1. Introduction
Along with the accelerated global transformation of the energy structure, high-energy-density and high-safety energy storage technologies have become critical to supporting the development of clean energy. Lithium-ion batteries (LIBs), as the current mainstream electrochemical energy storage technology, have been widely applied in portable electronic devices and electric vehicles[1]. However, because of the lithium-intercalation within the graphite anode in LIB, their development is constrained by low energy density, resulting in a specific capacity of only 372 mAh g-1[2] and thereby limiting further improvements in battery energy density [3].
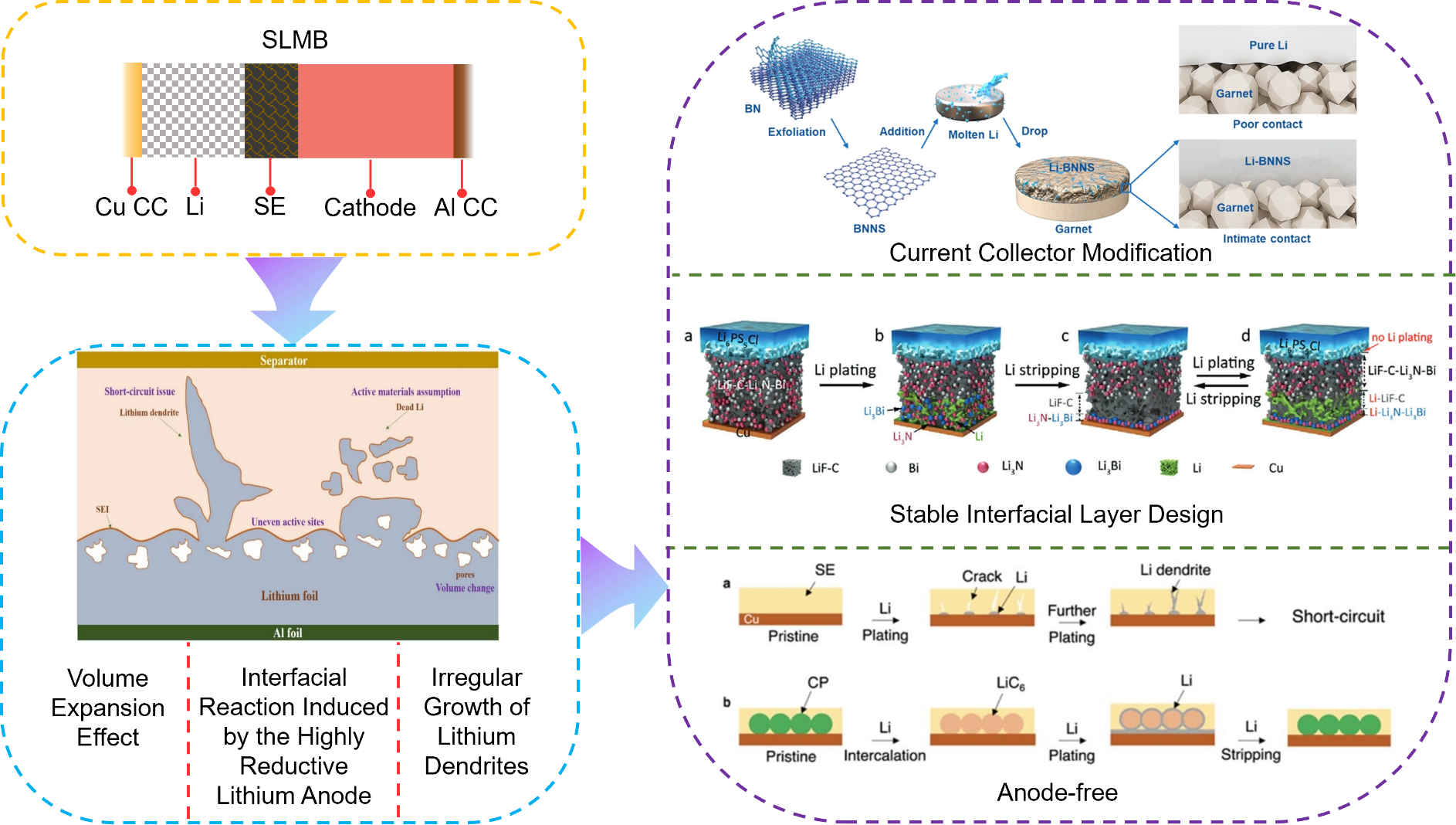
Figure 1: Challenges and modification strategies for the anode–electrolyte interface in SLMBs
Lithium metal anode (LMA) is regarded as the ideal choice for next-generation high-energy-density batteries due to its exceptionally high theoretical specific capacity (3860 mAh g-1) and the lowest redox potential (-3.04 V vs. SHE). However, conventional liquid lithium metal batteries (LMBs) face several challenges in practical applications. Firstly, the high reactivity of lithium metal triggers continuous side reactions with the liquid electrolyte, leading to the formation of an unstable solid electrolyte interphase (SEI) and thereby reducing coulombic efficiency (CE). Moreover, the growth of lithium dendrites and the gradual accumulation of “dead lithium” during cycling decrease the CE of the LMA, further exacerbating capacity fade. To solve these issues, LMBs require additional lithium (N/P>1) to compensate for the lithium loss during cycling. Nevertheless, the use of excess lithium increases safety risks and reduces the overall energy density of the LMBs [4-5].
As a result, under the dual requirements of safety and high energy density, the concept of anode-free lithium metal batteries (AFLMBs) has emerged. In the initial state of AFLMBs, no active lithium metal is present on the anode side. During the first charge, Li+ is extracted from the cathode material and deposit on the surface of the current collector (CC), forming a lithium metal layer. This design not only eliminates the safety risks associated with excess lithium metal but also significantly enhances the volumetric energy density of the battery. However, liquid-state AFLMBs still face challenges such as electrolyte decomposition and flammability, particularly under high current densities, which can rapidly deteriorate cycling performance. Additionally, the risk of internal short circuits due to dendrite growth remains [6]. The emergence of SE has opened up new opportunities for the development of AFLMBs. Compared with LE, SE is nonflammable, nonvolatile, and stable at high temperatures. For certain solid lithium-ion conductors, they exhibit a lithium-ion transference number (tLi+) close to 1. More importantly, the high elastic modulus and mechanical strength of SE can effectively suppress lithium dendrite growth, thereby enhancing battery safety and cycle life [7]. Anode-free solid-state lithium metal batteries (AFSSLBs) based on SE combine the high energy density advantage of AFLMBs with the superior safety of SE, becoming a current research hotspot.
Although AFSSLBs exhibit tremendous application potential, their practical performance remains constrained by issues such as poor solid–solid interfacial contact, degradation of the anode material structure, and compatibility problems between the SE and the current collectors. As shown in Figure 1, This review focuses on interfacial modification strategies between the SE and the anode, and explores the key scientific and technical issues common to both SLMBs with abundant “lithium inventory” and AFSSLBs. The aim is to provide theoretical guidance and practical solutions for the development of future high-energy-density and high-safety batteries.
