Z. Q, S. M, Q. Q, S. Y, Y. J, L. L, D. X, S. X, International Journal of Pharmaceutics. , 603 (2021) - 120709.
[60]. W. X, Z. W, S. Q, W. Y, T. Y, L. Z, W. X, S. L, L. C, L. J, Signal Transduction and Targeted Therapy. , (2021) - 33.
[61]. X. Jin, J. Zhou, Z. Zhang, H. Lv, Artificial Cells Nanomedicine and Biotechnology, 46 (2018) S931-S942.
[62]. Y.Q. Cui, P. Yang, P. Sun, Y.D. Yan, G.Y. Jin, J.S. Quan, Indian Journal of Pharmaceutical Sciences, 82 (2020) 149-156.
[63]. L. Yang, Z. Zhang, J. Hou, X. Jin, Z. Ke, D. Liu, M. Du, X. Jia, H. Lv, International Journal of Nanomedicine, 12 (2017) 7653-7667.
[64]. M. Zheng, C. Yue, Y. Ma, P. Gong, P. Zhao, C. Zheng, Z. Sheng, P. Zhang, Z. Wang, L. Cai, Acs Nano, 7 (2013) 2056-2067.
[65]. W. Fan, W. Bu, B. Shen, Q. He, Z. Cui, Y. Liu, X. Zheng, K. Zhao, J. Shi, Advanced Materials, 27 (2015) 4155-4161.









