1. Introduction
Osteoarthritis (OA), a joint syndrome with symptoms such as joint swelling, deformity, stiffness, weakness, pain, and tenderness, is significantly impairing patients' quality of life. As a degenerative joint disorder, OA develops with aging, overuse, and joint injuries. Its pathogenesis is complex, involving inflammatory responses, cartilage degradation, oxidative stress, and apoptosis.
A joint consists of bone and cartilage. Compared to bone tissue, Cartilage tissue is lack in cell number (primarily chondrocytes) and exhibit limited diversity. Cartilage tissue ECM is composed of approximately 70% to 80% waters, along with a significant amount of collagen and glycosaminoglycans. Cartilage lacks blood and lymphatic vessels, resulting in inefficient nutrient delivery and poor synthetic metabolism [1]. Consequently, self-repair after injury is minimal, necessitating reliance on exogenous pharmacological interventions. Current clinical treatments focus on pain relief and functional improvement but lack precision in halting disease progression. Moreover, continuous employ of nonsteroidal anti-inflammatory drugs (NSAIDs) is associated with massive side effects.
Marine Omega-3 PUFAs, including Docosahexaenoic Acid (DHA), Eicosapentaenoic Acid (EPA), etc., are bioactive compounds not endogenously synthesized in humans. Marine sources such as krill, microalgae, and deep-sea fish provide EPA and DHA in distinct forms (Table 1). Cod liver oil and tuna oil contain EPA and DHA mainly in triglyceride form, while krill oil conserve these PUFAs in phospholipid forms, and algal oil is rich mainly in DHA instead of EPA [2].
Table 1: Contents of EPA/DHA in marine extract [2]
Type of extract | Typical EPA/DHA content per g of extract (mg) | Contents | |
Cod liver oil | 200 | EPA & DHA | in triglyceride form |
Standard fish oil | 300 | ||
Fish oil concentrate | 450–600 | ||
Tuna oil | 460 | ||
Krill oil | 205 | in phospholipid form | |
Algal oil | 400 | DHA | |
Marine Omega-3 PUFAs modulate cellular membrane fluidity, signal transduction, and gene expression, exerting anti-inflammatory, immunoregulatory, and chondroprotective effects [2]. Recent studies have increasingly explored their potential in OA prevention and management. This review synthesizes molecular mechanisms and clinical evidence to explore the therapeutic potential of Marine Omega-3 PUFAs in OA, providing insights for future research and clinical applications.
2. Current status and treatment dilemmas of OA
2.1. Epidemiological overview
OA has become one of the most disabling chronic diseases worldwide. Clinically, the severity of OA pain is assessed in randomized controlled trials (RCT) using two primary metrics: the WOMAC and the visual analogue scale (VAS) [3]. OA affects both elderly and younger populations, with the global prevalence among individuals aged 30 and older increasing to 14.8% over recent decades. The risk of OA increases sharply with aging, making it the main reason for disability in adults aged 60 and older [4]. Authoritative clinical studies and statistical analyses have identified sedentary lifestyles, lack of exercise, and high-sugar/high-fat diets as major contributing factors to OA progression [5]. Furthermore, due to aging populations and the prevalence of obesity and physical inactivity, OA is increasingly affecting younger individuals. A global analysis revealed that between 1990 and 2021, only two out of 204 countries and regions experienced a decline in age-standardized incidence rates (ASR) of OA, while the majority showed upward trends. OA is closely linked to societal aging and population density, leading to joint dysfunction, reduced quality of life, and substantial socioeconomic burdens [6].
2.2. Limitations of current treatments
According to guidelines from the American College of Rheumatology, OA management primarily relies on symptomatic therapies, including non-pharmacological interventions (e.g., physical therapy, weight loss) and pharmacological treatments (e.g., NSAIDs, corticosteroids or hyaluronic acid). However, these approaches have significant drawbacks. Long-term NSAID use increases risks of gastrointestinal bleeding, cardiovascular events, and renal impairment. Corticosteroids deliver temporary anti-inflammatory relief but may cause cartilage degeneration with repeated injections. Hyaluronic acid injections exhibit variable efficacy among individuals and show limited effectiveness in advanced OA. Surgical interventions like joint replacement, while beneficial for end-stage patients, carry risks of postoperative infections, prosthesis loosening, and are less ideal for younger patients [7].