2. Causes of capacity loss
AFSSLBs significantly enhance the intrinsic safety of batteries by completely eliminating the design of excessive lithium metal anodes. Whereas, their performance degradation fundamentally stems from the kinetic imbalance of lithium supply and consumption. Unlike the "lithium inventory" buffering mechanism in SLMBs, the active lithium of AFSSLBs totally relies on the limited extraction from the cathode materials (such as NCM or lithium-rich manganese-based materials), while AFSSLBs lack the "lithium inventory" compensation mechanism present in traditional LMBs. This design makes the reversibility of lithium deposition/stripping become the decisive factor for system stability. If irreversible reactions occur at the anode interface (such as the formation of dead lithium or interfacial side reactions), it will directly contribute to continuous loss of active lithium and capacity loss (Figure 2). Experimental results confirm that the capacity loss in AFSSLBs is primarily attributed to two interconnected mechanisms: (1) The low coulombic efficiency of the highly reducing LMA (typically <99 %, resulting in a loss of approximately 1% active lithium per cycle) will significantly shortens cycle life; (2) The continuously increasing overpotentials during cycling induce dynamic reformation of the solid electrolyte interphase (SEI), with in-situ impedance spectroscopy analysis showing that the interfacial impedance increases by about 15% per cycle[8]. Notably, the mechanical stress accumulation of the rigid SEI in solid-state batteries leads to the expansion of microcracks, forming local barriers to lithium ion transport (ΔμLi+ >0.2 eV), which further exacerbates the spatially uneven deposition of lithium. This "SEI damage-deposition distortion" positive feedback mechanism results in an exponential decay in capacity retention[9]. Therefore, understanding the coupling of these two mechanisms is key to developing solutions.
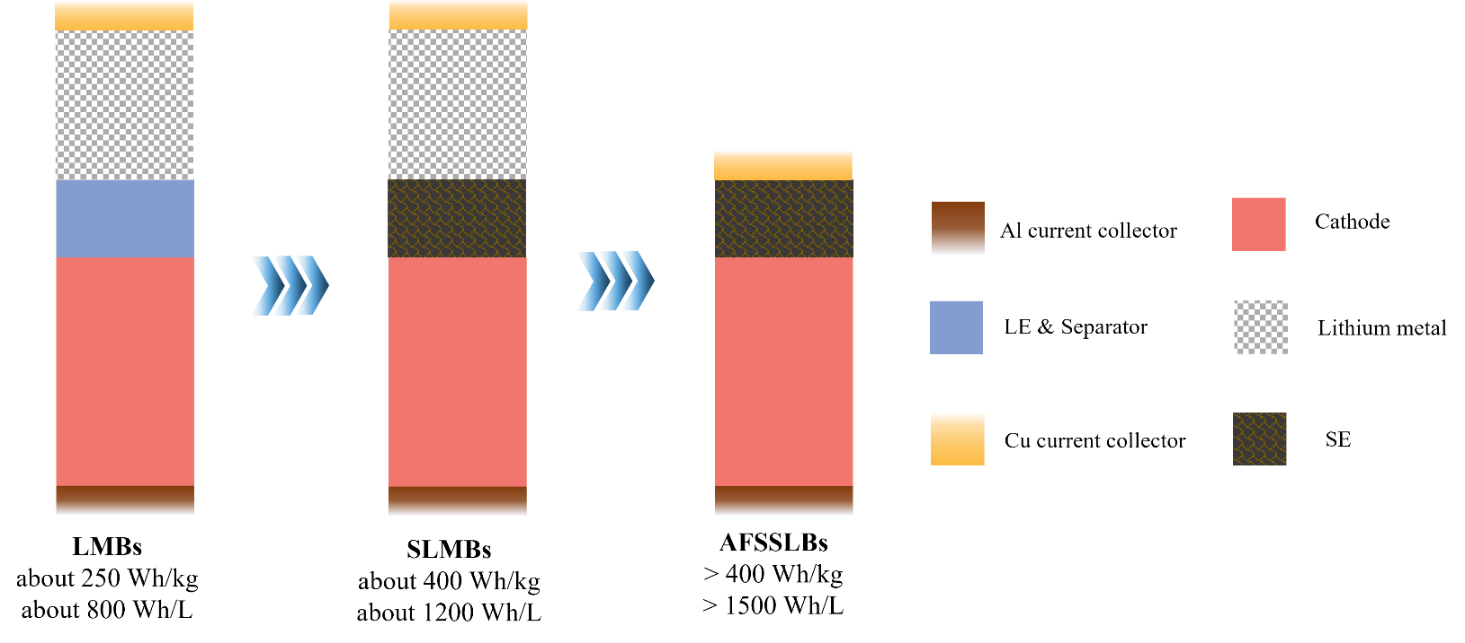
Figure 2: Battery architecture diagrams of LMBs, SLMBs, and AFSSLBs
2.1. Interfacial reaction induced by the highly reductive lithium anode
In liquid-state LMBs,the low redox potential of LMA (-3.04 V vs. SHE) continuously drives the reduction of ether/ester-based electrolytes, forming SEI composed of inorganic compounds such as Li2O, Li2S, Li3N. Although this passivation layer exhibits ionic conductivity, its electronic insulating properties are theoretically expected to suppress the continuous decomposition of the electrolyte [10]. This is of significant importance for enhancing the cycle life and safety of liquid-state LMBs. However, in SE matrices commonly used in SLMBs (e.g., Li6PS5Cl), reducible metal elements such as Ge, Ti, and Al are existing. XPS analysis indicates that these elements are reduced to their metallic states (e.g., Ge0) during cycling and subsequently react with Li to form highly conductive lithium alloys (e.g., Li22Ge5) through alloying reactions, leading to ongoing interfacial reduction [11]. Although the solid–solid contact in SLMBs reduces the active reaction area, the uneven deposition on the current collector causes the rough morphology of the deposited lithium metal to trigger localized hotspot reactions with SE [12]. Moreover, once the deposited lithium reacts with the solid electrolyte, there is no surplus lithium reserve in SLMBs to replenish it, resulting in a rapid and irreversible capacity loss. What is noticeable is that polymer SE (e.g., PEO-LiTFSI) can improve interfacial contact through the flexibility of their molecular chains. Their apparent electrochemical window (>4 V vs. Li/Li+) can effectively suppresses electrolyte decomposition. Synchrotron imaging has shown that the thickness of the interfacial by-product is only one-third that of sulfide electrolytes [13]. By introducing an artificial SEI design (such as a Li3N modification layer), the overpotential for lithium deposition can be reduced by over 60%, thereby providing a significant optimization pathway for interfacial engineering.
