1. Introduction
In recent years, the incidence of cancer has shown a continuous upward trend year by year. Due to its high incidence, high mortality rate, and high recurrence rate, cancer has become one of the greatest threats to human life and health and quality of life. According to statistics, in 2022, the number of new cancer cases in China reached 4.825 million, accounting for 24.17% of the global total [1]. In the past decade, the incidence of malignant tumors in China has increased by 3.9% annually, and the mortality rate has increased by 2.5% annually. Malignant tumors account for 23.91% of all causes of death among residents, and the prevention and control situation are severe [2]. The number of new cancer cases worldwide has been increasing year by year. According to statistics, the number of new cancer cases in the United States will reach 1.95 million in 2023, with a death toll of 600,000.
Therefore, cancer treatment remains a major global health challenge, with its incidence rate continuing to rise annually, posing a serious threat to global public health. With the development of nanomaterials and the diversification of their functions, their application in cancer treatment has gradually become widespread. This article will start from the methods of cancer treatment, introduce the structure and material properties of carbon-based nanomaterials, and further summarize their application in cancer phototherapy. Finally, based on the existing research results, the future development prospects and challenges of nanomaterials in the biomedical field will be discussed.
2. The current situation of cancer treatment
2.1. Traditional therapy
With the development of science and technology, various effective methods have been developed to combat tumors. The current main clinical tumor treatment methods include surgical treatment (mainly by surgically removing the lesions), radiotherapy, and chemotherapy. These traditional treatment methods are often widely used as important tools for tumor treatment in clinical applications. However, some inherent potential limitations, such as low treatment efficiency, high cost, strong toxic and side effects, and a high likelihood of triggering allergic reactions, cannot be underestimated. Surgery is a very effective method for treating early-stage cancers at present. However, it has limitations. During the surgical procedure, the tumor and its adjacent normal tissues need to be removed, and there are certain requirements for the patient's physical constitution and the type of tumor. In clinical practice, the high radiation of radiotherapy may induce DNA damage, which may further lead to cell apoptosis. The infusion of chemotherapy-related drugs may cause high toxicity to the cells in the body, resulting in relevant side effects [3].
2.2. Novel therapeutic approaches
In recent years, with the continuous deepening of the understanding of tumors and clinical diagnosis, various other effective tumor treatment modalities have emerged successively, such as photodynamic therapy, photothermal therapy, magnetothermal therapy, immunotherapy, gene therapy, etc. These approaches offer diversified options for tumor treatment and bring more hope to patients.
2.3. Phototherapy
2.3.1. Photothermal therapy
Photothermal therapy (PTT) is a method that utilizes photothermal conversion nanomaterials to convert the energy in the near-infrared light region (NIR, with a wavelength ranging from 700 to 1300 nm) into heat energy [4]. This process raises the temperature of the local tumor tissue to 40°C to 45°C or above 45°C, leading to the denaturation and necrosis of tumor cells, thus achieving the goal of tumor treatment [5].
2.3.2. Photodynamic therapy
Photodynamic therapy (PDT) is an ideal method for treating tumor and non-tumor degenerative diseases. PDT is a therapeutic approach based on the interaction between light and chemical substances, which in turn induces a killing effect. Its basic principle is that the photosensitizer (PS) is excited by light of a specific wavelength. After reacting with molecular oxygen, reactive oxygen species (ROS) are generated in the cells, which subsequently trigger cell necrosis and apoptosis. PDT has been widely applied in cancer treatment.
3. Carbon-based nanomaterials and phototherapy
Both photothermal therapy and photodynamic therapy require the identification of suitable photothermal conversion agents or photosensitizers. Different nanomaterials possess unique structures and properties, which can significantly enhance the effectiveness of phototherapy in various aspects, thus bringing new opportunities for tumor treatment. Carbon-based nanomaterials refer to materials in which at least one dimension of the dispersed phase has a scale of less than 100 nm. The dispersed phase can be composed of carbon atoms, heterogeneous atoms, or even nanopores. Currently, the mainly developed carbon-based nanomaterials include carbon quantum dots, carbon nanotubes, graphene, fullerenes, etc. These materials have remarkable characteristics, such as an ultra-high specific surface area, a large drug-loading capacity, strong light absorption ability, and high photothermal conversion efficiency, and they can be widely applied in photothermal and photodynamic therapies (Figure 1), etc. [6,7]. This paper will mainly introduce the structures, properties, and application progress in the field of cancer treatment of four representative carbon-based nanomaterials.