3. Mechanisms of Marine Omega-3 PUFAs in OA
Marine Omega-3 PUFAs, as natural anti-inflammatory agents, demonstrate potential in alleviating OA pain and delaying cartilage degradation. Key mechanisms include:
3.1. Suppression of pro-inflammatory mediators
As illustrated in Figure 1, high-sugar/high-fat diets exacerbate inflammatory responses by increasing intracellular lipid accumulation. Saturated fatty acids activate Toll-like receptors, triggering NF-κB signaling and subsequent transcription of pro-inflammatory genes. Hypoxia in adipose tissues further activates damage-associated molecular patterns (DAMPs) [8].
EPA and DHA enhance mitochondrial and peroxisomal fatty acid β-oxidation, reducing lipid droplet size. They upregulate the anti-inflammatory adipokine adiponectin via PPAR-γ-dependent pathways and inhibit NF-κB signaling through GPR120 binding [8]. Additionally, EPA and DHA metabolites, such as resolvins and protectins, competitively inhibit cyclooxygenase-2 (COX-2) activity, reducing leukotrienes and pro-inflammatory prostaglandins0, while promoting the synthesis of anti-inflammatory mediators (e.g., resolvin E1 and protectin D1) [9]. These specialized pro-resolving mediators (SPMs) suppress neutrophil infiltration and polarize macrophages toward an M2 anti-inflammatory phenotype via G protein-coupled receptor activation [10].
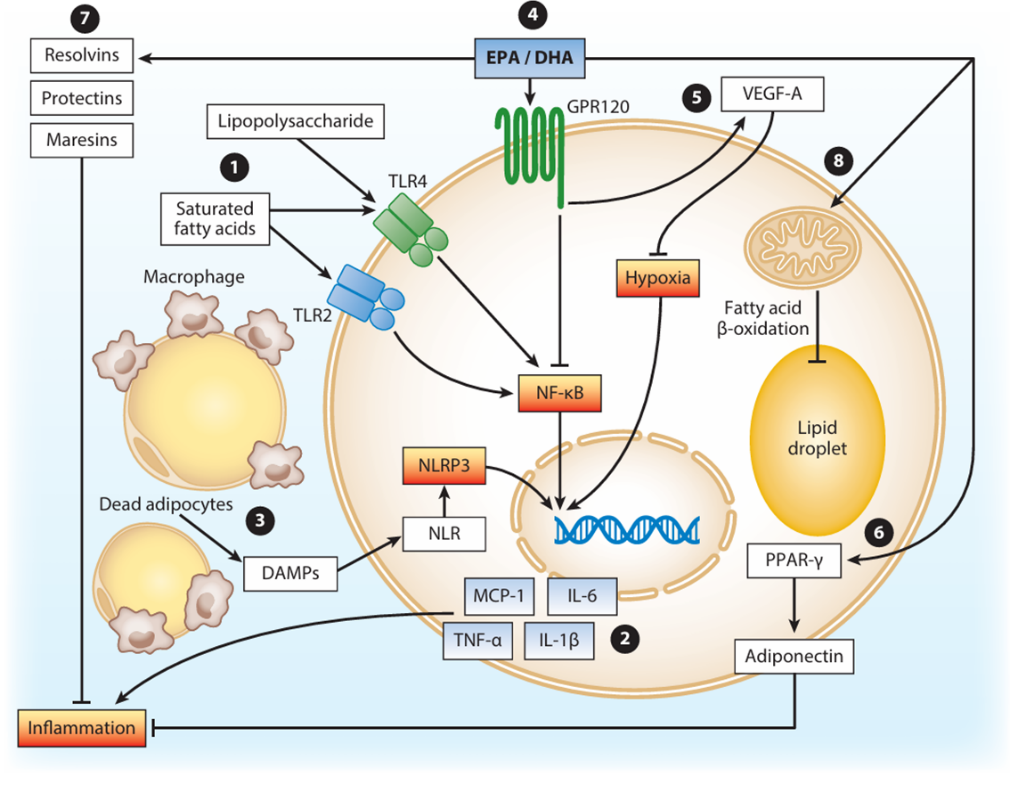
Figure 1: Omega-3 modulates inflammation triggered by obesity [8]
3.2. Additional mechanisms of Marine Omega-3 PUFAs
EPA and DHA enhance chondrocyte anabolism, thereby protecting cartilage integrity. These PUFAs modulate intracellular signaling pathways to stimulate proteoglycan and collagen synthesis. For example, DHA activates the PI3K/Akt pathway, promoting proteoglycan synthesis and improving cartilage elasticity and load-bearing capacity [10]. Additionally, EPA prevents nitric oxide overproduction and preserving cartilage structure [11].