2.2. Volume expansion effect
In conventional LMBs, the volume expansion of active materials is one of the key factors affecting cycling performance. Nevertheless, irreversible volume changes also occur between the electrolyte and electrode materials in SLMBs, which further compromise battery stability. During the charging process, lithium metal deposits irregularly on the anode and gradually accumulates, leading to continuous internal expansion of the battery. Moreover, due to the rigidity of the SE, the effects of volume change are further amplified. During discharging, the consumption of lithium metal on the anode results in a corresponding reduction in volume. Although deposited lithium can partially offset the volume loss, the non-fluidity of SE combined with the uneven growth of lithium dendrites gradually leads to the formation of voids between the electrode and the electrolyte during cycling, ultimately resulting in structural failure and even potential short circuits [14]. These issues severely affect the long-term cycling stability of the battery. Particularly under deep discharge conditions, if SE is unable to accommodate the volume changes of the lithium anode, significant fluctuations in battery thickness may occur. Moreover, the lithium metal on the anode side may also undergo side reactions with components, further exacerbating the issue of volume expansion.
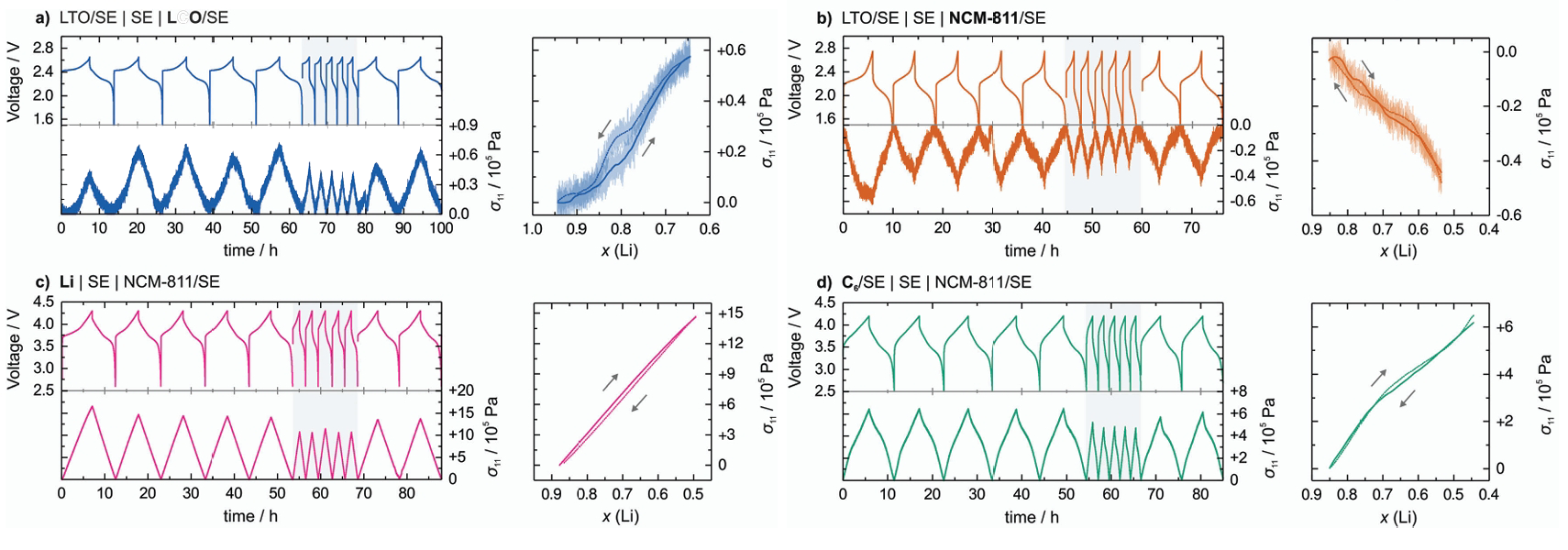
Figure 3: Net stress changes induced by constant current in SLMBs with different electrode configurations: (a) LTO/SE||SE||LCO/SE (b) LTO/SE||SE||NCM-811/SE (c) Li||SE||NCM-811/SE (d) LiC6/SE||NCM-811/SE. [15]
As shown in Figure 3, Koerver et al. [15] studied the pressure variations in all-solid-state lithium batteries with different electrode configurations during charge–discharge cycles and found that the battery pressure exhibits periodic changes throughout the cycling process. With continued charge–discharge cycling, the combined effects of lithium dendrite growth and interfacial side reactions further intensify the structural failure induced by volume expansion, thereby severely impacting the long-term stability of the battery.
2.3. Irregular growth of lithium dendrites
In solid-state batteries, solid electrolytes (SE) were initially thought to fully suppress dendrite growth. However, under high current densities, lithium dendrites can still form along grain boundaries, voids, and microcracks. This process, involving nucleation and propagation, results from a combination of mechanical stress, uneven ion transport, interfacial side reactions, and electron leakage [16]. Similar to liquid systems, dendrites nucleate at voids and grain boundaries, where low ionic conductivity leads to Li⁺ accumulation and local electric field distortions. Pre-existing cracks can expand into permeation channels, potentially causing short circuits. Unlike liquid electrolytes, electron leakage at interfaces, such as sulfide grain boundaries, can also lead to the formation of "bulk dendrites” [17].
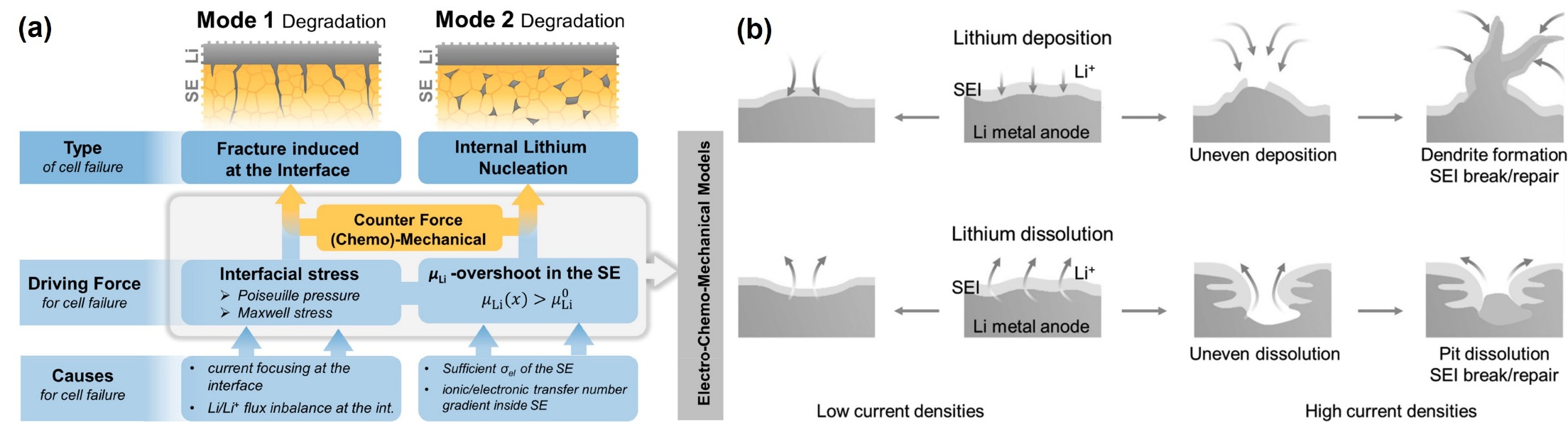
Figure 4: (a) Two failure modes of SLMBs induced by lithium dendrite growth in SE[18] (b) schematic illustration of lithium metal deposition/stripping during battery operation[6]
The failure modes of solid-state lithium metal batteries (SLMBs) caused by dendrites can be broadly categorized into two types (Figure 4a): interface-related issues, such as poor contact or void formation, and electron penetration, which leads to slow lithium deposition. When dendrites penetrate the SE and contact the cathode, they can cause short circuits or even explosions [18].