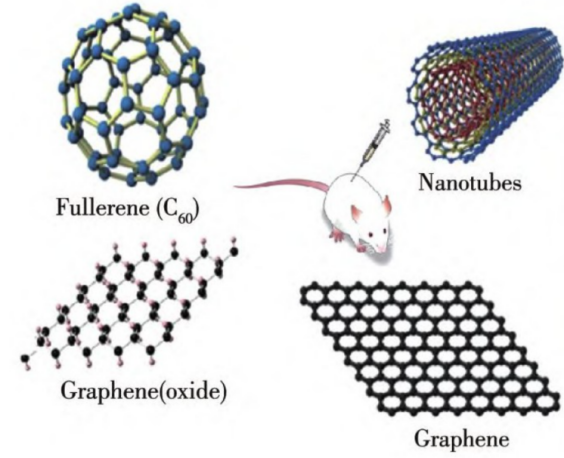
Figure 1: Applications of common carbon-based nanomaterials in phototherapy
3.1. Carbon quantum dots
3.1.1. Structure and material properties
Carbon quantum dots (CDs) have a lamellar structure composed of a honeycomb-like arrangement of carbon six-membered rings. Their composition includes carbon, hydrogen, oxygen, and nitrogen. They are a new type of zero-dimensional carbon-based nanomaterials with excellent optical properties and good biocompatibility. They have a wide range of sources and simple preparation methods. The advantages of CDs lie in their small particle size, good permeability, and high biological safety. Therefore, they can be modified with biological macromolecules loaded with anti-tumor drugs to establish relevant drug carrier systems. CDs can undergo different surface modifications depending on the raw materials [8,9]. In addition, most carbon dots exhibit absorption capacity in the near-infrared region and can be used for photothermal therapy of tumors.
3.1.2. Applications in phototherapy
Liu et al. developed an immune-inducing carbon dot hydrogel (iCD@Gel) using mannose-modified aluminum-doped carbon quantum dots as cross-linkers (Figure 2). The photothermal therapy mediated by aluminum-doped carbon dots triggered immunogenic cell death, thereby inducing a strong tumor-killing effect [10].
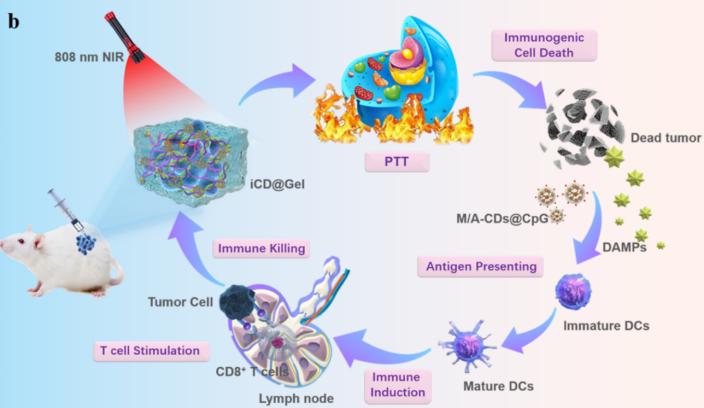
Figure 2: Schematic diagram of the photothermal therapy mediated by iCD@Gel
Yang Yumeng et al. [11] developed a nanoplatform for synergistic photothermal-photodynamic therapy targeting mitochondria, namely gold-doped carbon quantum dots T-CDs@Au. After intratumoral injection, T-CDs@Au/ALA was endocytosed by cells and enriched in mitochondria (Figure 3). Here, 5-ALA could be released and converted into protoporphyrin (Pp IX). Due to the excellent photodynamic responsiveness of Pp IX, a large amount of reactive oxygen species (ROS) was rapidly generated under 660 nm laser irradiation, which in turn killed tumor cells. Meanwhile, on the one hand, gold doping depleted glutathione (GSH) by forming Au-S bonds with the antioxidant GSH, enhancing the therapeutic effect of photodynamic therapy (PDT) [12-14]. On the other hand, gold doping also significantly improved the photothermal performance of T-CDs@Au/ALA, and further killed tumor cells by mediating local temperature increase.
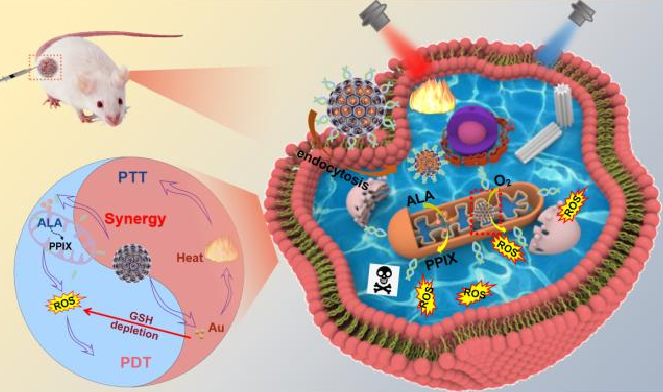
Figure 3: The in vivo antitumor applications of T-CDs@Au/ALA
3.2. Graphene and its derivatives
3.2.1. Structure and material properties
Graphene is a sheet-like substance formed by the arrangement of hexagonal aromatic rings of carbon atoms through sp2 hybridization. The lamellae form a layered structure through van der Waals forces and π-π interactions. In a single layer of graphene, each carbon atom has four valence electrons. Three of them form σ bonds with three adjacent carbon atoms, and the fourth electron is unpaired, forming a π bond. The delocalized π electrons run through the graphene lamella, endowing it with high electrical conductivity and high photothermal properties [15]. Since its first discovery, graphene and its derivatives [graphene oxide (GO), reduced graphene oxide (rGO), etc.] have also attracted extensive attention in biomedical fields such as phototherapy. In addition to the advantages of strong electrical conductivity, high thermal conductivity, and large specific surface area, some of them also have the advantage of a wide absorption band that can cover the near-infrared (NIR) region.