4. Research on Marine Omega-3 PUFAs applications
4.1. In vitro studies
An in vitro study applied a physical impact to human cartilage explants using a customized drop tower. EPA was added to the culture medium of the experimental group one day prior to this physical treatment. Cartilage explants subjected to contusion injury exhibited significant surface stretch and structural destruction. Untreated explants displayed extensive cartilage injury after contusion. In contrast, EPA-treated explants showed surface compression but significantly reduced structural damage compared to the contusion-only group. RT-qPCR and immunofluorescence were employed to measure type II collagen mRNA levels and protein expression, respectively. EPA-preconditioned explants maintained collagen secretion levels close to baseline under 30% mechanical deformation simulating extreme joint environments [12].
4.2. Animal experiments
In an OA mouse model induced by destabilizing the medial meniscus of the knee joint, mice fed a saturated fatty acid-rich diet developed aggravated OA symptoms, including ectopic ossification, synovitis, osteophyte formation, and fibrotic tissue. In contrast, mice fed an omega-3-rich diet demonstrated enhanced wound repair, as evidenced by histological sections and three-dimensional reconstructed images from Micro-CT [13].
4.3. Clinical trials
Marine Omega-3 PUFAs supplementation has shown therapeutic potential in clinical trials. A double-blind experiment, involving patients with mild-to-moderate knee OA, randomized participants to receive krill oil or placebo. Krill oil, derived from Antarctic krill, contains EPA, DHA, phospholipids, and astaxanthin, with phospholipids enhancing EPA/DHA absorption. The krill oil group consumed EPA and DHA daily, while the placebo contained 1.7% of daily fat intake to avoid influencing OA progression. Omega-3 indices were measured via blood tests, and non-steroidal anti-inflammatory drugs were minimized. The WOMAC questionnaire (24 items scored 0–10, total normalized to 100) revealed that after 6 months, the krill oil group had a significantly higher omega-3 index (+3.22%) and reduced pain scores (adjusted mean difference: −5.18; 95% CI: −10.0, −0.32; P = 0.04). Pain reduction was most pronounced in patients with high inflammation (hsCRP≥3 mg/L) [14].
A meta-analysis of 9 RCTs demonstrated that Marine Omega-3 PUFAs application significantly alleviated OA pain (n = 2070; mean difference = 22.89; 95% CI: 3.37–42.42) without severe adverse events [5].
5. Limitations and future prospects
Current research faces several limitations: Mechanistic studies predominantly focus on Marine Omega-3 PUFAs’ effects on general inflammatory responses or inflammatory cells (e.g., white adipocytes), with scarce investigations specifically targeting articular chondrocytes; Interactions between Marine Omega-3 PUFAs and systems such as gut microbiota, immunity, and aging remain unclear, potentially contributing to individual variability in therapeutic outcomes; Existing clinical trials exhibit substantial heterogeneity in Marine Omega-3 PUFAs dosages and lack long-term (>6 months) efficacy assessments, hindering the identification of optimal regimens. As one sort of fatty acids, when Marine Omega-3 PUFAs combined with lipid-soluble synthetic drugs or natural products possessing anti-inflammatory, chondroprotective, and pro-differentiation properties, could represent a theoretically viable therapeutic strategy for OA. However, current experimental studies predominantly focus on natural supplements containing Omega-3 or purified DHA/EPA derived from such supplements, with limited progress in pharmacological innovation targeting synergistic mechanisms.