The high reactivity of lithium, along with volume expansion and dendrite growth, complicates the formation of a stable anode interface. An ideal SEI should be chemically stable, thin, and hermetically sealed. As shown in Figure 4b, at lower current densities, lithium deposition maintains a smooth interface and stable SEI, but at higher currents, uneven deposition and volume effects can cause the SEI to crack, facilitating dendrite growth. Repeated cracking and repair of the SEI consume electrolyte, decreasing CE and increasing impedance [6].
Further issues arise as dendrite growth increases contact with SE, leading to more side reactions that irreversibly consume active lithium and electrolyte, reducing CE [19]. Additionally, dendrites can become encapsulated by insulating SEI products, forming "dead lithium," which further reduces CE. Accumulated dendrites and dead lithium create a porous interface, hindering Li⁺ and electron transport, increasing polarization, and lowering energy efficiency [20].
3. Modification strategies for the SLMBs interface
During operation of SLMBs, the lithium metal deposition and stripping often triggers several problems including interfacial reactions induced by the highly reducing LMA, volume expansion effects, the formation of lithium dendrites, “dead lithium” and so on. To solve these problems, current efforts are focused on designing stable interfacial layers, modifying current collectors, and the anode-free trends.
3.1. Current collector modification
In SLMBs, the current collector not only plays the core roles of current conduction and supporting electrode materials, but its interfacial properties and structural design also deeply influence Li+ transport kinetics, interfacial electrochemical stability and battery cycle life. Due to poor physical contact with lithium metal and intrinsic chemical inertness, traditional copper foil current collector tends to cause increased interfacial resistance and disordered lithium dendrite growth, thereby severely limiting battery performance.
In response to the above challenges, researchers have proposed structural modification strategies for current collectors to enable SLMBs operating stably at ambient temperature and pressure. The design of current collector based on three-dimensional frameworks achieves a triple optimization effect by establishing hierarchical porous structures: firstly, by enlarging the surface area of the current collector, the local current density is significantly reduced, suppressing the nucleation and growth of lithium dendrites; secondly, the porous framework provides an expansion buffering space that effectively mitigates volume fluctuations during lithium deposition and stripping process (volume change rate < 20 %); besides, the abundance of topological defects on the framework surface serves as lithiophilic sites, inducing uniform lithium deposition and promoting the formation of a stable SEI enriched with inorganic components (such as LiF and Li3N)[21]. Experiments have indicated that such modified current collectors can reduce the loss rate of active lithium during cycling while maintaining stable thickness.
Recent studies have developed various high-performance current collector framework material systems, mainly including: (1) Graphene and its derivatives: Graphene materials possess high electrical conductivity (~106 S cm-1) and extremely high specific surface area (~2630 m2 g-1), which are widely used to construct highly stable current collectors. (2) Hexagonal boron nitride (h-BN): Possessing not only a layered structure similar to graphene but also chemical inertness and high mechanical strength (tensile strength ~130 GPa) make it an ideal interfacial protective material. (3) 2D transition metal carbides (MoS₂, VS₂, etc.): With high ionic conductivity and catalytic activity, they can simultaneously serve as active materials and current collector modifiers [22].
Huang et al. [23] innovatively grew vertical graphene arrays (VG) on the surface of a commercial three-dimensional copper mesh, thereby constructing a composite anode (VGCM) with a dual 3D structure. Benefiting from the unique dual 3D architecture with vertically aligned structures, abundant topological defects and lithiophilic structure, VGCM provides uniformly distributed Li nucleation sites, guiding uniform lithium growth during the initial electroplating stage. During battery cycling, VGCM@Li||CSE||NCM cell exhibits lower polarization (Figure 5a) and demonstrates excellent electrochemical stability over 20 cycles (Figure 5b). Moreover, as shown in Figure 5c, VGCM@Li||CSE||NCM cell displays a higher initial capacity compared to Li||CSE||NCM cell and maintains a high reversible capacity after 50 cycles. Wen’s group [24] adopted boron nitride nanosheets (BNNS) to modify the lithium anode, constructing a Li–BNNS composite electrode system. The modified battery exhibited a much tighter interfacial contact, with the interfacial resistance drastically reduced from 560Ω cm2 to 9Ω cm2. Meanwhile,by leveraging the insulating properties of BNNS (electronic conductivity ~10-15 S/cm) together with the in situ-formed Li3N layer at the interface, electron leakage is blocked and uniform lithium-ion transport is promoted, thereby increasing the critical current density and suppressing the growth of lithium dendrites. In the LiFePO4 full cell system, the electrode maintained a capacity retention rate of over 90 % after 100 cycles at 0.5 C. And the lithium deposition morphology was dense and free of dendrites.
In addition to the aforementioned framework engineering strategies, stress regulation has emerged as a new research direction. Feng et al. [25] suppressed dendrite growth from the perspective of mitigating stress non-uniformity, designing a nanoporous metallic current collector (NP-Ni) to homogenize interfacial stress. As shown in the cross-sectional SEM images in (Figure 5e, f), SLMBs incorporating NP-Ni exhibit more uniform lithium deposition and greatly alleviate the formation of lithium dendrites, thereby achieving stable SLMBs performance. Current collectors with 3D multifunctional designs not only integrate ion transport, mechanical support, and interfacial stability, significantly enhancing the energy density, safety, and cycle life of SLMBs, but also improve anode stability and suppress lithium dendrite growth, thereby more closely mimicking the contact characteristics of liquid batteries compared with planar current collectors.