3.2.2. Applications in phototherapy
The ultra-high specific surface area of graphene and its derivatives can be used for drug delivery and is also conducive to functionalization with polyethylene glycol (PEG), hyaluronic acid (HA), etc. [16-18]. Graphene is insoluble in physiological media, but GO has better solubility in physiological solutions [19]. GO has light absorption from the ultraviolet (UV) to the NIR region [20]. Liu et al. [21] used PEGylated GO to load chlorin e6 (Ce6) for photothermal therapy (PTT)-enhanced photodynamic therapy (PDT). The PTT induced by GO under an 808 nm laser did not lead to cell death but caused mild local heating, which could improve the cell membrane permeability and greatly promote the intracellular uptake of Ce6, enhancing the PDT efficacy of Ce6. This study reveals the broad prospects of graphene in the combined treatment of cancer.
Ting et al. [22] developed a triple-effect nanosystem for synergistic photothermal, photodynamic, and chemotherapy. PEG-functionalized graphene oxide nanoparticles with protoporphyrin were synthesized through supramolecular interactions and covalently bound to the chemotherapeutic drug doxorubicin. Cell experiments showed that the triple-effect nanosystem significantly increased the uptake and cytotoxicity of the chemotherapeutic drug doxorubicin in breast cancer cells 4T1. In vivo experiments showed that, compared with the severe cardiotoxicity of doxorubicin, the triple-effect nanosystem had no toxic side effects and had a stronger antitumor effect.
3.3. Carbon nanotubes
3.3.1. Structure and material properties
Carbon nanotubes (CNTs) are formed by curling single-layer or multi-layer graphene sheets and have a long and hollow cylindrical structure. They have now been widely used in various industries. CNTs are suitable as drug carriers and can load a variety of targeting ligands and drugs. After appropriate modification, their toxicity can be reduced, endowing them with biocompatibility. According to their structures, CNTs can be divided into single-walled carbon nanotubes (SWNTs) and multi-walled carbon nanotubes (MWNTs). Both of them exhibit near-infrared (NIR) optical absorption and good photothermal conversion properties.
3.3.2. Applications in phototherapy
Single-walled carbon nanotubes (SWNTs) are more flexible than multi-walled carbon nanotubes (MWNTs), with fewer structural defects and higher solubility [23], so they are more commonly used in cancer treatment and have been applied in near-infrared (NIR)-induced photothermal therapy (PTT) and photodynamic therapy (PDT). Shen et al. [24] prepared the SWNTs-PEG-Fe₃O₄@CQDs nanocomposite, which exhibits both photodynamic and photothermal effects under 808 nm laser irradiation (Figure 4). It can load drugs and has the characteristic of pH/NIR photothermal-responsive drug release. The nanocomposite coupled with an aptamer is denoted as SWCNTs-PEG-Fe₃O₄ @CQDS/DOX-Apt, which can be used for the targeted dual-modal fluorescence/magnetic resonance imaging and the combined regimen of PTT/PDT/chemotherapy. SWNTs-PEG-Fe₃O₄@CQDs can be used to treat diseases such as cervical cancer that require specific drug targeting.