Future exploration should address the following dimensions: First of all, Marine Omega-3 PUFAs require a simple, feasible, accurate and efficient delivery method (In the previous clinical trial, it was just dissolved in oil and directly orally delivered), such as preparing nanocarriers to embed omega-3 through hydrogels, phospholipid complexes, collagen and other materials, so as to improve the absorption and utilization efficiency in the human body, targeting the joint tissue, and preventing the degradation of effective ingredients and the generation of harmful oxidation products; Explore the synergistic effects of Marine Omega-3 PUFAs with glucosamine, collagen, natural nutritional supplements, natural antioxidant agents, novel generation of anti-inflammatory drugs, etc., and explores multi-modal intervention mechanisms combining exercise therapy and hypoglycemic/lipid-lowering therapies, aiming to develop comprehensive, multi-level, and wide-ranging integrated therapeutic strategies for osteoarthritis management; Employ transcriptomic, proteomic, and metabolomic approaches to investigate Marine Omega-3 PUFAs’ regulation of metabolic reprogramming in joint tissue chondrocytes, focusing on energy metabolism, lipid biosynthesis, and oxidative stress pathways, while exploring its potential in modulating epigenetic mechanisms, autophagy, and cellular senescence to inform osteoarthritis therapeutics; Conduct unprecedented large-scale long-term clinical validation, which is conducting multi center RCTs to clarify the applicability and differences of optimal dosage, course of treatment, administration method, and combination therapy for patients with different subtypes of OA; Expand the aquaculture of bivalves, microalgae, etc., and other novel economic species while obtaining a large amount of natural Marine Omega-3 PUFAs can alleviate the problems of marine biological resources exhaustion and high production cost caused by the traditional fish/krill oil production. In addition to that, promoting the industrialized production of omega-3 by microbial fermentation could strictly control the molecular specifications (e.g., chain length, purity) of Omega-3, and can also carry out the transformation of chemical groups and increase functional groups with special functions. This fermentation system, which takes into account cost-effectiveness and ecological sustainability, will also be one of the critical keys to Marine Omega-3 PUFAs’ wide application.
6. Conclusion
As a chronic degenerative joint disease, OA urgently requires exploration of safe and effective novel intervention strategies due to its high prevalence and limitations in current treatments. Marine Omega-3 PUFAs (e.g., EPA, DHA), with their multi-target anti-inflammatory, cartilage-repairing, and metabolic-regulating properties, have emerged as a research hotspot in OA therapy. This article systematically elucidates the potential and mechanisms of Marine Omega-3 PUFAs in OA treatment by integrating molecular mechanisms and clinical evidence, while proposing future research directions. Omega-3 demonstrates significant efficacy in anti-inflammation, mechanical protection, metabolic regulation, tissue regeneration, and pain relief, holding promise as a novel therapeutic approach for OA.
Nonetheless, bridging the gap between basic study and therapeutic applications demands interdisciplinary collaboration and technological innovation to fully unlock their therapeutic potential. By synergizing marine biotechnology, pharmaceutical innovation, and precision medicine, Marine Omega-3 PUFAs may emerge as a sustainable, multi-target strategy for OA management. In the future, burgeoning exploration on molecular mechanisms, pharmaceutical development, and clinical trials in the fields of age-related diseases exemplified by osteoarthritis and marine-derived bioactive compounds represented by DHA and EPA will propel Marine Omega-3 PUFAs into uncharted therapeutic frontiers.
References
[1]. Huey DJ, Hu JC, Athanasiou KA. Unlike bone, cartilage regeneration remains elusive. Science. 2012 Nov 16;338(6109):917-21.
[2]. Djuricic I, Calder PC. Pros and Cons of Long-Chain Omega-3 Polyunsaturated Fatty Acids in Cardiovascular Health. Annu Rev Pharmacol Toxicol. 2023 Jan 20;63:383-406.