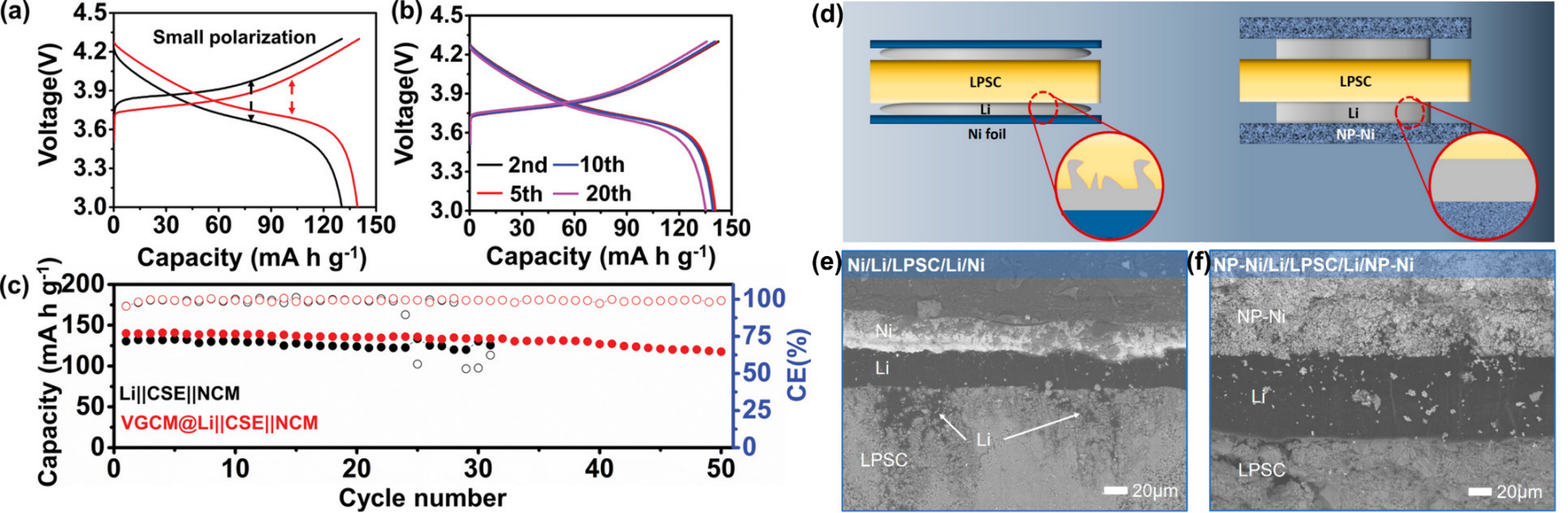
Figure 5: Electrochemical performance of SLMBs with VGCM composite anodes or lithium foil. (a) constant current charge–discharge curves (b) constant current charge–discharge curves (c) cycling stability test [23] (d) schematic diagrams of cells with/without NP-Ni (e, f) cross-sectional SEM images of Li||LPSC electrolyte interface with/without NP-Ni current collectors[25]
3.2. Stable interfacial layer design
The solid–solid contact interface between the SE and the lithium metal anode in SLMBs still faces three major challenges: (1) Physical contact defects: poor interfacial wettability (contact angle >90°) results in insufficient effective contact area, further leading to localized current concentration (j > 5 mA cm⁻²); (2) Chemical instability: SE components (e.g. S2- in sulfides and O2- in oxides) undergo irreversible reactions with lithium metal, forming a heterogeneous interfacial layer that significantly increases interfacial resistance; (3) Electrochemical kinetic limitation: high energy barriers for Li+ transport across the interface exacerbate the concentration polarization during lithium deposition/stripping [26].
To address these issues, researchers have proposed introducing coatings such as LiAlO2 or MoS2 at the interface or optimizing SE’s microstructure to reduce defects, thereby enhancing electrochemical stability and delaying dendrite penetration. The purpose of interfacial layer modification is to construct a stable SE-Li anode interface to mitigate issues such as the growth of lithium dendrites. Interfacial layer modification can generally be classified into composite anode design, surface modification and interlayer modification, which aim to tailor the SEI nanostructure by adjusting its composition (enhancing mechanical stability, uniformity, thermal/chemical stability, etc.) to achieve the desired modification.
Kong et al. [27] constructed a multiphase composite anode Li-Al alloy/Li3N/LiNO2 (AN@Li) by performing an in situ reaction with the addition of Al(NO3)3·9H2O into molten lithium. The Li-Al alloy, Li3N and LiNO2 contribute to lowering the interfacial energy and improving the adhesion between AN@Li and SE, thereby enhancing the wettability of AN@Li with SE (Figure 6a, b). The intimate contact between SE and anode impedes the nucleation and growth of dendrites. Moreover, it provides an efficient conductive pathway, facilitating Li+ diffusion within the anode. Experiments demonstrated that symmetric cells with the AN@Li electrode achieved a significantly higher critical current density (1.95 mA cm-2) (Figure 6c) and extended cycle life (6000 hours at 0.3 mA cm-2) (Figure 6d) compared with Li||AN@Li-LLZTO||Li. Besides, Figure 6e shows the compact contact of AN@Li||LLZTO. In full cells paired with LiFePO4 cathodes, a capacity retention of 96 % was maintained after 200 cycles at a 1 C rate.
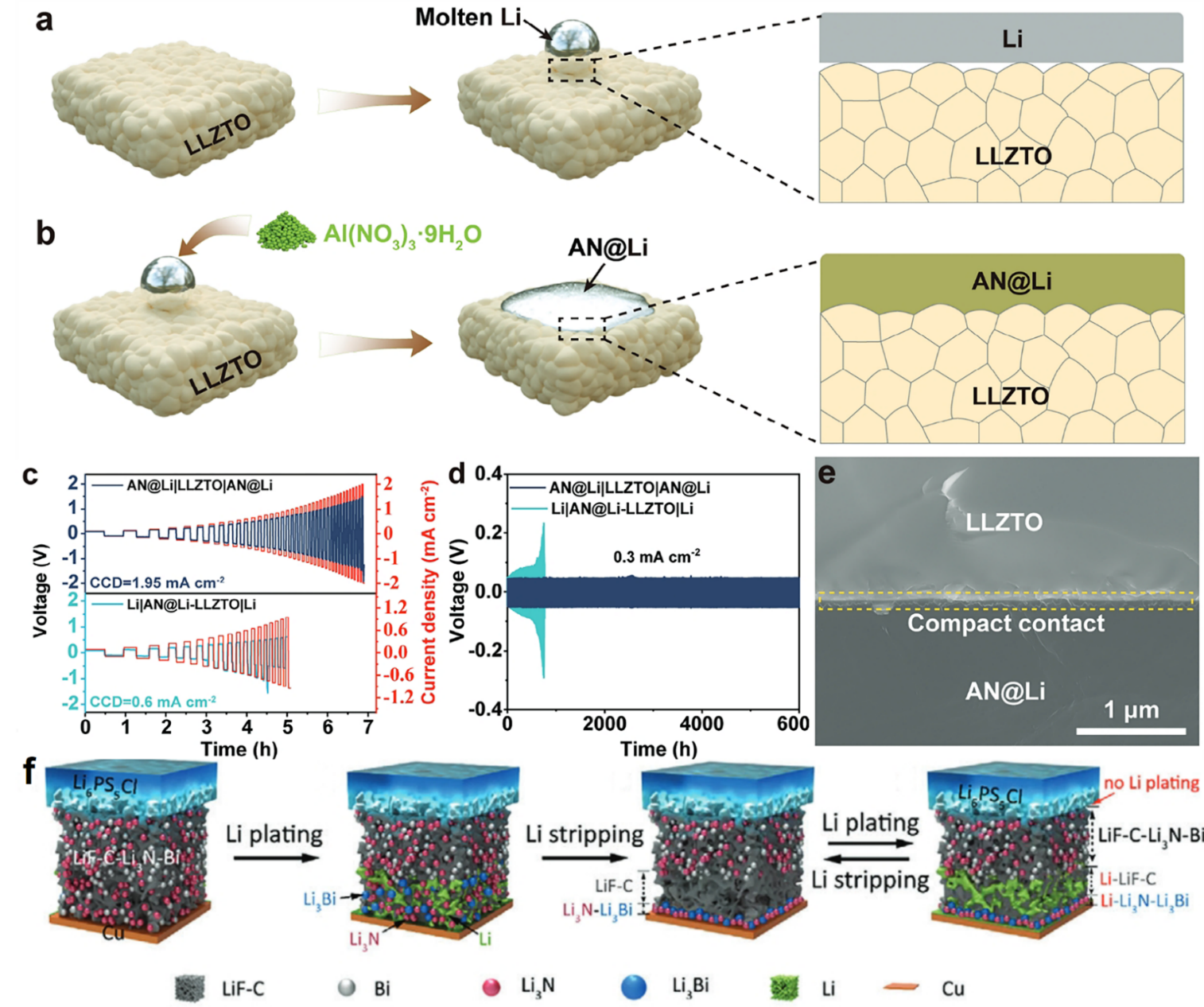
Figure 6: (a, b) Schematic diagrams illustrating the interfacial contact (c) CCD measurement of symmetric cells (d) constant current cycling tests of symmetric cells (e) cross-sectional SEM image of AN@Li||LLZTO[27] (f) action mechanism of LiF-C-Li3N-Bi layer[28]
In addition, researchers have utilized alloying reactions to achieve uniform lithium deposition at the SLMBs’ interfacial layer. Wan et al. [28] inserted a mixed ionic-electronic conductive (MIEC) lithium-repellent and porous LiF-C-Li3N-Bi nanocomposite interlayer between Li6PS5Cl SE and lithium anode, thereby in situ constructing a porous lithiophobic/lithiophilic interfacial layer. As shown in Figure 6f, during lithium deposition, the deposited lithium enters the porous LiF-C-Li3N-Bi layer and reacts with Bi to form a Li3Bi alloy. The lithiophilic Li3Bi and Li3N move towards the current collector along with the deposited lithium, accumulating to form a lithiophilic Li3Bi-Li3N layer that promotes uniform lithium deposition, while the highly lithiophobic LiF-C layer remains in place. This further drives the migration of lithium towards current collector, thereby suppressing the reduction of Li6PS5Cl and the growth of lithium dendrites. During lithium stripping, a dealloying reaction occurs to revert to the LiF-C-Li3N-Bi layer, thus enabling a reversible cycle. The developed lithiophobic/lithiophilic interface enables the Li|Li6PS5Cl|Li symmetric cell to cycle stably with a high capacity of 3.0 mAh cm-2 at a high current density of 3.0 mA cm-2.