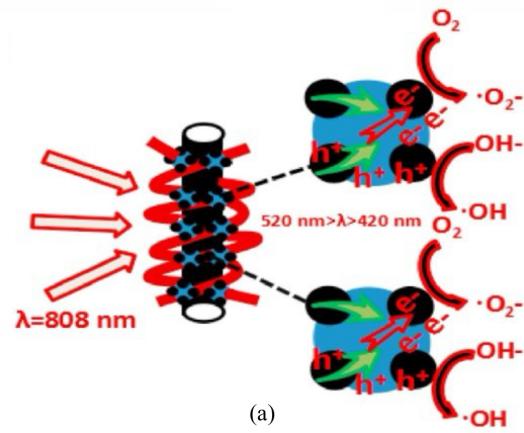
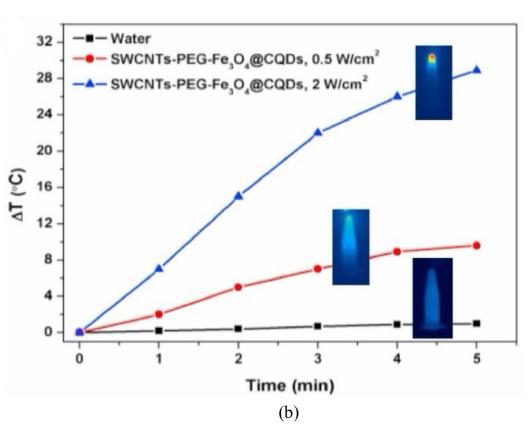
Figure 4: (a)The possible catalytic mechanism for SWCNTs-PEG-Fe3O4@CQDs under visible light. (b)Laser power dose-dependent temperature elevation of SWCNTs-PEG-Fe3O4@CQDs solution (50 µg/mL) under 808 nm laser irradiation for 5 min (initial temperature 22 °C)[24]
In addition, the nanocomposite coupled with the aptamer, SWCNTs-PEG-Fe₃O₄@CQDS /DOX-Apt, can be applied to the targeted dual-modal fluorescence/magnetic resonance imaging and the combined regimen of PTT/PDT/chemotherapy.
Fisher [25] et al. achieved significant photothermal ablation of human prostate cancer (PC3) and murine renal cancer (RENCA) cells using MWNTs under 1064 nm laser irradiation and also reduced the expression of heat shock proteins. Since the thermal induction of heat shock proteins can enhance the viability of tumor cells and make them resistant to PTT, this material can reduce the drug resistance of tumor cells. Lin Zhen [26] et al. constructed MWNTs functionalized with distearoylphosphatidylethanolamine polyethylene glycol 2000 (DSPE-PEG 2000). When MWNTs were combined with near-infrared laser irradiation for 2 minutes (at a power density of 5 W/cm²), it significantly caused a temperature rise and led to a remarkable decrease in cell viability. Therefore, this experiment confirms that the combination of MWNTs and near-infrared laser irradiation can significantly increase the temperature, and the generated high temperature can effectively kill tumor cells. Thus, these studies reveal the broad prospects of CNTs in the combined treatment of cancer.
3.4. Fullerene
3.4.1. Structure and material properties
Fullerene is a kind of football-shaped carbon nanomaterial (C60) composed of 60 or 70 carbon atoms, which was first discovered in 1985. Due to the poor hydrophilicity of fullerene under physiological conditions, its application in phototherapy is hindered. To overcome this obstacle, water-soluble fullerene derivatives can be developed by introducing functional groups, such as OH, COOH and NH2 [27]. Functionalized fullerenes have been proved to possess properties such as antioxidant activity, inhibition of angiogenesis, protection of the central nervous system, and suppression of tumor growth.
3.4.2. Applications in phototherapy
The team led by Moudgil [28] confirmed in 2010 that functionalized fullerene derivatives such as polyhydroxyfullerene (PHF) can be heated or ignited under continuous-wave near-infrared (NIR) irradiation with low intensity (<102 W·cm⁻²). PHF is a biocompatible and biodegradable water-soluble fullerene derivative. Moudgil et al. [29] studied its photothermal therapy and photoacoustic imaging properties. After intratumoral injection of PHF, when irradiated with 785 nm NIR light for 10 minutes, the average tumor area decreased by 32% within 2 hours. In addition, PHF has a size of only 1.3 nm and can be excreted through the kidneys.
Hu et al. [30] used graphene to deliver C60 fullerene to overcome the defects of C60. They prepared a nanocomposite (C60-PDA-rGO) composed of C60, polydopamine (PDA)-coated reduced graphene oxide (rGO), and folic acid (FA) by a simple method. PDA reduces and coats GO to obtain PDA-rGO with strong physiological solubility and biocompatibility, which is then reacted with the FA-C60 derivative (FFA) to prepare C60-PDA-rGO. They demonstrated that C60-PDA-rGO has an absorption band ranging from the ultraviolet to the NIR region. The combination of graphene, PDA, and FFA enhances light absorption and improves optical properties, and the targeting property of FA enhances cellular uptake. Under the irradiation of a xenon lamp emitting visible and NIR light, this composite exhibits synergistic photodynamic therapy (PDT)/photothermal therapy (PTT) activity, leading to a significant decrease in cell viability.