[3]. Deng W, Yi Z, Yin E, et al. Effect of omega-3 polyunsaturated fatty acids supplementation for patients with osteoarthritis: a meta-analysis. J Orthop Surg Res. 2023 May 24;18(1):381.
[4]. GBD 2021 Osteoarthritis Collaborators. Global, regional, and national burden of osteoarthritis, 1990-2020 and projections to 2050: a systematic analysis for the Global Burden of Disease Study 2021. Lancet Rheumatol. 2023;5(9):e508-e522.
[5]. Berenbaum F, Wallace IJ, Lieberman DE, et al. Modern-day environmental factors in the pathogenesis of osteoarthritis. Nat Rev Rheumatol. 2018 Nov;14(11):674-681
[6]. Li HZ, Liang XZ, Sun YQ, et al. Global, regional, and national burdens of osteoarthritis from 1990 to 2021: findings from the 2021 global burden of disease study. Front Med (Lausanne). 2024 Nov 14;11:1476853.
[7]. Hochberg MC, Altman RD, April KT, et al. American College of Rheumatology. American College of Rheumatology 2012 recommendations for the use of nonpharmacologic and pharmacologic therapies in osteoarthritis of the hand, hip, and knee. Arthritis Care Res (Hoboken). 2012 Apr;64(4):465-74.
[8]. Kalupahana NS, Goonapienuwala BL, Moustaid-Moussa N. Omega-3 Fatty Acids and Adipose Tissue: Inflammation and Browning. Annu Rev Nutr. 2020 Sep 23;40:25-49.
[9]. Calder PC. Marine omega-3 fatty acids and inflammatory processes: Effects, mechanisms and clinical relevance. Biochim Biophys Acta. 2015 Apr;1851(4):469-84. doi: 10.1016/j.bbalip.2014.08.010. Epub 2014 Aug 20. PMID: 25149823.
[10]. Calder PC. Omega-3 polyunsaturated fatty acids and inflammatory processes: nutrition or pharmacology? Br J Clin Pharmacol. 2013 Mar;75(3):645-62. doi: 10.1111/j.1365-2125.2012.04374.x. PMID: 22765297; PMCID: PMC3575932.
[11]. Abshirini M, Ilesanmi-Oyelere BL, Kruger MC. Potential modulatory mechanisms of action by long-chain polyunsaturated fatty acids on bone cell and chondrocyte metabolism. Prog Lipid Res. 2021 Jul;83:101113.
[12]. Chen F, Zhang Z, Wang W, et al. Omega-3 fatty acids protect cartilage from acute injurie by reducing the mechanical sensitivity of chondrocytes. J Orthop Surg Res. 2024 Sep 28;19(1):591. doi: 10.1186/s13018-024-05081-4. PMID: 39342268; PMCID: PMC11437636.
[13]. Wu CL, Jain D, McNeill JN, et al. Dietary fatty acid content regulates wound repair and the pathogenesis of osteoarthritis following joint injury. Ann Rheum Dis. 2015 Nov;74(11):2076-83.
[14]. Stonehouse W, Benassi-Evans B, Bednarz J, et al. Krill oil improved osteoarthritic knee pain in adults with mild to moderate knee osteoarthritis: a 6-month multicenter, randomized, double-blind, placebo-controlled trial. Am J Clin Nutr. 2022 Sep 2;116(3):672-685.
Cite this article
Tan,J. (2025). Exploring the Therapeutic Potential and Mechanisms of Marine Omega-3 PUFAs in Osteoarthritis. Theoretical and Natural Science,111,57-62.
Data availability
The datasets used and/or analyzed during the current study will be available from the authors upon reasonable request.
Disclaimer/Publisher's Note
The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of EWA Publishing and/or the editor(s). EWA Publishing and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
About volume
Volume title: Proceedings of ICBioMed 2025 Symposium: AI for Healthcare: Advanced Medical Data Analytics and Smart Rehabilitation
© 2024 by the author(s). Licensee EWA Publishing, Oxford, UK. This article is an open access article distributed under the terms and
conditions of the Creative Commons Attribution (CC BY) license. Authors who
publish this series agree to the following terms:
1. Authors retain copyright and grant the series right of first publication with the work simultaneously licensed under a Creative Commons
Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this
series.
2. Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the series's published
version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial
publication in this series.
3. Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and
during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See
Open access policy for details).
References
[1]. Huey DJ, Hu JC, Athanasiou KA. Unlike bone, cartilage regeneration remains elusive. Science. 2012 Nov 16;338(6109):917-21.
[2]. Djuricic I, Calder PC. Pros and Cons of Long-Chain Omega-3 Polyunsaturated Fatty Acids in Cardiovascular Health. Annu Rev Pharmacol Toxicol. 2023 Jan 20;63:383-406.
[3]. Deng W, Yi Z, Yin E, et al. Effect of omega-3 polyunsaturated fatty acids supplementation for patients with osteoarthritis: a meta-analysis. J Orthop Surg Res. 2023 May 24;18(1):381.
[4]. GBD 2021 Osteoarthritis Collaborators. Global, regional, and national burden of osteoarthritis, 1990-2020 and projections to 2050: a systematic analysis for the Global Burden of Disease Study 2021. Lancet Rheumatol. 2023;5(9):e508-e522.
[5]. Berenbaum F, Wallace IJ, Lieberman DE, et al. Modern-day environmental factors in the pathogenesis of osteoarthritis. Nat Rev Rheumatol. 2018 Nov;14(11):674-681
[6]. Li HZ, Liang XZ, Sun YQ, et al. Global, regional, and national burdens of osteoarthritis from 1990 to 2021: findings from the 2021 global burden of disease study. Front Med (Lausanne). 2024 Nov 14;11:1476853.
[7]. Hochberg MC, Altman RD, April KT, et al. American College of Rheumatology. American College of Rheumatology 2012 recommendations for the use of nonpharmacologic and pharmacologic therapies in osteoarthritis of the hand, hip, and knee. Arthritis Care Res (Hoboken). 2012 Apr;64(4):465-74.
[8]. Kalupahana NS, Goonapienuwala BL, Moustaid-Moussa N. Omega-3 Fatty Acids and Adipose Tissue: Inflammation and Browning. Annu Rev Nutr. 2020 Sep 23;40:25-49.
[9]. Calder PC. Marine omega-3 fatty acids and inflammatory processes: Effects, mechanisms and clinical relevance. Biochim Biophys Acta. 2015 Apr;1851(4):469-84. doi: 10.1016/j.bbalip.2014.08.010. Epub 2014 Aug 20. PMID: 25149823.
[10]. Calder PC. Omega-3 polyunsaturated fatty acids and inflammatory processes: nutrition or pharmacology? Br J Clin Pharmacol. 2013 Mar;75(3):645-62. doi: 10.1111/j.1365-2125.2012.04374.x. PMID: 22765297; PMCID: PMC3575932.
[11]. Abshirini M, Ilesanmi-Oyelere BL, Kruger MC. Potential modulatory mechanisms of action by long-chain polyunsaturated fatty acids on bone cell and chondrocyte metabolism. Prog Lipid Res. 2021 Jul;83:101113.
[12]. Chen F, Zhang Z, Wang W, et al. Omega-3 fatty acids protect cartilage from acute injurie by reducing the mechanical sensitivity of chondrocytes. J Orthop Surg Res. 2024 Sep 28;19(1):591. doi: 10.1186/s13018-024-05081-4. PMID: 39342268; PMCID: PMC11437636.
[13]. Wu CL, Jain D, McNeill JN, et al. Dietary fatty acid content regulates wound repair and the pathogenesis of osteoarthritis following joint injury. Ann Rheum Dis. 2015 Nov;74(11):2076-83.
[14]. Stonehouse W, Benassi-Evans B, Bednarz J, et al. Krill oil improved osteoarthritic knee pain in adults with mild to moderate knee osteoarthritis: a 6-month multicenter, randomized, double-blind, placebo-controlled trial. Am J Clin Nutr. 2022 Sep 2;116(3):672-685.