High-entropy alloy interface engineering has shown unique advantages: Zeng et al. [29] developed a high-entropy alloy coating for application on SEs. Due to the random distribution of different metal cations in high-entropy materials, slight but widespread distortions in the metal-oxygen bond length occur within the lattice, creating a locally disordered overlapping site-energy distribution between Li+ transport sites. This allows for rapid Li+ migration, significantly enhancing the ionic conductivity of SE and site percolation.
3.3. Anode-free configuration design
In recent years, anode-free solid-state lithium batteries (AFSSLBs) have gained significant attention due to their high energy density, low cost, and improved safety. These batteries enhance safety by eliminating excess lithium metal anodes. Unlike conventional lithium metal batteries, which rely on a "lithium reservoir" buffering mechanism, AFSSLBs depend entirely on limited lithium extracted from the cathode materials, such as NCM or lithium-rich manganese-based compounds. The absence of a lithium reserve often leads to shorter cycle life, rapid capacity decay, and lower ionic conductivity. Therefore, improving the reversibility of lithium deposition/stripping and preventing dead lithium formation are critical for enhancing cycle stability.
To address these challenges, researchers have focused on modifying current collectors. For instance, Huang et al.[30] designed a carbon-reinforced ionic-electronic composite current collector, which forms a 3D ionic-electronic network when combined with SE, providing nucleation sites and accommodation space for lithium. This modification improved cycle life (>5000 cycles), stable lithium deposition, and high areal capacity (>8 mAh cm-2). Additionally, Liu et al.[31] introduced an Ag-C composite current collector, where nano-Ag particles (~50 nm) reduced the lithium nucleation overpotential to 1.5 mV. The improved contact area and uniform lithium-ion flux distribution helped achieve stable lithium deposition, with an areal capacity exceeding 7.0 mAh cm–2 and stable cycling for over 200 cycles at 0.25 mA cm–2.
4. Conclusion
As a key avenue for achieving high-energy-density and high-safety energy storage systems, the development of SLMBs still faces multiple challenges: continuous degradation of the SEI caused by poor interfacial contact along with side reactions between LMA and SE; lithium dendrite growth which leads to volume expansion and raises the risk of internal short circuits; the limited lithium source in anode‐free systems that affects cycling stability.
In response to these bottlenecks, current research is focusing on multidimensional modification strategies, including: optimizing the structure of current collectors (such as adopting three-dimensional frameworks, graphene, BN nanosheets and other materials) to enlarge the reactive interface, reduce local current densities, and improve the uniformity of lithium deposition; constructing artificial SEIs or regulate the composition of interfacial layers through alloying reactions, with the aim of forming a stable, dense, and ionically conductive protective interface; innovations in anode‐free systems which enhancing the reversibility of lithium deposition through the synergistic integration of composite current collectors and interface engineering.
Overall, the modification and optimization of SLMBs must be advanced in concert across materials innovation, interface engineering, and structural design. Future breakthroughs require a balanced approach to material design and mechanistic investigation, leveraging cross-scale interface optimization and multi-strategy integration to drive substantive progress in achieving SLMBs with high safety, long cycle life, and commercial viability.
References
[1]. Lu, Y., Chen, H., Wang, L., Yu, Z., Huang, Y., Yu, X., Wang, Y. and Roskilly, A.P. (2021) Energy Storage Driving towards a Clean Energy Future. Energy Reports, 7, 8128-8130.
[2]. Schmuch, R., Wagner, R., Hörpel, G., Placke, T. and Winter, M. (2018) Performance and Cost of Materials for Lithium-Based Rechargeable Automotive Batteries. Nature Energy, 3, 267-278.
[3]. XU J, CAI X, CAI S, et al. High‐Energy Lithium‐Ion Batteries: Recent Progress and a Promising Future in Applications[J/OL]. ENERGY & ENVIRONMENTAL MATERIALS, 2023, 6(5): e12450. DOI:10.1002/eem2.12450.