Fu Sheng [31] et al. obtained water-soluble C60-COOH through the Bingel cycloaddition reaction, loaded MnO2 nanoparticles through a hydrothermal reaction, and finally connected the tumor-targeting molecule folic acid using aminated polyethylene glycol to synthesize a multifunctional nanocomposite C60-Mn-PEG-FA. It is stably dispersed in an aqueous solution and generates reactive oxygen species (ROS) under light induction to achieve the effect of photodynamic therapy for tumors. At the same time, the loaded MnO2 nanoparticles can decompose to generate O2 in the hypoxic environment of the tumor, providing an oxygen source for photodynamic therapy to improve the efficiency of photodynamic therapy. Experiments and various characterizations have proven that C60-Mn-PEG-FA can realize the monitoring and treatment of tumor cells and is expected to become a potential integrated diagnostic and therapeutic agent for application in the field of clinical treatment.
4. Challenge and prospect
In conclusion, various types of carbon-based nanomaterials have become an important class of candidate materials in cancer phototherapy, and there have been numerous reports of remarkable achievements in both in vitro and in vivo experiments. However, in the process of further promoting these achievements for clinical application, many challenges still remain. From the material perspective, these carbon nanomaterials still face deficiencies such as poor biocompatibility and degradability, poor stability, long-term toxicity, and the inability to precisely control the size and shape of nanoparticles. At present, photothermal therapy also faces issues such as limited light penetration depth, low delivery efficiency of photothermal conversion agents, and unnecessary damage to normal tissues caused by overheating in the tumor area. Photodynamic therapy has relatively poor selectivity, and a limited phototoxic reaction may occur in the surrounding normal tissues. In phototherapy experiments, the laser power range often exceeds the safe range for continuous laser irradiation of the skin, and strong laser irradiation can cause irreversible damage to healthy cells and tissues [23].
Therefore, in the future of tumor photothermal therapy (PTT) and photodynamic therapy (PTD) based on nanomaterials, there are many potential development opportunities worth grasping: There is an urgent need to develop biodegradable nanomaterials and conduct comprehensive studies on their potential in vivo toxicity. Design multifunctional treatment platforms with diagnostic and therapeutic functions using a single nanostructure. Develop nanoplatforms that can be rapidly excreted from normal organs after systemic administration while effectively remaining in the tumor site. New methods, technologies, and devices to improve penetrability, etc. Combine PTT or PDT with other treatment methods to produce additional or even synergistic effects, thereby improving the cure rate of tumors, reducing toxic and side effects, and decreasing tumor recurrence and metastasis.
5. Conclusion
In summary, carbon-based nanomaterials represented by carbon dots, carbon nanotubes, graphene, and fullerenes have indeed shown extremely broad application prospects in the fields of photothermal therapy and photodynamic therapy for cancer treatment. Their unique structures, such as the hollow cylindrical shape of carbon nanotubes and the hexagonal layered structure of graphene, endow them with extraordinary material properties. These properties include a high specific surface area, excellent light absorption ability, and efficient photothermal conversion rate. For example, carbon dots can be designed to have strong fluorescence properties, which can be used for both imaging and drug delivery in phototherapy. However, despite the many promising properties of these nanomaterials, issues such as the biosafety of nanomaterials still remain significant barriers to their further clinical application. Concerns about potential long-term toxicity, difficulties in precisely controlling the size and shape of nanoparticles, and problems with biodegradability all urgently need to be addressed. In addition, how to achieve non-invasive diagnosis and cure of tumors while avoiding toxic side effects is also an urgent challenge faced by researchers today.
Nevertheless, the outlook remains optimistic. With the continuous improvement of the preparation technology of nanomaterials, scientists are now able to synthesize materials with more precise structures and properties. The continuous progress of application research has enabled a deeper understanding of the interaction between these nanomaterials and biological systems. Moreover, the continuous optimization of the two therapies, photothermal therapy (PTT) and photodynamic therapy (PTD), in terms of light sources, treatment protocols, etc., is also in progress. It is expected that biomedical research on nanomaterials will achieve more breakthroughs in the field of cancer treatment in the future.
References
[1]. Guangwen Cao. Epidemiological Characteristics, Current Status of Prevention and Control, and Future Response Strategies of Cancer in China [J]. Academic Journal of Naval Medical University, 2025, 46(03): 279-290. 10.16781/j.CN31-2187/R.20250050.
[2]. Maomao Cao, Wanqing Chen. The Epidemiology and Current Status of Prevention and Control of Malignant Tumors in China. Chinese Journal of Clinical Oncology, 46 (2019): 145-149.
[3]. Jiazhi Duan. Construction and Research of a Magnetic Nanomaterial-Mediated Tumor Targeted Diagnosis/Treatment System [D]. Shandong University, 2021. 10.27272/d. cnki. gshdu. 2021. 005956.
[4]. Ren Z., Cui J., Sun Q., et al. Polyethylene glycol-modified nanoscale conjugated polymer for the photothermal therapy of lung cancer [J]. Nanotechnology, 2022, 33(45):1-12.
[5]. Shi X., Tan Y., Liu Y., et al. Research Progress of Photothermal Nanomaterials in Multimodal Tumor Therapy [J]. Front Oncol, 2022,12:1-27.
[6]. Zhou W., Du M., Wang J., et al. Organic nanomaterials for near-infrared light-triggered photothermal/thermodynamic combination therapy [J]. Dyes and Pigments, 2022, 205:110499.
[7]. Zhao S., Yu X., Qian Y., et al. Multifunctional magnetic iron oxide nanoparticles: an advanced platform for cancer theranostics [J]. Theranostics, 2020, 10(14): 6278-6309.
[8]. Du J., Xu N., Fan J., et al. Carbon Dots for In Vivo Bioimaging and Theranostics [J]. Small, 2019, 15(32): e1805087.
[9]. Ali H., Ghosh S., Jana N. R. Fluorescent carbon dots as intracellular imaging probes [J]. Wiley Interdiscip Rev Nanomed Nanobiotechnol, 2020, 12(4): e1617.
[10]. Liu S., Zhang M., Yu H., et al. Immunoinducible Carbon Dot-Incorporated Hydrogels as a Photothermal-Derived Antigen Depot to Trigger a Robust Antitumor Immune Response [J]. ACS Appl Mater Interfaces, 2023, 15(6): 7700-12.
[11]. Mengyu Yang. Mitochondria-Targeted Gold-Doped Porous Carbon Quantum Dots for Photothermal Ablation and Photodynamic Therapy of Breast Cancer [D]. Jiangsu University, 2023. 10.27170/d.cnki.gjsuu.2023.001451.
[12]. Yang X. F., Wang A., QIiao B., et al. Single-atom catalysts: a new frontier inheterogeneous catalysis [J]. Acc Chem Res, 2013, 46(8): 1740-8.
[13]. Del Valle A. C., Yeh C. K., Huang Y. F. Near Infrared-Activatable Platinum-Decorated Gold Nanostars for Synergistic Photothermal/Ferroptotic Therapy in Combating Cancer Drug Resistance [J]. Adv Healthc Mater, 2020, 9(20): e2000864.
[14]. Peng C., Liang Y., Su N., et al. Dual nanoenzymes loaded hollow mesoporousorganotantalum nanospheres for chemo-radio sensitization [J]. J Control Release, 2022, 347: 369-78.
[15]. Chunlian Zhong, Changjian Fang, Guiyu Zhou, et al. Research on Graphene Oxide Nanoparticles as a Clinical Drug Carrier in Tumor Treatment [J]. Chinese Pharmacological Bulletin, 2024, 40(08): 1413-1418.
[16]. Yu F., Quan F., Xu J., et al. Breast cancer prognosis signature: linking risk stratification to disease subtypes [J]. Brief Bioinform, 2019, 20(6): 2130-40.
[17]. Mcdonald E. S., Clark A. S., Tchou J., et al. Clinical Diagnosis and Management of Breast Cancer [J]. J Nucl Med, 2016, 57 Suppl 1: 9S-16S.
[18]. Bellon J. R., Burstein H. J., Frank E. S., et al. Multidisciplinary considerations in the treatment of triple-negative breast cancer [J]. CA Cancer J Clin, 2020, 70(6): 432-42.
[19]. Kerr A. J., Dodwell D., Mcgale P., et al. Adjuvant and neoadjuvant breast cancer treatments: A systematic review of their effects on mortality [J]. Cancer Treat Rev, 2022,105: 102375.
[20]. Masuda N., Lee S. J., Ohtani S., et al. Adjuvant Capecitabine for Breast Cancer after Preoperative Chemotherapy [J]. N Engl J Med, 2017, 376(22): 2147-59.
[21]. Kandasamy G., Maity D. Multifunctional theranostic nanoparticles for biomedical cancer treatments: A comprehensive review. Mater.Sci. Eng. C Mater. Biol. Appl., 2021,127:112199
[22]. Su T., Cheng F., Yan J., et al. Hierarchical nanocomposites of graphene oxide and PEGylated protoporphyrin as carriers to load d oxorubicin hydrochloride for trimodal synergistic therapy [J]. J Mater Chem B, 2018, 6(28):4687-96.
[23]. Wang Y. F., Meng H. M., Li Z. H. Near⁃infrared inorganic nanomaterial⁃based nanosystems for photothermal therapy. Nanoscale, 2021, 13(19):8751⁃8772.
[24]. Zhang M., Wang W. T., et al. Magnetofluorescent Fe3O4/carbon quantum dots coated single-walled carbon nanotubes as dual-modal targeted imaging and chemo/photo-dynamic/photothermal triple-modal therapeutic agents. Chem. Eng.J., 2018, 338:526-538.