[4]. Lin L, Qin K, Li M, et al. Spinel-related Li2Ni0. 5Mn1. 5O4 cathode for 5-V anode-free lithium metal batteries[J]. Energy Storage Materials, 2022, 45: 821-827.
[5]. Albertus P, Babinec S, Litzelman S, et al. Status and challenges in enabling the lithium metal electrode for high-energy and low-cost rechargeable batteries[J]. Nature Energy, 2018, 3(1): 16-21.
[6]. Yang C , Fu K , Zhang Y ,et al.Protected Lithium‐Metal Anodes in Batteries: From Liquid to Solid[J].Advanced Materials, 2017, 29(36):1701169.DOI:10.1002/adma.201701169.
[7]. Yang C, Xie H, Ping W, et al. An electron/ion dual‐conductive alloy framework for high‐rate and high‐capacity solid‐state lithium‐metal batteries[J]. Advanced Materials, 2019, 31(3): 1804815.
[8]. Huang W Z, Zhao C Z, Wu P, et al. Anode‐free solid‐state lithium batteries: a review[J]. Advanced Energy Materials, 2022, 12(26): 2201044.
[9]. Yu W, Lin K Y, Boyle D T, et al. Electrochemical formation of bis (fluorosulfonyl) imide-derived solid-electrolyte interphase at Li-metal potential[J]. Nature Chemistry, 2024: 1-10.
[10]. Ding J F, Xu R, Yan C, et al. A review on the failure and regulation of solid electrolyte interphase in lithium batteries[J]. Journal of Energy Chemistry, 2021, 59: 306-319.
[11]. Kim K J, Balaish M, Wadaguchi M, et al. Solid‐state Li–metal batteries: challenges and horizons of oxide and sulfide solid electrolytes and their interfaces[J]. Advanced Energy Materials, 2021, 11(1): 2002689.
[12]. Zhao D, Wang J, Wang P, et al. Regulating the composition distribution of layered SEI film on Li-ion battery anode by LiDFBOP[J]. Electrochimica Acta, 2020, 337: 135745.
[13]. Brooks D J, Merinov B V, Goddard III W A, et al. Atomistic description of ionic diffusion in PEO–LiTFSI: Effect of temperature, molecular weight, and ionic concentration[J]. Macromolecules, 2018, 51(21): 8987-8995.
[14]. Wang Q, Liu B, Shen Y, et al. Confronting the challenges in lithium anodes for lithium metal batteries[J]. Advanced Science, 2021, 8(17): 2101111.
[15]. Koerver R, Zhang W, De Biasi L, et al. Chemo-mechanical expansion of lithium electrode materials–on the route to mechanically optimized all-solid-state batteries[J]. Energy & Environmental Science, 2018, 11(8): 2142-2158.
[16]. Esmizadeh S, Cabras L, Serpelloni M, et al. A review on modeling of nucleation and growth of Li dendrites in solid electrolytes[J]. Journal of Energy Storage, 2024, 97: 112897.
[17]. Wu B, Wang S, Lochala J, et al. The role of the solid electrolyte interphase layer in preventing Li dendrite growth in solid-state batteries[J]. Energy & Environmental Science, 2018, 11(7): 1803-1810.
[18]. Krauskopf T, Richter F H, Zeier W G, et al. Physicochemical concepts of the lithium metal anode in solid-state batteries[J]. Chemical reviews, 2020, 120(15): 7745-7794.
[19]. Jagger B, Pasta M. Solid electrolyte interphases in lithium metal batteries[J]. Joule, 2023, 7(10): 2228-2244.
[20]. Ke X, Wang Y, Dai L, et al. Cell failures of all-solid-state lithium metal batteries with inorganic solid electrolytes: Lithium dendrites[J]. Energy Storage Materials, 2020, 33: 309-328.
[21]. Chen X R, Chen X, Yan C, et al. Role of lithiophilic metal sites in lithium metal anodes[J]. Energy & Fuels, 2021, 35(15): 12746-12752.
[22]. Ma Q, Zheng Y, Luo D, et al. 2D materials for all‐solid‐state lithium batteries[J]. Advanced Materials, 2022, 34(16): 2108079.
[23]. Huang S, Yang H, Hu J, et al. Early lithium plating behavior in confined nanospace of 3D lithiophilic carbon matrix for stable solid‐state lithium metal batteries[J]. Small, 2019, 15(43): 1904216.
[24]. Wen J, Huang Y, Duan J, et al. Highly adhesive Li-BN nanosheet composite anode with excellent interfacial compatibility for solid-state Li metal batteries[J]. ACS nano, 2019, 13(12): 14549-14556.
[25]. Feng S, Yeerella R H, Zhou J, et al. Homogenizing interfacial stress by nanoporous metal current collector to enable stable all-solid-state Li metal battery[J]. ACS Energy Letters, 2024, 9(2): 748-757.
[26]. Sun Z, Liu M, Zhu Y, et al. Issues concerning interfaces with inorganic solid electrolytes in all-solid-state lithium metal batteries[J]. Sustainability, 2022, 14(15): 9090.
[27]. Kong W, Wang S, Liu H, et al. Compositional Engineering of Lithium Metal Anode for High‐Performance Garnet Type Solid‐State Lithium Battery[J]. Small Methods, 2024: 2400910.
[28]. Wan H, Zhang B, Liu S, et al. Interface Design for High‐Performance All‐Solid‐State Lithium Batteries[J]. Advanced Energy Materials, 2024, 14(19): 2303046
[29]. Zeng Y, Ouyang B, Liu J, et al. High-entropy mechanism to boost ionic conductivity[J]. Science, 2022, 378(6626): 1320-1324.
[30]. Huang W Z, Liu Z Y, Xu P, et al. High-areal-capacity anode-free all-solid-state lithium batteries enabled by interconnected carbon-reinforced ionic-electronic composites[J]. Journal of Materials Chemistry A, 2023, 11(24): 12713-12718.
[31]. Liu Z, Huang W, Xiao Y, et al. Nanocomposite current collectors for anode-free all-solid-state lithium batteries[J]. Acta Physico-Chimica Sinica, 2024, 40(3): 2305040.
Cite this article
Bao,Z. (2025). Interfacial Engineering in Solid-State Lithium Metal Batteries: Degradation Mechanisms and Dynamic Regulation Strategies. Theoretical and Natural Science,109,8-17.
Data availability
The datasets used and/or analyzed during the current study will be available from the authors upon reasonable request.
Disclaimer/Publisher's Note
The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of EWA Publishing and/or the editor(s). EWA Publishing and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
About volume
Volume title: Proceedings of CONF-MPCS 2025 Symposium: Leveraging EVs and Machine Learning for Sustainable Energy Demand Management
© 2024 by the author(s). Licensee EWA Publishing, Oxford, UK. This article is an open access article distributed under the terms and
conditions of the Creative Commons Attribution (CC BY) license. Authors who
publish this series agree to the following terms:
1. Authors retain copyright and grant the series right of first publication with the work simultaneously licensed under a Creative Commons
Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this
series.
2. Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the series's published
version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial
publication in this series.
3. Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and
during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See
Open access policy for details).
References
[1]. Lu, Y., Chen, H., Wang, L., Yu, Z., Huang, Y., Yu, X., Wang, Y. and Roskilly, A.P. (2021) Energy Storage Driving towards a Clean Energy Future. Energy Reports, 7, 8128-8130.
[2]. Schmuch, R., Wagner, R., Hörpel, G., Placke, T. and Winter, M. (2018) Performance and Cost of Materials for Lithium-Based Rechargeable Automotive Batteries. Nature Energy, 3, 267-278.
[3]. XU J, CAI X, CAI S, et al. High‐Energy Lithium‐Ion Batteries: Recent Progress and a Promising Future in Applications[J/OL]. ENERGY & ENVIRONMENTAL MATERIALS, 2023, 6(5): e12450. DOI:10.1002/eem2.12450.
[4]. Lin L, Qin K, Li M, et al. Spinel-related Li2Ni0. 5Mn1. 5O4 cathode for 5-V anode-free lithium metal batteries[J]. Energy Storage Materials, 2022, 45: 821-827.
[5]. Albertus P, Babinec S, Litzelman S, et al. Status and challenges in enabling the lithium metal electrode for high-energy and low-cost rechargeable batteries[J]. Nature Energy, 2018, 3(1): 16-21.
[6]. Yang C , Fu K , Zhang Y ,et al.Protected Lithium‐Metal Anodes in Batteries: From Liquid to Solid[J].Advanced Materials, 2017, 29(36):1701169.DOI:10.1002/adma.201701169.
[7]. Yang C, Xie H, Ping W, et al. An electron/ion dual‐conductive alloy framework for high‐rate and high‐capacity solid‐state lithium‐metal batteries[J]. Advanced Materials, 2019, 31(3): 1804815.
[8]. Huang W Z, Zhao C Z, Wu P, et al. Anode‐free solid‐state lithium batteries: a review[J]. Advanced Energy Materials, 2022, 12(26): 2201044.
[9]. Yu W, Lin K Y, Boyle D T, et al. Electrochemical formation of bis (fluorosulfonyl) imide-derived solid-electrolyte interphase at Li-metal potential[J]. Nature Chemistry, 2024: 1-10.
[10]. Ding J F, Xu R, Yan C, et al. A review on the failure and regulation of solid electrolyte interphase in lithium batteries[J]. Journal of Energy Chemistry, 2021, 59: 306-319.
[11]. Kim K J, Balaish M, Wadaguchi M, et al. Solid‐state Li–metal batteries: challenges and horizons of oxide and sulfide solid electrolytes and their interfaces[J]. Advanced Energy Materials, 2021, 11(1): 2002689.
[12]. Zhao D, Wang J, Wang P, et al. Regulating the composition distribution of layered SEI film on Li-ion battery anode by LiDFBOP[J]. Electrochimica Acta, 2020, 337: 135745.
[13]. Brooks D J, Merinov B V, Goddard III W A, et al. Atomistic description of ionic diffusion in PEO–LiTFSI: Effect of temperature, molecular weight, and ionic concentration[J]. Macromolecules, 2018, 51(21): 8987-8995.
[14]. Wang Q, Liu B, Shen Y, et al. Confronting the challenges in lithium anodes for lithium metal batteries[J]. Advanced Science, 2021, 8(17): 2101111.
[15]. Koerver R, Zhang W, De Biasi L, et al. Chemo-mechanical expansion of lithium electrode materials–on the route to mechanically optimized all-solid-state batteries[J]. Energy & Environmental Science, 2018, 11(8): 2142-2158.
[16]. Esmizadeh S, Cabras L, Serpelloni M, et al. A review on modeling of nucleation and growth of Li dendrites in solid electrolytes[J]. Journal of Energy Storage, 2024, 97: 112897.
[17]. Wu B, Wang S, Lochala J, et al. The role of the solid electrolyte interphase layer in preventing Li dendrite growth in solid-state batteries[J]. Energy & Environmental Science, 2018, 11(7): 1803-1810.
[18]. Krauskopf T, Richter F H, Zeier W G, et al. Physicochemical concepts of the lithium metal anode in solid-state batteries[J]. Chemical reviews, 2020, 120(15): 7745-7794.
[19]. Jagger B, Pasta M. Solid electrolyte interphases in lithium metal batteries[J]. Joule, 2023, 7(10): 2228-2244.
[20]. Ke X, Wang Y, Dai L, et al. Cell failures of all-solid-state lithium metal batteries with inorganic solid electrolytes: Lithium dendrites[J]. Energy Storage Materials, 2020, 33: 309-328.
[21]. Chen X R, Chen X, Yan C, et al. Role of lithiophilic metal sites in lithium metal anodes[J]. Energy & Fuels, 2021, 35(15): 12746-12752.
[22]. Ma Q, Zheng Y, Luo D, et al. 2D materials for all‐solid‐state lithium batteries[J]. Advanced Materials, 2022, 34(16): 2108079.
[23]. Huang S, Yang H, Hu J, et al. Early lithium plating behavior in confined nanospace of 3D lithiophilic carbon matrix for stable solid‐state lithium metal batteries[J]. Small, 2019, 15(43): 1904216.
[24]. Wen J, Huang Y, Duan J, et al. Highly adhesive Li-BN nanosheet composite anode with excellent interfacial compatibility for solid-state Li metal batteries[J]. ACS nano, 2019, 13(12): 14549-14556.
[25]. Feng S, Yeerella R H, Zhou J, et al. Homogenizing interfacial stress by nanoporous metal current collector to enable stable all-solid-state Li metal battery[J]. ACS Energy Letters, 2024, 9(2): 748-757.
[26]. Sun Z, Liu M, Zhu Y, et al. Issues concerning interfaces with inorganic solid electrolytes in all-solid-state lithium metal batteries[J]. Sustainability, 2022, 14(15): 9090.
[27]. Kong W, Wang S, Liu H, et al. Compositional Engineering of Lithium Metal Anode for High‐Performance Garnet Type Solid‐State Lithium Battery[J]. Small Methods, 2024: 2400910.
[28]. Wan H, Zhang B, Liu S, et al. Interface Design for High‐Performance All‐Solid‐State Lithium Batteries[J]. Advanced Energy Materials, 2024, 14(19): 2303046
[29]. Zeng Y, Ouyang B, Liu J, et al. High-entropy mechanism to boost ionic conductivity[J]. Science, 2022, 378(6626): 1320-1324.
[30]. Huang W Z, Liu Z Y, Xu P, et al. High-areal-capacity anode-free all-solid-state lithium batteries enabled by interconnected carbon-reinforced ionic-electronic composites[J]. Journal of Materials Chemistry A, 2023, 11(24): 12713-12718.
[31]. Liu Z, Huang W, Xiao Y, et al. Nanocomposite current collectors for anode-free all-solid-state lithium batteries[J]. Acta Physico-Chimica Sinica, 2024, 40(3): 2305040.