[25]. Fisher J. W., Sarkar S., et al. Photothermal response of human and murine cancer cells to multiwalled carbon nanotubes after laser irradiation. Cancer Res., 2010, 70 (23):9855-9864.
[26]. Zhen Lin. Photothermal Therapy Mediated by Multi-Walled Carbon Nanotubes Combined with Chemotherapy for Bone Metastatic Tumors [D]. Southern Medical University, 2016.
[27]. Younis M. R., He G., Qu J. L., Lin J., Huang P., Xia X. H. Inorganic nanomaterials with intrinsic singlet oxygen generation for photody-namic therapy. Adv. Sci., 2021, 8(21):e2102587.
[28]. Krishna V., Stevens N., Koopman B., Moudgil B. Optical heating and rapid transformation of functionalized fullerenes. Nat. Nanotechnol., 2010, 5(5):330-334.
[29]. Krishna V., Singh A., et al. Polyhy-droxy fullerenes for non⁃invasive cancer imaging and therapy. Small, 2010, 6(20):2236-2241.
[30]. Hu Z., Zhao F., Wang Y. F., et al. Facile fabrication of a C60⁃polydopamine⁃graphene nanohybridfor single light induced photothermal and photodynamic therapy.Chem. Commun., 2014, 50(74):10815⁃10818.
[31]. Sheng Fu. Preparation of Fullerene-Based Nanomaterials and Their Applications in Photodynamic Therapy and Biological Imaging [D]. South-Central University for Nationalities, 2021.
Cite this article
Liang,X. (2025). Applications of Carbon-based Nanomaterials in the Field of Cancer Phototherapy. Theoretical and Natural Science,109,64-72.
Data availability
The datasets used and/or analyzed during the current study will be available from the authors upon reasonable request.
Disclaimer/Publisher's Note
The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of EWA Publishing and/or the editor(s). EWA Publishing and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
About volume
Volume title: Proceedings of CONF-MPCS 2025 Symposium: Leveraging EVs and Machine Learning for Sustainable Energy Demand Management
© 2024 by the author(s). Licensee EWA Publishing, Oxford, UK. This article is an open access article distributed under the terms and
conditions of the Creative Commons Attribution (CC BY) license. Authors who
publish this series agree to the following terms:
1. Authors retain copyright and grant the series right of first publication with the work simultaneously licensed under a Creative Commons
Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this
series.
2. Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the series's published
version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial
publication in this series.
3. Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and
during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See
Open access policy for details).
References
[1]. Guangwen Cao. Epidemiological Characteristics, Current Status of Prevention and Control, and Future Response Strategies of Cancer in China [J]. Academic Journal of Naval Medical University, 2025, 46(03): 279-290. 10.16781/j.CN31-2187/R.20250050.
[2]. Maomao Cao, Wanqing Chen. The Epidemiology and Current Status of Prevention and Control of Malignant Tumors in China. Chinese Journal of Clinical Oncology, 46 (2019): 145-149.
[3]. Jiazhi Duan. Construction and Research of a Magnetic Nanomaterial-Mediated Tumor Targeted Diagnosis/Treatment System [D]. Shandong University, 2021. 10.27272/d. cnki. gshdu. 2021. 005956.
[4]. Ren Z., Cui J., Sun Q., et al. Polyethylene glycol-modified nanoscale conjugated polymer for the photothermal therapy of lung cancer [J]. Nanotechnology, 2022, 33(45):1-12.
[5]. Shi X., Tan Y., Liu Y., et al. Research Progress of Photothermal Nanomaterials in Multimodal Tumor Therapy [J]. Front Oncol, 2022,12:1-27.
[6]. Zhou W., Du M., Wang J., et al. Organic nanomaterials for near-infrared light-triggered photothermal/thermodynamic combination therapy [J]. Dyes and Pigments, 2022, 205:110499.
[7]. Zhao S., Yu X., Qian Y., et al. Multifunctional magnetic iron oxide nanoparticles: an advanced platform for cancer theranostics [J]. Theranostics, 2020, 10(14): 6278-6309.
[8]. Du J., Xu N., Fan J., et al. Carbon Dots for In Vivo Bioimaging and Theranostics [J]. Small, 2019, 15(32): e1805087.
[9]. Ali H., Ghosh S., Jana N. R. Fluorescent carbon dots as intracellular imaging probes [J]. Wiley Interdiscip Rev Nanomed Nanobiotechnol, 2020, 12(4): e1617.
[10]. Liu S., Zhang M., Yu H., et al. Immunoinducible Carbon Dot-Incorporated Hydrogels as a Photothermal-Derived Antigen Depot to Trigger a Robust Antitumor Immune Response [J]. ACS Appl Mater Interfaces, 2023, 15(6): 7700-12.
[11]. Mengyu Yang. Mitochondria-Targeted Gold-Doped Porous Carbon Quantum Dots for Photothermal Ablation and Photodynamic Therapy of Breast Cancer [D]. Jiangsu University, 2023. 10.27170/d.cnki.gjsuu.2023.001451.
[12]. Yang X. F., Wang A., QIiao B., et al. Single-atom catalysts: a new frontier inheterogeneous catalysis [J]. Acc Chem Res, 2013, 46(8): 1740-8.
[13]. Del Valle A. C., Yeh C. K., Huang Y. F. Near Infrared-Activatable Platinum-Decorated Gold Nanostars for Synergistic Photothermal/Ferroptotic Therapy in Combating Cancer Drug Resistance [J]. Adv Healthc Mater, 2020, 9(20): e2000864.
[14]. Peng C., Liang Y., Su N., et al. Dual nanoenzymes loaded hollow mesoporousorganotantalum nanospheres for chemo-radio sensitization [J]. J Control Release, 2022, 347: 369-78.
[15]. Chunlian Zhong, Changjian Fang, Guiyu Zhou, et al. Research on Graphene Oxide Nanoparticles as a Clinical Drug Carrier in Tumor Treatment [J]. Chinese Pharmacological Bulletin, 2024, 40(08): 1413-1418.
[16]. Yu F., Quan F., Xu J., et al. Breast cancer prognosis signature: linking risk stratification to disease subtypes [J]. Brief Bioinform, 2019, 20(6): 2130-40.
[17]. Mcdonald E. S., Clark A. S., Tchou J., et al. Clinical Diagnosis and Management of Breast Cancer [J]. J Nucl Med, 2016, 57 Suppl 1: 9S-16S.
[18]. Bellon J. R., Burstein H. J., Frank E. S., et al. Multidisciplinary considerations in the treatment of triple-negative breast cancer [J]. CA Cancer J Clin, 2020, 70(6): 432-42.
[19]. Kerr A. J., Dodwell D., Mcgale P., et al. Adjuvant and neoadjuvant breast cancer treatments: A systematic review of their effects on mortality [J]. Cancer Treat Rev, 2022,105: 102375.
[20]. Masuda N., Lee S. J., Ohtani S., et al. Adjuvant Capecitabine for Breast Cancer after Preoperative Chemotherapy [J]. N Engl J Med, 2017, 376(22): 2147-59.
[21]. Kandasamy G., Maity D. Multifunctional theranostic nanoparticles for biomedical cancer treatments: A comprehensive review. Mater.Sci. Eng. C Mater. Biol. Appl., 2021,127:112199
[22]. Su T., Cheng F., Yan J., et al. Hierarchical nanocomposites of graphene oxide and PEGylated protoporphyrin as carriers to load d oxorubicin hydrochloride for trimodal synergistic therapy [J]. J Mater Chem B, 2018, 6(28):4687-96.
[23]. Wang Y. F., Meng H. M., Li Z. H. Near⁃infrared inorganic nanomaterial⁃based nanosystems for photothermal therapy. Nanoscale, 2021, 13(19):8751⁃8772.
[24]. Zhang M., Wang W. T., et al. Magnetofluorescent Fe3O4/carbon quantum dots coated single-walled carbon nanotubes as dual-modal targeted imaging and chemo/photo-dynamic/photothermal triple-modal therapeutic agents. Chem. Eng.J., 2018, 338:526-538.
[25]. Fisher J. W., Sarkar S., et al. Photothermal response of human and murine cancer cells to multiwalled carbon nanotubes after laser irradiation. Cancer Res., 2010, 70 (23):9855-9864.
[26]. Zhen Lin. Photothermal Therapy Mediated by Multi-Walled Carbon Nanotubes Combined with Chemotherapy for Bone Metastatic Tumors [D]. Southern Medical University, 2016.
[27]. Younis M. R., He G., Qu J. L., Lin J., Huang P., Xia X. H. Inorganic nanomaterials with intrinsic singlet oxygen generation for photody-namic therapy. Adv. Sci., 2021, 8(21):e2102587.
[28]. Krishna V., Stevens N., Koopman B., Moudgil B. Optical heating and rapid transformation of functionalized fullerenes. Nat. Nanotechnol., 2010, 5(5):330-334.
[29]. Krishna V., Singh A., et al. Polyhy-droxy fullerenes for non⁃invasive cancer imaging and therapy. Small, 2010, 6(20):2236-2241.
[30]. Hu Z., Zhao F., Wang Y. F., et al. Facile fabrication of a C60⁃polydopamine⁃graphene nanohybridfor single light induced photothermal and photodynamic therapy.Chem. Commun., 2014, 50(74):10815⁃10818.
[31]. Sheng Fu. Preparation of Fullerene-Based Nanomaterials and Their Applications in Photodynamic Therapy and Biological Imaging [D]. South-Central University for Nationalities, 2021.









